Molecular Mimicry Mapping in Streptococcus pneumoniae: Cues for Autoimmune Disorders and Implications for Immune Defense Activation
Abstract
:1. Introduction
2. Material and Methods
2.1. Mimicry Prediction
2.2. Pathway Enrichment
2.3. Mimic-Based Vaccine Construct Design
2.4. Validation of Immune Reaction
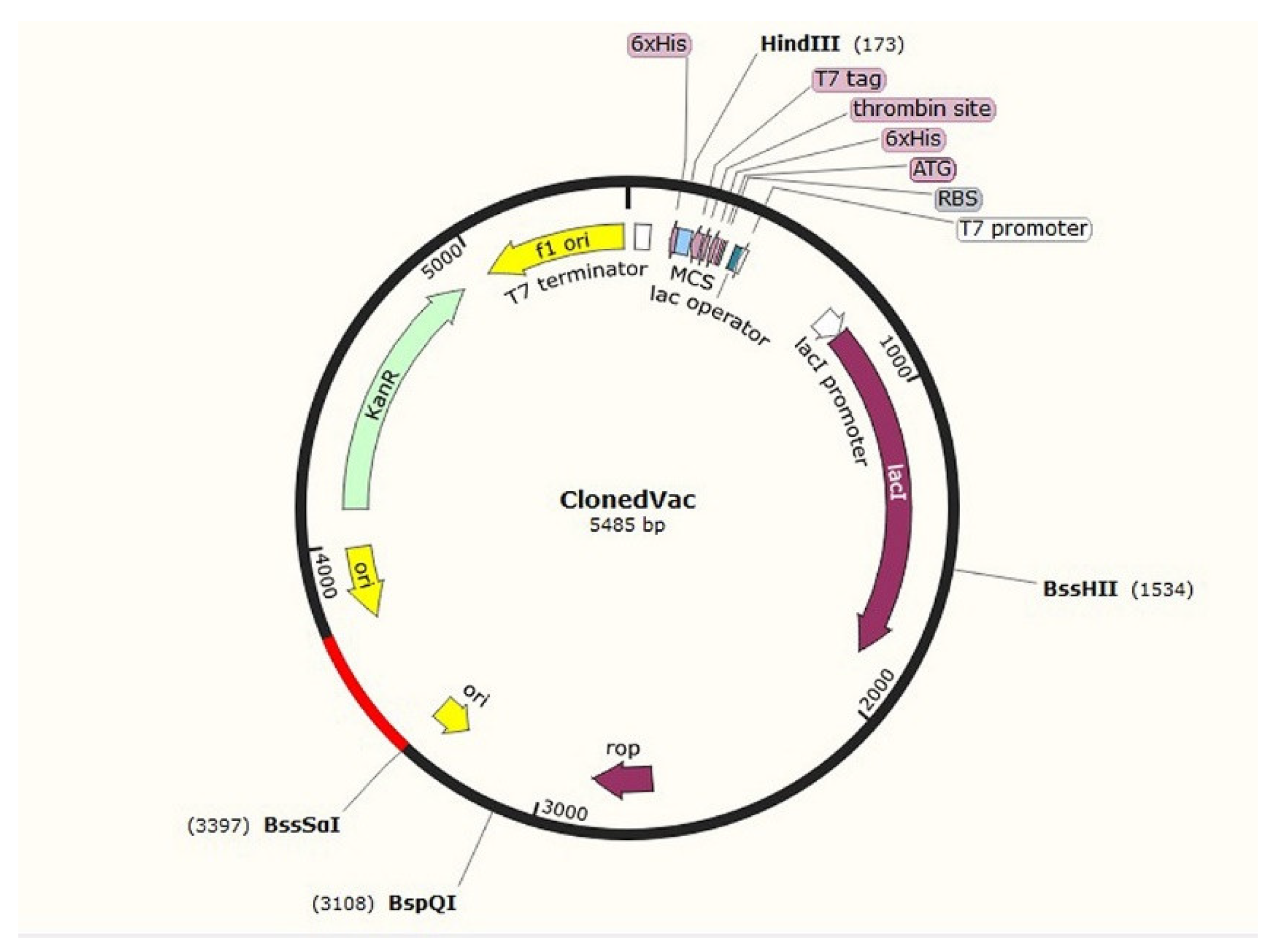
2.5. Cloning
3. Results
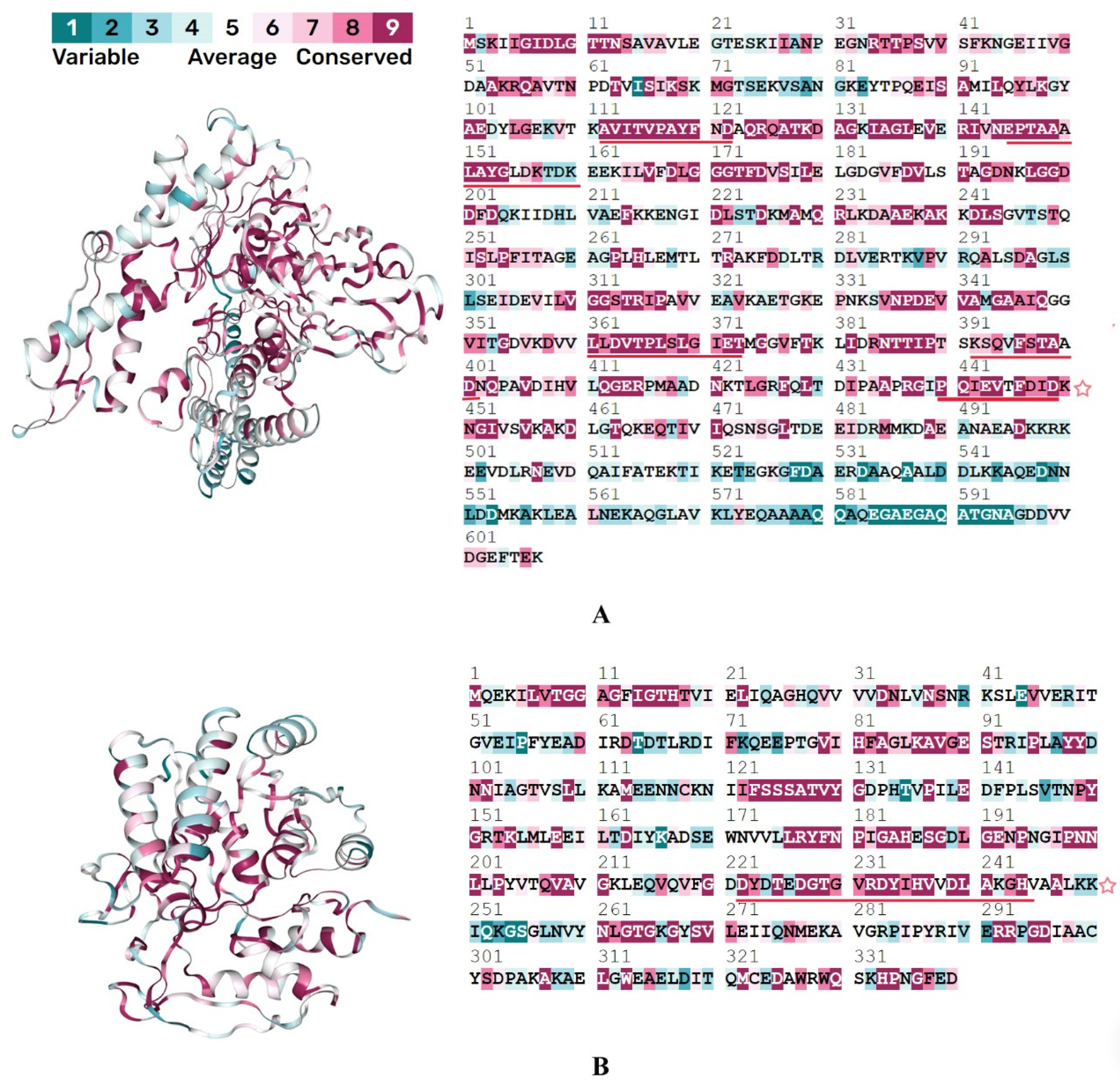
3.1. Mimic Prediction
3.2. Pathway Analysis of Homologs
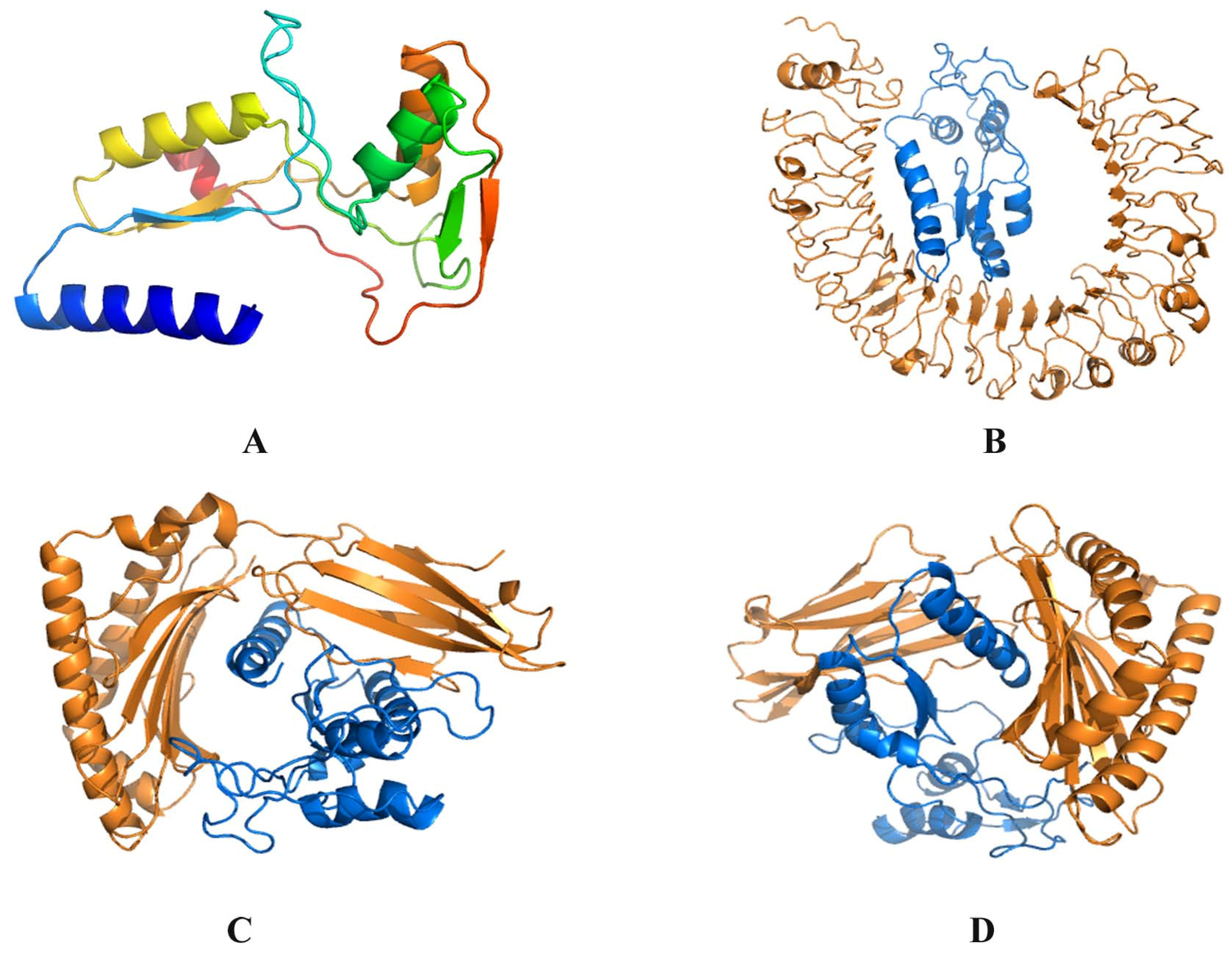
3.3. Mimic-Based Vaccine Design
3.4. C8 Modeling and Docking
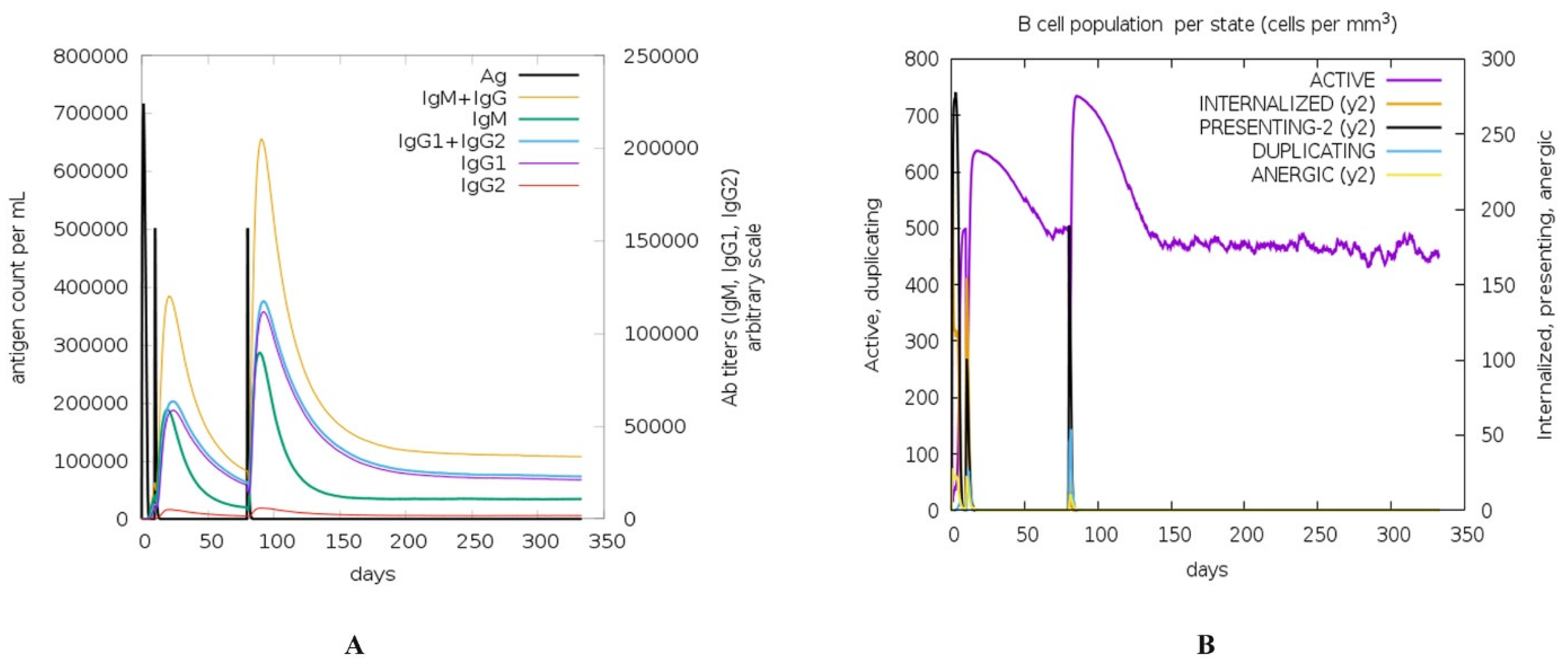
3.5. Immune Response Simulation
3.6. Cloning C8
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burckhardt, I.; Sebastian, K.; Mauder, N.; Kostrzewa, M.; Burckhardt, F.; Zimmermann, S. Analysis of Streptococcus pneumoniae using Fourier-transformed infrared spectroscopy allows prediction of capsular serotype. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1883–1890. [Google Scholar] [CrossRef] [Green Version]
- Brueggemann, A.B.; van Rensburg, M.J.J.; Shaw, D.; McCarthy, N.D.; Jolley, K.A.; Maiden, M.C.; van Der Linden, M.P.; Amin-Chowdhury, Z.; Bennett, D.E.; Borrow, R.; et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: A prospective analysis of surveillance data. Lancet Digit. Health 2021, 3, e360–e370. [Google Scholar] [PubMed]
- Liu, C.; Han, Z. Progress of invasive pneumococcal disease in children. Int. J. Pediatr. 2021, 6, 262–266. [Google Scholar]
- Reta, O. Nasopharyngeal Carriage Rate of Streptococcus pneumoniae Associated Risk Factors and Antimicrobial Susceptibility among Adult Individuals in Hawassa Prison. Ph.D. Thesis, Hawassa University, Hawassa, Ethiopia, 2022. [Google Scholar]
- Yani, F.F.; Julianty, R.J.; Tafroji, W.; Linosefa, L.; Ihsan, I.; Masnadi, N.R.; Safari, D. Nasopharyngeal carriage of Streptococcus pneumoniae strains and antimicrobial susceptibility carried by hospitalized pneumonia patients and healthy children under-five years olds in Padang, Indonesia. Access Microbiol. 2023, 5, 000584. [Google Scholar] [CrossRef]
