Nondestructive Methods of Pathogen Detection: Importance of Mosquito Integrity in Studies of Disease Transmission and Control
Abstract
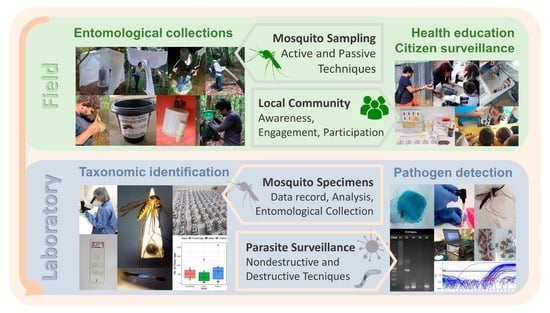
:1. Introduction
2. Why Do Mosquito Systematics Matter?
2.1. Mosquito Systematics and Taxonomy
2.2. Classical Taxonomy x Integrated Approaches
2.3. Culicidae as a Biodiversity Component
3. Pathogens Transmitted by Mosquitoes
Life Cycle of Parasites and Viruses in the Mosquito
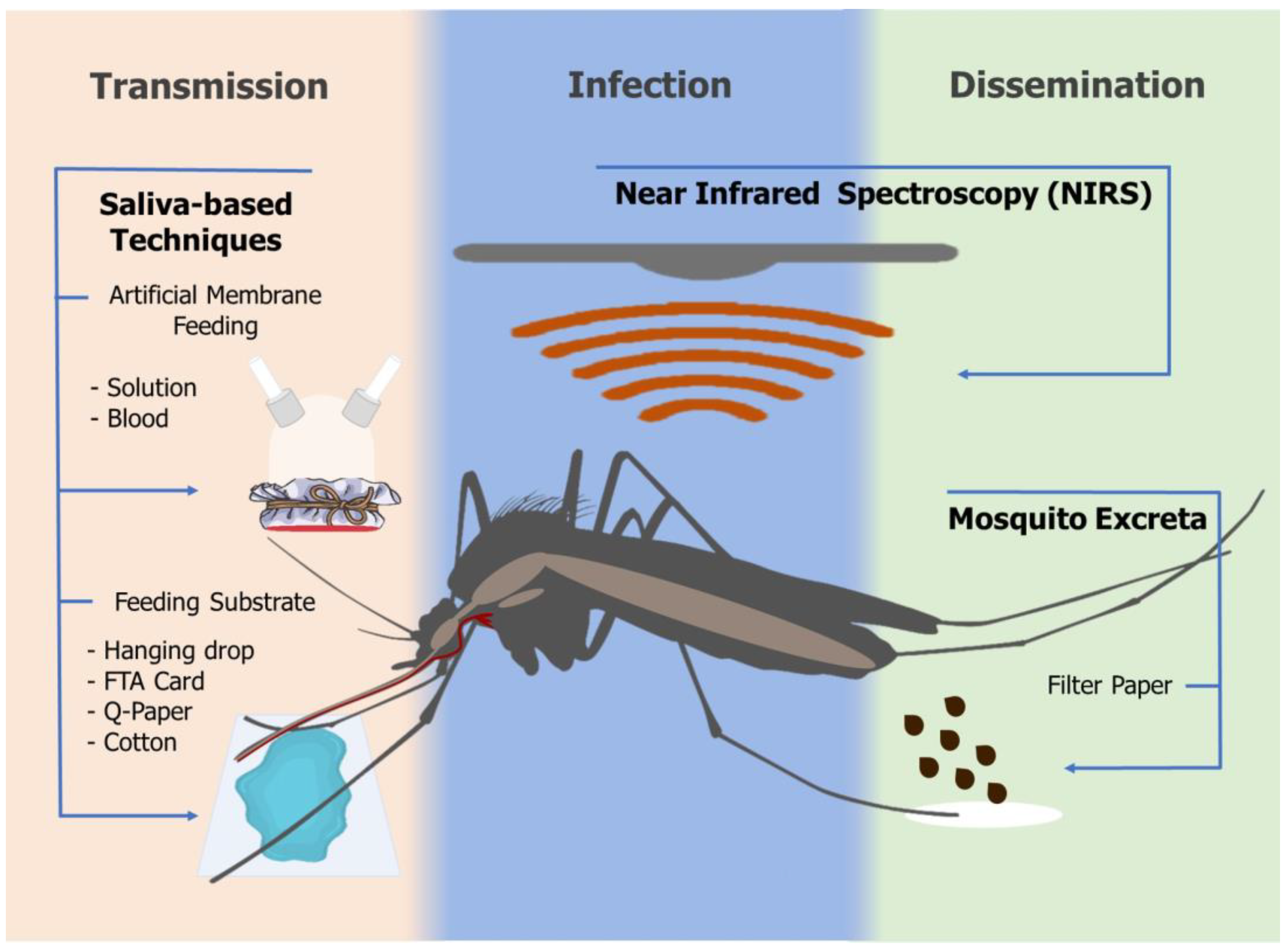
4. Nondestructive Approaches to Pathogen Detection in Mosquitoes
4.1. Saliva-Based Technique—Feeding Substrate
4.1.1. FTA Cards
4.1.2. Q-Paper
4.1.3. Cotton Substrates
4.1.4. Hanging Drop Method
4.2. Saliva-Based Technique—Artificial Membrane Feeding (AMF)
4.2.1. AMF—Blood-Feeding
4.2.2. AMF—Non-Blood-Feeding
4.3. Mosquito Excreta
4.4. Near-Infrared Spectroscopy (NIRS)
5. Scientific Horizons Expanded: Museums, Biorepositories, and Nondestructive DNA Extraction
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gubler, D.J. Resurgent vector-borne diseases as a global health problem. Emerg. Infect. Dis. 1998, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Swei, A.; Couper, L.I.; Coffey, L.L.; Kapan, D.; Bennett, S. Patterns, drivers, and challenges of vector-borne disease emergence. Vector Borne Zoonotic Dis. 2020, 20, 159–170. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Vector Control Response 2017–2030; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Christophers, S. Aedes aegypti (L.) the Yellow Fever Mosquito: Its Life History, Bionomics and Structure; Cambridge University Press: London, UK, 1960. [Google Scholar]
- Lehane, M.J. The Biology of Blood-Sucking in Insects, 2nd ed.; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Foster, W.A.; Walker, E.D. Mosquitoes (Culicidae). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Elsevier: London, UK, 2019; Chapter 15; pp. 261–325. [Google Scholar]
- Barker, C.M.; Reisen, W.K. Epidemiology of vector-borne diseases. In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Elsevier: London, UK, 2019; Chapter 4; pp. 33–49. [Google Scholar]
- Almeida, A.P.G.; Fouque, F.; Launois, P.; Sousa, C.A.; Silveira, H. From the laboratory to the field: Updating capacity building in medical entomology. Trends Parasitol. 2017, 33, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Connelly, R. Highlights of medical entomology 2018: The importance of sustainable surveillance of vectors and vector-borne pathogens. J. Med. Entomol. 2019, 56, 1183–1187. [Google Scholar] [CrossRef] [Green Version]
- Eldridge, B.F.; Edman, J.D. Medical Entomology: A Textbook on Public Health and Veterinary Problems Caused by Arthropods, Revised ed.; Science+Business Media: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Braz Sousa, L.; Fricker, S.; Webb, C.E.; Baldock, K.L.; Williams, C.R. Citizen Science Mosquito Surveillance by Ad Hoc Observation Using the iNaturalist Platform. Int. J. Environ. Res. Public Health 2022, 19, 6337. [Google Scholar] [CrossRef]
- Sousa, L.B.; Fricker, S.R.; Doherty, S.S.; Webb, C.E.; Baldock, K.L.; Williams, C.R. Citizen science and smartphone e-entomology enables low-cost upscaling of mosquito surveillance. Sci. Total Environ. 2020, 704, 135349. [Google Scholar] [CrossRef]
- Carney, R.M.; Mapes, C.; Low, R.D.; Long, A.; Bowser, A.; Durieux, D.; Rivera, K.; Dekramanjian, B.; Bartumeus, F.; Guerrero, D.; et al. Integrating global citizen science platforms to enable next-generation surveillance of invasive and vector mosquitoes. Insects 2022, 13, 675. [Google Scholar] [CrossRef]
- Pedro, P.M.; Amorim, A.; Rojas, M.V.R.; Luizi Sá, I.; Ribeiro Galardo, A.K.; Santos Neto, N.F.; Pires de Carvalho, D.; Nabas Ribeiro, K.A.; Pepe Razzolini, M.T.; Sallum, M.A.M. Culicidae-centric metabarcoding through targeted use of D2 ribosomal DNA primers. PeerJ 2020, 8, e9057. [Google Scholar] [CrossRef]
- Pedro, P.M.; Rodrigues de Sá, I.L.; Rojas, M.V.R.; Amorim, J.A.; Ribeiro Galardo, A.K.; Santos Neto, N.F.; Furtado, N.V.R.; Pires de Carvalho, D.; Nabas Ribeiro, K.A.; de Paiva, M.; et al. Efficient Monitoring of Adult and Immature Mosquitoes through Metabarcoding of Bulk Samples: A Case Study for Non-Model Culicids With Unique Ecologies. J. Med. Entomol. 2021, 58, 1210–1218. [Google Scholar] [CrossRef]
- Mechai, S.; Bilodeau, G.; Lung, O.; Roy, M.; Steeves, R.; Gagne, N.; Baird, D.; Lapen, D.R.; Ludwig, A.; Ogden, N.H. Mosquito identification from bulk samples using DNA metabarcoding: A protocol to support mosquito-borne disease surveillance in Canada. J. Med. Entomol. 2021, 58, 1686–1700. [Google Scholar] [CrossRef]