- Mitchell, T.J.; Dalziel, C.E. The Biology of Pneumolysin; Springer: Dordrecht, The Netherlands, 2014; pp. 145–160. [Google Scholar]
- Bedeley, E.; Gori, A.; Yeboah-Manu, D.; Diallo, K. Control of Streptococcal infections: Is a common vaccine target achievable against Streptococcus agalactiae and Streptococcus pneumoniae. Front. Microbiol. 2021, 12, 658824. [Google Scholar] [CrossRef]
- Kim, L.; McGee, L.; Tomczyk, S.; Beall, B. Biological and epidemiological features of antibiotic-resistant Streptococcus pneumoniae in pre-and post-conjugate vaccine eras: A United States perspective. Clin. Microbiol. Rev. 2016, 29, 525–552. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.R.; Link-Gelles, R.; Schaffner, W.; Lynfield, R.; Lexau, C.; Bennett, N.M.; Petit, S.; Zansky, S.M.; Harrison, L.H.; Reingold, A.; et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: Analysis of multisite, population-based surveillance. Lancet Infect. Dis. 2015, 15, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Maraqa, N.F. Pneumococcal infections. Pediatr. Rev. 2014, 35, 299–310. [Google Scholar] [CrossRef]
- Cillóniz, C.; Ardanuy, C.; Vila, J.; Torres, A. What is the clinical relevance of drug-resistant pneumococcus? Curr. Opin. Pulm. Med. 2016, 22, 227–234. [Google Scholar] [CrossRef]
- Rojas, M.; Restrepo-Jiménez, P.; Monsalve, D.M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramírez-Santana, C.; Leung, P.S.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.-M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018, 95, 100–123. [Google Scholar] [CrossRef]
- Lang, S.N.; Germerodt, S.; Glock, C.; Skerka, C.; Zipfel, P.F.; Schuster, S. Molecular crypsis by pathogenic fungi using human factor H. A numerical model. PLoS ONE 2019, 14, e0212187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carapetis, J.R.; Beaton, A.; Cunningham, M.W.; Guilherme, L.; Karthikeyan, G.; Mayosi, B.M.; Sable, C.; Steer, A.; Wilson, N.; Wyber, R.; et al. Acute rheumatic fever and rheumatic heart disease. Nat. Rev. Dis. Prim. 2016, 2, 15084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamaa, A.; Abdallah, A.N.; Bahr, M.M.; El-Tookhy, O. A review: Multiple Sclerosis Treatment: Current Strategies and Future Hopes. Alex. J. Veter.-Sci. 2020, 66, 30. [Google Scholar] [CrossRef]
- Olsen, J.A.; Akirav, E.M. Remyelination in multiple sclerosis: Cellular mechanisms and novel therapeutic approaches. J. Neurosci. Res. 2015, 93, 687–696. [Google Scholar] [CrossRef]
- Begum, S.; Aiman, S.; Ahmad, S.; Samad, A.; Almehmadi, M.; Allahyani, M.; Aljuaid, A.; Afridi, S.G.; Khan, A. Molecular Mimicry Analyses Unveiled the Human Herpes Simplex and Poxvirus Epitopes as Possible Candidates to Incite Autoimmunity. Pathogens 2022, 11, 1362. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Begum, S.; Khan, A.; Afridi, S.G.; Sahibzada, M.U.K.; Atwah, B.; Alhindi, Z.; Khan, H. An insight in Salmonella typhi associated autoimmunity candidates’ prediction by molecular mimicry. Comput. Biol. Med. 2022, 148, 105865. [Google Scholar] [CrossRef]
- He, L.; Zhu, J. Computational tools for epitope vaccine design and evaluation. Curr. Opin. Virol. 2015, 11, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Ruff, K.M.; Pappu, R.V. AlphaFold and implications for intrinsically disordered proteins. J. Mol. Biol. 2021, 433, 167208. [Google Scholar] [CrossRef]
- Gelly, J.-C.; Joseph, A.P.; Srinivasan, N.; de Brevern, A.G. iPBA: A tool for protein structure comparison using sequence alignment strategies. Nucleic Acids Res. 2011, 39, W18–W23. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Skolnick, J. TM-align: A protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 2005, 33, 2302–2309. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, S.; Abovsky, M.; Pastrello, C.; Jurisica, I. pathDIP: An annotated resource for known and predicted human gene-pathway associations and pathway enrichment analysis. Nucleic Acids Res. 2017, 45, D419–D426. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.-T.; Mathias, S.; Bologa, C.; Brunak, S.; Fernandez, N.; Gaulton, A.; Hersey, A.; Holmes, J.; Jensen, L.J.; Karlsson, A.; et al. Pharos: Collating protein information to shed light on the druggable genome. Nucleic Acids Res. 2017, 45, D995–D1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhavan, M.; Mustafa, S. En route to peptide therapeutics for COVID 19: Harnessing potential antigenic mimicry between viral and human proteins. Trans. Indian Natl. Acad. Eng. 2020, 5, 411–415. [Google Scholar] [CrossRef]
- Doytchinova, I.A.; Flower, D.R. VaxiJen: A server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. 2007, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Dhanda, S.K.; Mahajan, S.; Paul, S.; Yan, Z.; Kim, H.; Jespersen, M.C.; Jurtz, V.; Andreatta, M.; Greenbaum, J.A.; Marcatili, P.; et al. IEDB-AR: Immune epitope database—Analysis resource in 2019. Nucleic Acids Res. 2019, 47, W502–W506. [Google Scholar] [CrossRef] [Green Version]
- Armon, A.; Graur, D.; Ben-Tal, N. ConSurf: An algorithmic tool for the identification of functional regions in proteins by surface mapping of phylogenetic information. J. Mol. Biol. 2001, 307, 447–463. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003. [Google Scholar] [CrossRef]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein–protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef] [Green Version]
- Castiglione, F.; Bernaschi, M. C-immsim: Playing with the immune response. In Proceedings of the Sixteenth International Symposium on Mathematical Theory of Networks and Systems (MTNS2004), Leuven, Belgium, 5–9 July 2004. [Google Scholar]