- Piper, A.M.; Batovska, J.; Cogan, N.O.; Weiss, J.; Cunningham, J.P.; Rodoni, B.C.; Blacket, M.J. Prospects and challenges of implementing DNA metabarcoding for high-throughput insect surveillance. GigaScience 2019, 8, giz092. [Google Scholar] [CrossRef] [Green Version]
- Batovska, J.; Lynch, S.E.; Cogan, N.O.I.; Brown, K.; Darbro, J.M.; Kho, E.A.; Blacket, M.J. Effective mosquito and arbovirus surveillance using metabarcoding. Mol. Ecol. Resour. 2018, 18, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Giraldo-Calderón, G.I.; Harb, O.S.; Kelly, S.A.; Rund, S.S.; Roos, D.S.; McDowell, M.A. VectorBase. org updates: Bioinformatic resources for invertebrate vectors of human pathogens and related organisms. Curr. Opin. Insect. Sci. 2022, 50, 100860. [Google Scholar] [CrossRef]
- Scarpassa, V.M.; Batista, E.T.; da Costa Ferreira, V.; dos Santos Neto, V.A.; Roque, R.A.; Tadei, W.P.; Ferreira, F.A.D.; da Costa, F.M. DNA barcoding suggests new species for the Mansonia subgenus (Mansonia, Mansoniini, Culicidae, Diptera) in the area surrounding the Jirau hydroelectric dam, Porto Velho municipality, Rondônia state, Brazil. Acta Trop. 2022, 233, 106574. [Google Scholar] [CrossRef]
- Do Nascimento, B.L.S.; da Silva, F.S.; Nunes-Neto, J.P.; de Almeida Medeiros, D.B.; Cruz, A.C.R.; da Silva, S.P.; Silva, L.H.S.; Monteiro, H.A.O.; Dias, D.D.; Vieira, D.B.R.; et al. First description of the mitogenome and phylogeny of Culicinae species from the Amazon region. Genes 2021, 12, 1983. [Google Scholar] [CrossRef]
- Muñoz-Gamba, A.S.; Laiton-Donato, K.; Perdomo-Balaguera, E.; Castro, L.R.; Usme-Ciro, J.A.; Parra-Henao, G. Molecular characterization of mosquitoes (Diptera: Culicidae) from the Colombian rainforest. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, e24. [Google Scholar] [CrossRef]
- Silva-do-Nascimento, T.F.; Sánchez-Ribas, J.; Oliveira, T.M.; Bourke, B.P.; Oliveira-Ferreira, J.; Rosa-Freitas, M.G.; Lourenço-de-Oliveira, R.; Marinho-e-Silva, M.; Sallum, M.A.M. Molecular Analysis Reveals a High Diversity of Anopheline Mosquitoes in Yanomami Lands and the Pantanal Region of Brazil. Genes 2021, 12, 1995. [Google Scholar] [CrossRef]
- Suarez, A.V.; Tsutsui, N.D. The value of museum collections for research and society. BioScience. 2004, 54, 66–74. [Google Scholar] [CrossRef]
- Sá, M.R. Scientific collections, tropical medicine and the development of Entomology in Brazil: The contribution of Instituto Oswaldo Cruz. Parassitologia 2008, 50, 187–197. [Google Scholar]
- Talaga, S.; Duchemin, J.B.; Girod, R.; Dusfour, I. The Culex mosquitoes (Diptera: Culicidae) of French Guiana: A comprehensive review with the description of three new species. J. Med. Entomol. 2021, 58, 182–221. [Google Scholar] [CrossRef]
- Saraiva, J.F.; Scarpassa, V.M. Anopheles (Nyssorhynchus) tadei: A new species of the Oswaldoi-konderi complex (Diptera, Anophelinae) and its morphological and molecular distinctions from An. konderi sensu stricto. Acta Trop. 2021, 221, 106004. [Google Scholar] [CrossRef] [PubMed]
- Talaga, S.; Gendrin, M. Three new species of Culex (Melanoconion) (Diptera: Culicidae) from French Guiana based on morphological and molecular data. Zootaxa 2022, 5205, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Sant’Ana, D.C.; Sallum, M.A.M. A new species of the Nuneztovari Complex of Nyssorhynchus (Diptera: Culicidae) from the western Brazilian Amazon. Zootaxa 2022, 5134, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Sant’ana, D.C.; Sallum, M.A.M. A new species of the Arthuri Complex of the Strodei Subgroup of Nyssorhynchus (Diptera: Culicidae). Zootaxa 2022, 5175, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, R.S.G.; Hutchings, R.W.; Menezes, I.S.; Sallum, M.A.M. Mosquitoes (Diptera: Culicidae) from the southwestern Brazilian Amazon: Liberdade and Gregório Rivers. J. Med. Entomol. 2020, 57, 1793–1811. [Google Scholar] [CrossRef]
- Morales Viteri, D.; Herrera-Varela, M.; Albuja, M.; Quiroga, C.; Diaz, G.; del Aguila Morante, C.; Ramirez, D.; Vinetz, J.M.; Bickersmith, S.A.; Conn, J.E. New records of Anopheles benarrochi B (Diptera: Culicidae) in malaria hotspots in the Amazon regions of Ecuador and Peru. J. Med. Entomol. 2021, 58, 1234–1240. [Google Scholar] [CrossRef]
- Simpson, G.G. Principles of animal taxonomy. In Principles of Animal Taxonomy; Columbia University Press: New York, NY, USA, 1961. [Google Scholar]
- Hennig, W. Phylogenetic Systematics; University of Illinois Press: Champaign, IL, USA, 1999. [Google Scholar]
- Felsenstein, J. Phylogenies and the comparative method. Am. Nat. 1985, 125, 1–15. [Google Scholar] [CrossRef]