- Sanami, S.; Alizadeh, M.; Nosrati, M.; Dehkordi, K.A.; Azadegan-Dehkordi, F.; Tahmasebian, S.; Nosrati, H.; Arjmand, M.-H.; Ghasemi-Dehnoo, M.; Rafiei, A.; et al. Exploring SARS-COV-2 structural proteins to design a multi-epitope vaccine using immunoinformatics approach: An in silico study. Comput. Biol. Med. 2021, 133, 104390. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.E.; El-Far, S.W.; Embaby, A.M. Cloning, expression, and in silico structural modeling of cholesterol oxidase of Acinetobacter sp. strain RAMD in E. coli. FEBS Open Bio 2021, 11, 2560–2575. [Google Scholar] [CrossRef] [PubMed]
- Ooi, J.D.; Jiang, J.-H.; Eggenhuizen, P.J.; Chua, L.L.; van Timmeren, M.; Loh, K.L.; O’Sullivan, K.M.; Gan, P.Y.; Zhong, Y.; Tsyganov, K.; et al. A plasmid-encoded peptide from Staphylococcus aureus induces anti-myeloperoxidase nephritogenic autoimmunity. Nat. Commun. 2019, 10, 3392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Routsias, J.G.; Tzioufas, A.G. The role of chaperone proteins in autoimmunity. Ann. N. Y. Acad. Sci. 2006, 1088, 52–64. [Google Scholar] [CrossRef]
- Fourie, K.R.; Wilson, H.L. Understanding GroEL and DnaK stress response proteins as antigens for bacterial diseases. Vaccines 2020, 8, 773. [Google Scholar] [CrossRef]
- Caradonna, S.J.; Cheng, Y. Induction of uracil-DNA glycosylase and dUTP nucleotidohydrolase activity in herpes simplex virus-infected human cells. J. Biol. Chem. 1981, 256, 9834–9837. [Google Scholar] [CrossRef]
- Stuart, D.; Upton, C.; Higman, M.; Niles, E.; McFadden, G. A poxvirus-encoded uracil DNA glycosylase is essential for virus viability. J. Virol. 1993, 67, 2503–2512. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Li, F.; Zhang, J.; Wang, L.; Wang, J.; Wen, Z.; Wang, Z.; Shuai, L.; Wang, X.; Ge, J.; et al. The ATPase ATP6V1A facilitates rabies virus replication by promoting virion uncoating and interacting with the viral matrix protein. J. Biol. Chem. 2021, 296, 100096. [Google Scholar] [CrossRef]
- Zhang, P.; Minardi, L.M.; Kuenstner, J.T.; Zekan, S.M.; Kruzelock, R. Seroprevalence of anti-microbial antibodies in the normal healthy population with implications in chronic diseases. BioRxiv 2019, 693655. [Google Scholar] [CrossRef]