- Mayr, E. The Growth of Biological Thought: Diversity, Evolution, and Inheritance; Harvard University Press: Cambridge, MA, USA, 1982. [Google Scholar]
- De Queiroz, K. The general lineage concept of species, species criteria, and the process of speciation. In Endless Forms: Species and Speciation; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Frankham, R.; Ballou, J.D.; Dudash, M.R.; Eldridge, M.D.; Fenster, C.B.; Lacy, R.C.; Mendelson, J.R.; Porton, I.J.; Ralls, K.; Ryder, O.A. Implications of different species concepts for conserving biodiversity. Biol. Conserv. 2012, 153, 25–31. [Google Scholar] [CrossRef]
- Failloux, A.B.; Bouattour, A.; Faraj, C.; Gunay, F.; Haddad, N.; Harrat, Z.; Jancheska, E.; Kanani, K.; Kenawy, M.A.; Kota, M.; et al. Surveillance of viruses transmitted by arthropods and their vectors in the Mediterranean and Black Sea regions within the MediLabSecure network. Curr. Trop. Med. Rep. 2017, 4, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Erlank, E.; Koekemoer, L.L.; Coetzee, M. The importance of morphological identification of African anopheline mosquitoes (Diptera: Culicidae) for malaria control programmes. Malar. J. 2018, 17, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Daly, H.V. Endangered species: Doctoral students in systematic entomology. Am. Entomol. 1995, 41, 55–59. [Google Scholar] [CrossRef]
- Cuisance, D.; Rioux, J.A. Current status of medical and veterinary entomology in France: Endangered discipline or promising science? Comp. Immun. Microbiol. Infect. Dis. 2004, 27, 377–392. [Google Scholar] [CrossRef]
- Stribling, J.B.; Moulton, S.R., II; Lester, G.T. Determining the quality of taxonomic data. J. N. Am. Benthol. Soc. 2003, 22, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Stribling, J.B.; Pavlik, K.L.; Holdsworth, S.M.; Leppo, E.W. Data quality, performance, and uncertainty in taxonomic identification for biological assessments. J. N. Am. Benthol. Soc. 2008, 27, 906–919. [Google Scholar] [CrossRef]
- Stribling, J.B. Partitioning error sources for quality control and comparability analysis in biological monitoring and assessment. In Modern Approaches to Quality Control; InTech: Rijeka, Croatia, 2011; pp. 59–84. [Google Scholar]
- Fontenille, D.; Almeras, L.; Garros, C. Concepts et méthodes d’identification des espèces d’arthropodes. In Entomologie Médicale et Vétérinaire; IRD Editions & Quae Editions: Marseille, France, 2017; pp. 61–78. [Google Scholar]
- Nayduch, D.; Fryxell, R.T.; Olafson, P.U. Molecular tools used in medical and veterinary entomology. In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Elsevier: London, UK, 2019; Chapter 28; pp. 673–694. [Google Scholar]
- Jourdain, F.; Picard, M.; Sulesco, T.; Haddad, N.; Harrat, Z.; Sawalha, S.S.; Günay, F.; Kanani, K.; Shaibi, T.; Akhramenko, D.; et al. Identification of mosquitoes (Diptera: Culicidae): An external quality assessment of medical entomology laboratories in the MediLabSecure Network. Parasite Vectors 2018, 11, 43. [Google Scholar] [CrossRef] [Green Version]
- Dayrat, B. Towards integrative taxonomy. Biol. J. Linn. Soc. 2005, 85, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Will, K.W.; Mishler, B.D.; Wheeler, Q.D. The perils of DNA barcoding and the need for integrative taxonomy. Syst. Biol. 2005, 54, 844–851. [Google Scholar] [CrossRef]
- Padial, J.M.; De La Riva, I. A response to recent proposals for integrative taxonomy. Biol. J. Linn. Soc. 2010, 101, 747–756. [Google Scholar] [CrossRef] [Green Version]
- Yeates, D.K.; Seago, A.; Nelson, L.; Cameron, S.L.; Joseph, L.E.O.; Trueman, J.W. Integrative taxonomy, or iterative taxonomy? Syst. Entomol. 2011, 36, 209–217. [Google Scholar] [CrossRef]
- De Queiroz, K. Species concepts and species delimitation. Syst. Biol. 2007, 56, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Forattini, O.P. Culicidologia Médica, 1st ed.; EDUSP: São Paulo, Brazil, 1996. [Google Scholar]
- Reinert, J.F.; Harbach, R.E.; Kitching, I.A.N.J. Phylogeny and classification of Ochlerotatus and allied taxa (Diptera: Culicidae: Aedini) based on morphological data from all life stages. Zool. J. Linn. Soc. 2008, 153, 29–114. [Google Scholar] [CrossRef] [Green Version]
- Coetzee, M.; Koekemoer, L.L. Molecular systematics and insecticide resistance in the major African malaria vector Anopheles funestus. Annu. Rev. Entomol. 2013, 58, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Harbach, R.E.; Kitching, I.J. Phylogeny and classification of the Culicidae (Diptera). Syst. Entomol. 1998, 23, 327–370. [Google Scholar] [CrossRef]
- Harbach, R.E. Mosquito Taxonomic Inventory. 2023. Available online: https://mosquito-taxonomic-inventory.myspecies.info/ (accessed on 3 February 2023).
- Suesdek, L. Microevolution of medically important mosquitoes—A review. Acta Trop. 2019, 191, 162–171. [Google Scholar] [CrossRef]
- Hutchings, R.S.G.; Sallum, M.A.M.; Ferreira, R.L.M.; Hutchings, R.W. Mosquitoes of the Jaú National Park and their potential importance in Brazilian Amazonia. Med. Vet. Entomol. 2005, 19, 428–441. [Google Scholar] [CrossRef]
- Hutchings, R.S.G.; Sallum, M.A.M.; Hutchings, R.W. Mosquito (Diptera: Culicidae) diversity of a forest-fragment mosaic in the Amazon rain forest. J. Med. Entomol. 2011, 48, 173–187. [Google Scholar] [CrossRef]
- World Health Organization. A Global Brief on Vector-Borne Diseases; WHO: Geneve, Switzerland, 2014. [Google Scholar]
- McMillan, J.R.; Blakney, R.A.; Mead, D.G.; Koval, W.T.; Coker, S.M.; Waller, L.A.; Kitron, U.; Vazquez-Prokopec, G.M. Linking the vectorial capacity of multiple vectors to observed patterns of West Nile virus transmission. J. Appl. Ecol. 2019, 56, 956–965. [Google Scholar] [CrossRef]
- Rios, F.G.F.; do Nascimento, V.A.; Naveca, F.G.; Vieira, D.S.; Julião, G.R. Arbovirus detection in synanthropic mosquitoes from the Brazilian Amazon and in mosquito saliva using Flinders Technology Associates cards. Microbes Infect. 2023, 25, 105046. [Google Scholar] [CrossRef]
- Serra, O.P.; Cardoso, B.F.; Ribeiro, A.L.M.; Santos, F.A.L.D.; Slhessarenko, R.D. Mayaro virus and dengue virus 1 and 4 natural infection in culicids from Cuiabá, state of Mato Grosso, Brazil. Mem. Inst. Oswaldo Cruz 2016, 111, 20–29. [Google Scholar] [CrossRef]
- Da Silva Neves, N.A.; Da Silva Ferreira, R.; Morais, D.O.; Pavon, J.A.R.; de Pinho, J.B.; Slhessarenko, R.D. Chikungunya, Zika, Mayaro, and Equine Encephalitis virus detection in adult Culicinae from South Central Mato Grosso, Brazil, during the rainy season of 2018. Braz. J. Microbiol. 2022, 53, 63–70. [Google Scholar] [CrossRef]
- Alencar, J.; Ferreira de Mello, C.; Brisola Marcondes, C.; Érico Guimarães, A.; Toma, H.K.; Queiroz Bastos, A.; Freitas Silva, S.O.; Lisboa Machado, S. Natural infection and vertical transmission of Zika virus in sylvatic mosquitoes Aedes albopictus and Haemagogus leucocelaenus from Rio de Janeiro, Brazil. Infect. Dis. Trop. Med. 2021, 6, 99. [Google Scholar] [CrossRef]
- Stanzani, L.M.D.A.; Motta, M.D.A.; Erbisti, R.S.; Abreu, F.V.S.D.; Nascimento-Pereira, A.C.; Ferreira-de-Brito, A.; Neves, M.S.A.S.; Pereira, G.R.; Pereira, G.R.; dos Santos, C.B.; et al. Back to Where It Was First Described: Vectors of Sylvatic Yellow Fever Transmission in the 2017 Outbreak in Espírito Santo, Brazil. Viruses 2022, 14, 2805. [Google Scholar] [CrossRef]
- De Curcio, J.S.; Salem-Izacc, S.M.; Neto, L.M.P.; Nunes, E.B.; Anunciação, C.E.; de Paula Silveira-Lacerda, E. Detection of Mayaro virus in Aedes aegypti mosquitoes circulating in Goiania-Goias-Brazil. Microbes Infect. 2022, 24, 104948. [Google Scholar] [CrossRef]
- Marcondes, C.B. Arthropod Borne Diseases; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Cardoso, B.F.; Serra, O.P.; Heinen, L.B.D.S.; Zuchi, N.; Souza, V.C.D.; Naveca, F.G.; dos Santos, A.M.M.; Slhessarenko, R.D. Detection of Oropouche virus segment S in patients and in Culex quinquefasciatus in the state of Mato Grosso, Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 745–754. [Google Scholar] [CrossRef]
- Pereira-Silva, J.W.; Ríos-Velásquez, C.M.; Lima, G.R.D.; Marialva dos Santos, E.F.; Belchior, H.C.M.; Luz, S.L.B.; Naveca, F.G.; Pessoa, F.A.C. Distribution and diversity of mosquitoes and Oropouche-like virus infection rates in an Amazonian rural settlement. PLoS ONE 2021, 16, e0246932. [Google Scholar] [CrossRef]
- Hoyos-López, R.; Suaza-Vasco, J.; Rúa-Uribe, G.; Uribe, S.; Gallego-Gómez, J.C. Molecular detection of flaviviruses and alphaviruses in mosquitoes (Diptera: Culicidae) from coastal ecosystems in the Colombian Caribbean. Mem. Inst. Oswaldo Cruz 2016, 111, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Beranek, M.D.; Gallardo, R.; Almiron, W.R.; Contigiani, M.S. First detection of Mansonia titillans (Diptera: Culicidae) infected with St. Louis encephalitis virus (Flaviviridae: Flavivirus) and Bunyamwera serogroup (Peribunyaviridae: Orthobunyavirus) in Argentina. J. Vector Ecol. 2018, 43, 340–343. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, A.; Ruiz, S.; Herrero, L.; Moreno, J.; Molero, F.; Magallanes, A.; Sánchez-Seco, M.P.; Figuerola, J.; Tenorio, A. West Nile and Usutu viruses in mosquitoes in Spain, 2008–2009. Am. J. Trop. Med. Hyg. 2011, 85, 178. [Google Scholar] [CrossRef]
- Benbetka, S.; Hachid, A.; Benallal, K.E.; Benbetka, C.; Khaldi, A.; Bitam, I.; Harrat, Z. First field evidence infection of Culex perexiguus by West Nile virus in Sahara Oasis of Algeria. J. Vector Borne Dis. 2018, 55, 305. [Google Scholar] [CrossRef]
- Unlu, I.; Kramer, W.L.; Roy, A.F.; Foil, L.D. Detection of West Nile virus RNA in mosquitoes and identification of mosquito blood meals collected at alligator farms in Louisiana. J. Med. Entomol. 2010, 47, 625–633. [Google Scholar] [CrossRef]
- Van den Eynde, C.; Sohier, C.; Matthijs, S.; De Regge, N. Japanese encephalitis virus interaction with mosquitoes: A review of vector competence, vector capacity and mosquito immunity. Pathogens 2022, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Roesch, F.; Fajardo, A.; Moratorio, G.; Vignuzzi, M. Usutu virus: An arbovirus on the rise. Viruses 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Lu, G.; Li, J.; Kelly, P.; Li, M.; Wang, J.; Zhang, Y.; Wu, H.; Wang, C. Molecular Detection of Rickettsia felis and Rickettsia bellii in Mosquitoes. Vector Borne Dis. 2019, 19, 802–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melaun, C.; Zotzmann, S.; Santaella, V.G.; Werblow, A.; Zumkowski-Xylander, H.; Kraiczy, P.; Klimpel, S. Occurrence of Borrelia burgdorferi sl in different genera of mosquitoes (Culicidae) in Central Europe. Ticks Tick Borne Dis. 2016, 7, 256–263. [Google Scholar] [CrossRef]
- Reinert, J.F. List of abbreviations for currently valid generic-level taxa in family Culicidae (Diptera). Eur. Mosq. Bull. 2009, 27, 68–76. [Google Scholar]
- Simões, M.L.; Caragata, E.P.; Dimopoulos, G. Diverse host and restriction factors regulate mosquito–pathogen interactions. Trends Parasitol. 2018, 34, 603–616. [Google Scholar] [CrossRef]
- Rückert, C.; Ebel, G.D. How do virus–mosquito interactions lead to viral emergence? Trends Parasitol. 2018, 34, 310–321. [Google Scholar] [CrossRef]
- Chan, M.; Johansson, M.A. The incubation periods of dengue viruses. PLoS ONE 2012, 7, e50972. [Google Scholar] [CrossRef]
- CDC—Centers for Disease Control and Prevention. Malaria, Biology, Lifecycle. 2018. Available online: https://www.cdc.gov/malaria/about/biology/index.html (accessed on 28 January 2023).