- Zhang, P.; Minardi, L.M.; Todd Kuenstner, J.; Zekan, S.M.; Zhu, F.; Hu, Y.; Kruzelock, R. Cross—Reactivity of antibodies against microbial proteins to human tissues as basis of Crohn’s disease and other autoimmune diseases. bioRxiv 2017, 116574. [Google Scholar] [CrossRef] [Green Version]
- Syed, S.; Viazmina, L.; Mager, R.; Meri, S.; Haapasalo, K. Streptococci and the complement system: Interplay during infection, inflammation and autoimmunity. FEBS Lett. 2020, 594, 2570–2585. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.L.; Jarocki, V.M.; Charles, I.G.; Djordjevic, S.P. The diverse functional roles of elongation factor Tu (EF-Tu) in microbial pathogenesis. Front. Microbiol. 2019, 10, 2351. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Choi, H.J.; Kim, S.Y.; Hong, K.S.; Min, S.K.; Nam, M.H.; Kim, C.W.; Koh, Y.H.; Seo, J.B. Proteomic and biochemical analyses reveal the activation of unfolded protein response, ERK-1/2 and ribosomal protein S6 signaling in experimental autoimmune myocarditis rat model. BMC Genom. 2011, 12, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.M.; Jackson, M.D.; Akerley, B.J. Suppression of alternative lipooligosaccharide glycosyltransferase activity by UDP-galactose epimerase enhances murine lung infection and evasion of serum IgM. Front. Cell. Infect. Microbiol. 2019, 9, 160. [Google Scholar] [CrossRef] [Green Version]
- Cladel, N.M.; Jiang, P.; Li, J.J.; Peng, X.; Cooper, T.K.; Majerciak, V.; Balogh, K.K.; Meyer, T.J.; Brendle, S.A.; Budgeon, L.R.; et al. Papillomavirus can be transmitted through the blood and produce infections in blood recipients: Evidence from two animal models. Emerg. Microbes Infect. 2019, 8, 1108–1121. [Google Scholar] [CrossRef] [Green Version]
- Francesco, C.; Everly, C.D.M.; Alberto, J.M. HSPD1 (heat shock 60 kDa protein 1). Atlas Genet. Cytogenet. Oncol Haematol 2015, 19, 575–585. [Google Scholar]