- Rajendram, D.; Ayenza, R.; Holder, F.M.; Moran, B.; Long, T.; Shah, H.N. Long-term storage and safe retrieval of DNA from microorganisms for molecular analysis using FTA matrix cards. J. Microbiol. Methods 2006, 67, 582–592. [Google Scholar] [CrossRef]
- Smith, L.M.; Burgoyne, L.A. Collecting, archiving and processing DNA from wildlife samples using FTA® databasing paper. BMC Ecol. 2004, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Cardona-Ospina, J.A.; Villalba-Miranda, M.F.; Palechor-Ocampo, L.A.; Mancilla, L.I.; Sepúlveda-Arias, J.C. A systematic review of FTA cards® as a tool for viral RNA preservation in fieldwork: Are they safe and effective? Prev. Vet. Med. 2019, 172, 104772. [Google Scholar] [CrossRef]
- Hall-Mendelin, S.; Ritchie, S.A.; Johansen, C.A.; Zborowski, P.; Cortis, G.; Dandridge, S.; Hall, R.A.; Van den Hurk, A.F. Exploiting mosquito sugar feeding to detect mosquito-borne pathogens. Proc. Natl. Acad. Sci. USA 2010, 107, 11255–11259. [Google Scholar] [CrossRef] [Green Version]
- Van den Hurk, A.F.; Hall-Mendelin, S.; Johansen, C.A.; Warrilow, D.; Ritchie, S.A. Evolution of mosquito-based arbovirus surveillance systems in Australia. J. Biotechnol. Biomed. 2012, 2012, 325659. [Google Scholar] [CrossRef] [Green Version]
- Wipf, N.C.; Guidi, V.; Tonolla, M.; Ruinelli, M.; Müller, P.; Engler, O. Evaluation of honey-baited FTA cards in combination with different mosquito traps in an area of low arbovirus prevalence. Parasite Vectors 2019, 12, 554. [Google Scholar] [CrossRef] [Green Version]
- Van den Hurk, A.F.; Hall-Mendelin, S.; Townsend, M.; Kurucz, N.; Edwards, J.; Ehlers, G.; Chris Rodwell, C.; Moore, F.A.; Mcmahon, J.L.; Northill, J.A.; et al. Applications of a sugar-based surveillance system to track arboviruses in wild mosquito populations. Vector-Borne Zoonotic Dis. 2014, 14, 66–73. [Google Scholar] [CrossRef]
- Guedes, D.R.; Paiva, M.H.; Donato, M.M.; Barbosa, P.P.; Krokovsky, L.; Rocha, S.W.D.S.; Saraiva, K.L.A.; Crespo, M.M.; Rezende, T.M.T.; Wallau, G.L.; et al. Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg. Microbes Infect. 2017, 6, e69. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, A.L.; van den Hurk, A.F.; Meyer, D.B.; Ritchie, S.A. Searching for the proverbial needle in a haystack: Advances in mosquito-borne arbovirus surveillance. Parasite Vectors 2018, 11, 320. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.J.; Kerlin, T.; Hall-Mendelin, S.; Van Den Hurk, A.F.; Cortis, G.; Doggett, S.L.; Toi, C.; Fall, K.; Mcmahon, J.L.; Townsend, M.; et al. Development and field evaluation of the sentinel mosquito arbovirus capture kit (SMACK). Parasite Vectors 2015, 8, 509. [Google Scholar] [CrossRef] [Green Version]
- Flies, E.J.; Toi, C.; Weinstein, P.; Doggett, S.L.; Williams, C.R. Converting mosquito surveillance to arbovirus surveillance with honey-baited nucleic acid preservation cards. Vector-Borne Zoonotic Dis. 2015, 15, 397–403. [Google Scholar] [CrossRef]
- Girod, R.; Guidez, A.; Carinci, R.; Issaly, J.; Gaborit, P.; Ferrero, E.; Ardillon, V.; Fontaine, A.; Dusfour, I.; Briolant, S. Detection of Chikungunya virus circulation using sugar-baited traps during a major outbreak in French Guiana. PLoS Negl. Trop. Dis. 2016, 10, e0004876. [Google Scholar] [CrossRef] [Green Version]
- Brugman, V.A.; Kristan, M.; Gibbins, M.P.; Angrisano, F.; Sala, K.A.; Dessens, J.T.; Blagborough, A.M.; Walker, T. Detection of malaria sporozoites expelled during mosquito sugar feeding. Sci. Rep. 2018, 8, 7545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourniol, L.; Madec, Y.; Mousson, L.; Vazeille, M.; Failloux, A.B. A laboratory-based study to explore the use of honey-impregnated cards to detect chikungunya virus in mosquito saliva. PLoS ONE 2021, 16, e0249471. [Google Scholar] [CrossRef] [PubMed]
- Lothrop, H.D.; Wheeler, S.S.; Fang, Y.; Reisen, W.K. Use of scented sugar bait stations to track mosquito-borne arbovirus transmission in California. J. Med. Entomol. 2014, 49, 1466–1472. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, A.L.; Van Den Hurk, A.F.; Mackay, I.M.; Yang, A.S.; Hewitson, G.R.; McMahon, J.L.; Boddey, J.A.; Ritchie, S.A.; Erickson, S.M. Malaria surveillance from both ends: Concurrent detection of Plasmodium falciparum in saliva and excreta harvested from Anopheles mosquitoes. Parasite Vectors 2019, 12, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, A.L.; Hall-Mendelin, S.; Doggett, S.L.; Hewitson, G.R.; Mcmahon, J.L.; Ritchie, S.A.; Van Den Hurk, A.F. Mosquito excreta: A sample type with many potential applications for the investigation of Ross River virus and West Nile virus ecology. PLoS Negl. Trop. Dis. 2018, 12, e0006771. [Google Scholar] [CrossRef]
- Sakuma, C.; Kanuka, H. A simple and affordable method for estimating the fluid volume a mosquito sucks using food dyes. Trop. Med. Health 2021, 49, 13. [Google Scholar] [CrossRef]
- Yang, F.; Song, X.; Yan, L. Preparation of cationic waste paper and its application in poisonous dye removal. Water Sci. Technol. 2013, 67, 2560–2567. [Google Scholar] [CrossRef]