- Vojdani, A.; Bazargan, M.; Vojdani, E.; Samadi, J.; Nourian, A.A.; Eghbalieh, N.; Cooper, E.L. Heat shock protein and gliadin peptide promote development of peptidase antibodies in children with autism and patients with autoimmune disease. Clin. Vaccine Immunol. 2004, 11, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Lopez, J.A.M.; Cordero, J.F.B.; Russo, U.D.B.; Villarreal, M.A.G.; Hernández, F.A.T.; Bonilla, J.A.A.; Amaya, G.P.B.; Dumaguala, M.R.J.; Valencia, C.C.R. Molecular Mimetism between Cystic Fibrosis and P. aeruginosa and S. aureus, Possible Cause of Autoimmunity? In-Silico Analysis. J. Biomed. Sci. 2022, 4, 2066–2072. [Google Scholar] [CrossRef]
- Choy, E.; Rose-John, S. Interleukin-6 as a multifunctional regulator: Inflammation, immune response, and fibrosis. J. Scleroderma Relat. Disord. 2017, 2, S1–S5. [Google Scholar] [CrossRef] [Green Version]
- Ghoreschi, K.; Thomas, P.; Breit, S.; Dugas, M.; Mailhammer, R.; van Eden, W.; van der Zee, R.; Biedermann, T.; Prinz, J.; Mack, M.; et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat. Med. 2003, 9, 40–46. [Google Scholar] [CrossRef]
- Mao, Y.-M.; Zhao, C.-N.; Leng, J.; Leng, R.-X.; Ye, D.-Q.; Zheng, S.G.; Pan, H.-F. Interleukin-13: A promising therapeutic target for autoimmune disease. Cytokine Growth Factor Rev. 2019, 45, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Huang, J.; Zhong, H.; Shen, N.; Faggioni, R.; Fung, M.; Yao, Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2014, 141, 125–139. [Google Scholar] [CrossRef]
- Cusick, M.F.; Libbey, J.E.; Fujinami, R.S. Molecular mimicry as a mechanism of autoimmune disease. Clin. Rev. Allergy Immunol. 2012, 42, 102–111. [Google Scholar] [CrossRef]
- Herrmann, I.; Kellert, M.; Spreer, A.; Gerber, J.; Eiffert, H.; Prinz, M.; Nau, R. Minocycline delays but does not attenuate the course of experimental autoimmune encephalomyelitis in Streptococcus pneumoniae-infected mice. J. Antimicrob. Chemother. 2007, 59, 74–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borella, E.; Domeneghetti, M.; Palma, L.; Doria, A. Streptococcus pneumoniae and Autoimmunity: A Systematic Review of the Literature: How Pneumococcal Infection Might Be Related to Rheumatic Diseases. Infect. Autoimmun. 2015, 535–550. [Google Scholar] [CrossRef]