- Glushakova, L.G.; Alto, B.W.; Kim, M.S.; Bradley, A.; Yaren, O.; Benner, S.A. Detection of chikungunya viral RNA in mosquito bodies on cationic (Q) paper based on innovations in synthetic biology. J. Virol. Methods 2017, 246, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Glushakova, L.G.; Alto, B.W.; Kim, M.-S.; Wiggins, K.; Eastmond, B.; Moussatche, P.; Burkett-Cadena, N.D.; Benner, S.A. Optimization of cationic (Q)-paper for detection of arboviruses in infected mosquitoes. J. Virol. Methods. 2018, 261, 71–79. [Google Scholar] [CrossRef]
- Wiggins, K.; Eastmond, B.; Alto, B.W. Transmission potential of Mayaro virus in Florida Aedes aegypti and Aedes albopictus mosquitoes. Med. Vet. Entomol. 2018, 32, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Glushakova, L.G.; Alto, B.W.; Kim, M.S.; Hutter, D.; Bradley, A.; Bradley, K.M.; Burkett-Cadena, N.D.; Benner, S.A. Multiplexed kit based on Luminex technology and achievements in synthetic biology discriminates Zika, chikungunya, and dengue viruses in mosquitoes. BMC Infec. Dis. 2019, 19, 418. [Google Scholar] [CrossRef]
- Danforth, M.E.; Reisen, W.K.; Barker, C.M. Detection of arbovirus transmission via sugar feeding in a laboratory setting. J. Med. Entomol. 2018, 55, 1575–1579. [Google Scholar] [CrossRef]
- Guissou, E.; Waite, J.L.; Jones, M.; Bell, A.S.; Suh, E.; Yameogo, K.B.; Djègbè, N.; Da, D.F.; Hien, F.D.S.D.; Yerbanga, R.S.; et al. A non-destructive sugar-feeding assay for parasite detection and estimating the extrinsic incubation period of Plasmodium falciparum in individual mosquito vectors. Sci. Rep. 2021, 11, 9344. [Google Scholar] [CrossRef]
- Gubler, D.J.; Rosen, L. A simple technique for demonstrating transmission of dengue virus by mosquitoes without the use of vertebrate hosts. Am. J. Trop. Med. Hyg. 1976, 25, 146–150. [Google Scholar] [CrossRef]
- Gubler, D.J.; Novak, R.J.; Vergne, E.; Colon, N.A.; Velez, M.; Fowler, J. Aedes (Gymnometopa) mediovittatus (Diptera: Culicidae), a potential maintenance vector of dengue viruses in Puerto Rico. J. Med. Entomol. 1985, 22, 469–475. [Google Scholar] [CrossRef]
- Styer, L.M.; Bernard, K.A.; Kramer, L.D. Enhanced early West Nile virus infection in young chickens infected by mosquito bite: Effect of viral dose. Am. J. Trop. Med. Hyg. 2006, 75, 337–345. [Google Scholar] [CrossRef]
- Styer, L.M.; Meola, M.A.; Kramer, L.D. West Nile virus infection decreases fecundity of Culex tarsalis females. J. Med. Entomol. 2007, 44, 1074–1085. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, F.; Chiles, R.E.; Fang, Y.I.N.G.; Reisen, W.K. Methods for studying the vector competence of Culex tarsalis for western equine encephalomyelitis virus. J. Am. Mosq. Control Assoc. 2004, 20, 277–282. [Google Scholar]
- Goddard, L.B.; Roth, A.E.; Reisen, W.K.; Scott, T.W. Vector competence of California mosquitoes for West Nile virus. Emerg. Infect. Dis. 2002, 8, 1385. [Google Scholar] [CrossRef]
- Romano, D.; Stefanini, C.; Canale, A.; Benelli, G. Artificial blood feeders for mosquitoes and ticks—Where from, where to? Acta Trop. 2018, 183, 43–56. [Google Scholar] [CrossRef]
- Pereira-Silva, J.W.; Martins-Campos, K.M.; Ferreira-Neto, J.V.; Lacerda, M.V.G.; Pessoa, F.A.C.; Ríos-Velásquez, C.M. Amazonian Anopheles with low numbers of oocysts transmit Plasmodium vivax sporozoites during a blood meal. Sci. Rep. 2022, 12, 19442. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Sorensen, M.R.; Markle, E.D.; Clarkson, T.C.; Knight, A.L.; Savran, M.J.; Foy, B.D. Characterizing and quantifying arbovirus transmission by Aedes aegypti using forced salivation and analysis of bloodmeals. Insects 2021, 12, 304. [Google Scholar] [CrossRef] [PubMed]
- Sri-In, C.; Weng, S.C.; Shiao, S.H.; Tu, W.C. A simplified method for blood feeding, oral infection, and saliva collection of the dengue vector mosquitoes. PLoS ONE 2020, 15, e0233618. [Google Scholar] [CrossRef] [PubMed]
- Erickson, S.M.; Fischer, K.; Weil, G.J.; Christensen, B.M.; Fischer, P.U. Distribution of Brugia malayi larvae and DNA in vector and non-vector mosquitoes: Implications for molecular diagnostics. Parasite Vectors 2009, 2, 56. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, A.; Jiolle, D.; Moltini-Conclois, I.; Lequime, S.; Lambrechts, L. Excretion of dengue virus RNA by Aedes aegypti allows non-destructive monitoring of viral dissemination in individual mosquitoes. Sci. Rep. 2016, 6, 24885. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.A.; Pilotte, N.; Minetti, C.; Williams, S.A.; Reimer, L.J. A superhydrophobic cone to facilitate the xenomonitoring of filarial parasites, malaria, and trypanosomes using mosquito excreta/feces. Gates Open Res. 2018, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Minetti, C.; Pilotte, N.; Zulch, M.; Canelas, T.; Tettevi, E.J.; Veriegh, F.B.; Osei-Atweneboana, M.Y.; Williams, S.A.; Reimer, L.J. Field evaluation of DNA detection of human filarial and malaria parasites using mosquito excreta/feces. PLoS Negl. Trop. Dis. 2020, 14, e0008175. [Google Scholar] [CrossRef] [Green Version]
- Pryce, J.; Pilotte, N.; Menze, B.; Sirois, A.R.; Zulch, M.; Agbor, J.P.; Williams, S.A.; Wondji, C.S.; Reimer, L. Integrated xenosurveillance of Loa loa, Wuchereria bancrofti, Mansonella perstans and Plasmodium falciparum using mosquito carcasses and faeces: A pilot study in Cameroon. PLoS Negl. Trop. Dis. 2022, 16, e0010868. [Google Scholar] [CrossRef]
- Ramírez, A.L.; Hall-Mendelin, S.; Hewitson, G.R.; Mcmahon, J.L.; Staunton, K.M.; Ritchie, S.A.; Van Den Hurk, A.F. Stability of West Nile Virus (Flaviviridae: Flavivirus) RNA in Mosquito Excreta. J. Med. Entomol. 2019, 56, 1135–1138. [Google Scholar] [CrossRef]
- L’ambert, G.; Gendrot, M.; Briolant, S.; Nguyen, A.; Pages, S.; Bosio, L.; Palomo, V.; Gomez, N.; Benoit, N.; Savini, H.; et al. Analysis of trapped mosquito excreta as a noninvasive method to reveal biodiversity and arbovirus circulation. Mol. Ecol. Resour. 2023, 23, 410–423. [Google Scholar] [CrossRef]
- Pasquini, C. Near Infrared Spectroscopy: Fundamentals, Practical Aspects and Analytical Applications. J. Braz. Chem. Soc. 2003, 14, 198–219. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.B.; Naiker, M. Seeing red: A review of the use of near-infrared spectroscopy (NIRS) in entomology. Appl. Spectrosc. Rev. 2020, 55, 810–839. [Google Scholar] [CrossRef]
- Lambert, B.; Sikulu-Lord, M.T.; Mayagaya, V.S.; Devine, G.; Dowell, F.; Churcher, T.S. Monitoring the age of mosquito populations using near-infrared spectroscopy. Sci. Rep. 2018, 8, 5274. [Google Scholar] [CrossRef] [Green Version]
- Joy, T.; Chen, M.; Arnbrister, J.; Williamson, D.; Li, S.; Nair, S.; Brophy, M.; Garcia, V.M.; Walker, K.; Ernst, K.; et al. Assessing near-infrared spectroscopy (NIRS) for evaluation of Aedes aegypti population age structure. Insects 2022, 13, 360. [Google Scholar] [CrossRef]
- Sikulu, M.T.; Majambere, S.; Khatib, B.O.; Ali, A.S.; Hugo, L.E.; Dowell, F.E. Using a near-infrared spectrometer to estimate the age of Anopheles mosquitoes exposed to pyrethroids. PLos ONE 2014, 9, e90657. [Google Scholar] [CrossRef] [Green Version]
- Liebman, K.; Swamidoss, I.; Vizcaino, L.; Lenhart, A.; Dowell, F.; Wirtz, R. The influence of diet on the use of near-infrared spectroscopy to determine the age of female Aedes aegypti mosquitoes. Am. J. Trop. Med. Hyg. 2015, 92, 1070–1075. [Google Scholar] [CrossRef] [Green Version]
- Milali, M.P.; Kiware, S.S.; Govella, N.J.; Okumu, F.; Bansal, N.; Bozdag, S.; Charlwood, J.D.; Maia, M.F.; Ogoma, S.B.; Dowell, F.E.; et al. An autoencoder and artificial neural network-based method to estimate parity status of wild mosquitoes from near-infrared spectra. PLoS ONE 2020, 15, e0234557. [Google Scholar] [CrossRef]
- Fernandes, J.N.; Dos Santos, L.M.; Chouin-Carneiro, T.; Pavan, M.G.; Garcia, G.A.; David, M.R.; Beier, J.C.; Dowell, F.E.; Maciel-De-Freitas, R.; Sikulu-Lord, M.T. Rapid, noninvasive detection of Zika virus in Aedes aegypti mosquitoes by near-infrared spectroscopy. Sci. Adv. 2018, 4, eaat0496. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.M.; Mutsaers, M.; Garcia, G.A.; David, M.R.; Pavan, M.G.; Petersen, M.T.; Corrêa-Antônio, J.; Couto-Lima, D.; Maes, L.; Dowell, F.; et al. High throughput estimates of Wolbachia, Zika and chikungunya infection in Aedes aegypti by near-infrared spectroscopy to improve arbovirus surveillance. Commun. Biol. 2021, 4, 67. [Google Scholar] [CrossRef]
- Garcia, G.A.; Lord, A.R.; Santos, L.M.; Kariyawasam, T.N.; David, M.R.; Couto-Lima, D.; Tátila-Ferreira, A.; Pavan, M.G.; Sikulu-Lord, M.T.; Maciel-de-Freitas, R. Rapid and Non-Invasive Detection of Aedes aegypti Co-Infected with Zika and Dengue Viruses Using Near Infrared Spectroscopy. Viruses 2022, 15, 11. [Google Scholar] [CrossRef]
- Santos, M.C.; Viana, J.L.; Monteiro, J.D.; Freire, R.C.; Freitas, D.L.; Câmara, I.M.; da Silva, G.J.S.; Gama, R.A.; Araújo, J.M.G.; Lima, K.M. Infrared spectroscopy (NIRS and ATR-FTIR) together with multivariate classification for non-destructive differentiation between female mosquitoes of Aedes aegypti recently infected with dengue vs. uninfected females. Acta Trop. 2022, 235, 106633. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.F.; Kapulu, M.; Muthui, M.; Wagah, M.G.; Ferguson, H.M.; Dowell, F.E.; Baldini, F.; Ranford-Cartwright, L. Detection of Plasmodium falciparum infected Anopheles gambiae using near-infrared spectroscopy. Malar. J. 2019, 18, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da, D.F.; McCabe, R.; Somé, B.M.; Esperança, P.M.; Sala, K.A.; Blight, J.; Blagborough, A.M.; Dowell, F.; Yerbanga, S.R.; Lefèvre, T.; et al. Detection of Plasmodium falciparum in laboratory-reared and naturally infected wild mosquitoes using near-infrared spectroscopy. Sci. Rep. 2021, 11, 10289. [Google Scholar] [CrossRef] [PubMed]
- Ong, O.T.; Kho, E.A.; Esperança, P.M.; Freebairn, C.; Dowell, F.E.; Devine, G.J.; Churcher, T.S. Ability of near-infrared spectroscopy and chemometrics to predict the age of mosquitoes reared under different conditions. Parasite Vectors 2020, 13, 160. [Google Scholar] [CrossRef]
- Esperança, P.M.; Blagborough, A.M.; Da, D.F.; Dowell, F.E.; Churcher, T.S. Detection of Plasmodium berghei infected Anopheles stephensi using near-infrared spectroscopy. Parasit. Vectors 2018, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Turney, S.; Cameron, E.R.; Cloutier, C.A.; Buddle, C.M. Non-repeatable science: Assessing the frequency of voucher specimen deposition reveals that most arthropod re-search cannot be verified. PeerJ 2015, 3, e1168. [Google Scholar] [CrossRef]
- Thompson, C.W.; Phelps, K.L.; Allard, M.W.; Cook, J.A.; Dunnum, J.L.; Ferguson, A.W.; Gelang, M.; Khan, F.A.A.; Paul, D.L.; Reeder, D.M.; et al. Preserve a voucher specimen! The criti-cal need for integrating natural history collections in infectious disease studies. mBio 2021, 12, e02698-20. [Google Scholar] [CrossRef]
- Dunnum, J.L.; Yanagihara, R.; Johnson, K.M.; Armien, B.; Batsaikhan, N.; Morgan, L.; Cook, J.A. Biospecimen repositories and integrated databases as critical infrastruc-ture for pathogen discovery and pathobiology research. PLoS Negl. Trop. Dis. 2017, 11, e0005133. [Google Scholar] [CrossRef] [Green Version]
- Astorga, F.; Groom, Q.; Shimabukuro, P.H.F.; Manguin, S.; Noesgaarde, D.; Orrell, T.; Sinka, M.; Hirsch, T.; Schigel, D. Biodiversity data supports research on human infectious diseases: Global trends, challenges, and opportunities. One Health 2023, 16, 100484. [Google Scholar] [CrossRef]
- Giantsis, I.A.; Chaskopoulou, A.; Bon, M.C. Mild-Vectolysis: A nondestructive DNA extraction method for vouchering sand flies and mosquitoes. J. Med. Entomol. 2016, 53, 692–695. [Google Scholar] [CrossRef]
- Korlević, P.; McAlister, E.; Mayho, M.; Makunin, A.; Flicek, P.; Lawniczak, M.K. A Minimally Morphologically Destructive Approach for DNA Retrieval and Whole-Genome Shotgun Sequencing of Pinned Historic Dipteran Vector Species. Genome Biol. Evol. 2021, 13, evab226. [Google Scholar] [CrossRef]
- Justi, S.A.; Soghigian, J.; Pecor, D.B.; Caicedo-Quiroga, L.; Rutvisuttinunt, W.; Li, T.; Stevens, L.; Dorn, P.L.; Wiegmann, B.; Linton, Y.M. From e-voucher to genomic data: Preserving archive specimens as demonstrated with medically important mosquitoes (Diptera: Culicidae) and kissing bugs (Hemiptera: Reduviidae). PLoS ONE 2021, 16, e0247068. [Google Scholar]
- Dhananjeyan, K.J.; Paramasivan, R.; Tewari, S.C.; Rajendran, R.; Thenmozhi, V.; Leo, S.V.; Venkatesh, A.; Tyagi, B.K. Molecular identification of mosquito vectors using ge-nomic DNA isolated from eggshells, larval and pupal exuvium. Trop. Biomed. 2010, 27, 47–53. [Google Scholar]
| Group | Pathogen Species | Main Vector Species 1 | Vector Mosquito Species 2 | Country or Continent 3 | References 4 |
|---|---|---|---|---|---|
| Virus | DENV | Ae. aegypti, Ae. albopictus | Cx. quinquefasciatus, Cx. bidens, Cx. interfor, Psorophora spp., Ps. varipes, Ps. albigenu, Sa. chloropterus | Brazil | [64,65] |
| ZIKV | Ae. aegypti, Ae. albopictus | Cx. quinquefasciatus, Hg. leucocelaenus | Brazil | [64,66,67] | |
| CHIKV | Ae. aegypti, Ae. albopictus | Cx. quinquefasciatus, Ps. ferox, Ps. albigenu | Brazil | [66] | |
| YFV | Haemagogus spp., Sabethes spp., Aedes spp. | Ae. albopictus, Ae. aureolineatus, Sa. identicus, Sh. fluviatilis | Colombia | [67,68] | |
| MAYV | Haemagogus spp. | Ae. aegypti, Ae. albopictus, Cx. quinquefasciatus | Brazil | [66,69] | |
| OROV | Culicoides paraensis (biting midge) | Cx. quinquefasciatus, Cq. venezuelensis, Oc. serratus, Ps. cingulata, Hg. tropicalis | Brazil | [70,71,72] | |
| VEEV | Cx. (Melanoconion) spp. | Ae. scapularis, Ae. aegypti, Ae. albopictus, Culex spp., De. atlanticus, Ma. titillans, Ps. confinnis Haemagogus spp., Sabethes spp., Deinocerites spp., Anopheles spp. | Brazil, Colombia | [70,73] | |
| SLEV | Cx. quinquefasciatus, Cx. pipiens s.l., Cx. nigripalpus, Cx. tarsalis | Culex spp., Ma. Titillans | Colombia | [73,74] | |
| WNV | Cx. pipiens s.l., Cx. univittatus | Culex spp., Cx. perexiguus, An. Crucians, An. Quadrimaculatus, Cq. Perturbans, Cx. coronator, Cx. erraticus, Cx. nigripalpus, Cx. quinquefasciatus, Ma. Titillans, Ae. sollicitans, Ps. Columbiae, Ur. Lowii | Colombia, Spain, Algeria, USA | [73,75,76,77] | |
| JEV | Culex tritaeniorhynchus, Culex annulirostris | Aedes spp., Anopheles spp., Ar. Subalbatus, Cq. Ochracea, Culex spp., Mansonia spp. | Australia/Oceania, Europe, Asia | [78] | |
| USUV | Cx. pipiens complex | Ae. albopictus, Cx. neavei, Cx. quinquefasciatus, Ae. japonicus, Ae. vexans, An. Maculipennis, An. Plumbeus, Cq. Richiardii, Cs. annulate, Ochlerotatus spp. | Europe | [79] | |
| Bacteria | Rickettsia felis | Ctenocephalides felis (flea) | An. Sinensis, Cx. pipiens s.l., Ae. albopictus, Ar. Subalbatus | China | [80] |
| Borrelia burgdorferi complex | Ixodes spp. (tick) | Aedes spp., Culiseta spp., Culex spp., Ochlerotatus spp. | Germany | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meireles, A.C.A.; Rios, F.G.F.; Feitoza, L.H.M.; da Silva, L.R.; Julião, G.R. Nondestructive Methods of Pathogen Detection: Importance of Mosquito Integrity in Studies of Disease Transmission and Control. Pathogens 2023, 12, 816. https://doi.org/10.3390/pathogens12060816
Meireles ACA, Rios FGF, Feitoza LHM, da Silva LR, Julião GR. Nondestructive Methods of Pathogen Detection: Importance of Mosquito Integrity in Studies of Disease Transmission and Control. Pathogens. 2023; 12(6):816. https://doi.org/10.3390/pathogens12060816
Chicago/Turabian StyleMeireles, Anne Caroline Alves, Flávia Geovana Fontineles Rios, Luiz Henrique Maciel Feitoza, Lucas Rosendo da Silva, and Genimar Rebouças Julião. 2023. "Nondestructive Methods of Pathogen Detection: Importance of Mosquito Integrity in Studies of Disease Transmission and Control" Pathogens 12, no. 6: 816. https://doi.org/10.3390/pathogens12060816
APA StyleMeireles, A. C. A., Rios, F. G. F., Feitoza, L. H. M., da Silva, L. R., & Julião, G. R. (2023). Nondestructive Methods of Pathogen Detection: Importance of Mosquito Integrity in Studies of Disease Transmission and Control. Pathogens, 12(6), 816. https://doi.org/10.3390/pathogens12060816