- Oksenhendler, E.; Spaan, A.N.; Neven, B.; Stolzenberg, M.-C.; Fusaro, M.; Casanova, J.-L.; Rieux-Laucat, F.; Boisson, B.; Magérus, A. Autoimmune lymphoproliferative syndrome presenting with invasive Streptococcus pneumoniae infection. J. Clin. Immunol. 2020, 40, 543–546. [Google Scholar] [CrossRef]
- Koppe, U.; Suttorp, N.; Opitz, B. Recognition of Streptococcus pneumoniae by the innate immune system. Cell. Microbiol. 2012, 14, 460–466. [Google Scholar] [CrossRef]
- Fournier, B.; Philpott, D.J. Recognition of Staphylococcus aureus by the innate immune system. Clin. Microbiol. Rev. 2005, 18, 521–540. [Google Scholar] [CrossRef] [Green Version]
- Marriott, H.M.; Mitchell, T.J.; Dockrell, D. Pneumolysin: A double-edged sword during the host-pathogen interaction. Curr. Mol. Med. 2008, 8, 497–509. [Google Scholar] [CrossRef]
- Lamb, D.J.; El-Sankary, W.; Ferns, G.A. Molecular mimicry in atherosclerosis: A role for heat shock proteins in immunisation. Atherosclerosis 2003, 167, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Loshaj-Shala, A.; Regazzoni, L.; Daci, A.; Orioli, M.; Brezovska, K.; Panovska, A.P.; Beretta, G.; Suturkova, L. Guillain Barré syndrome (GBS): New insights in the molecular mimicry between C. jejuni and human peripheral nerve (HPN) proteins. J. Neuroimmunol. 2015, 289, 168–176. [Google Scholar] [CrossRef]
- Bhardwaj, T.; Haque, S.; Somvanshi, P. In silico identification of molecular mimics involved in the pathogenesis of Clostridium botulinum ATCC 3502 strain. Microb. Pathog. 2018, 121, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Kaludercic, N.; Giorgio, V. The dual function of reactive oxygen/nitrogen species in bioenergetics and cell death: The role of ATP synthase. Oxidative Med. Cell. Longev. 2016, 2016, 3869610. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-J.; Goretzki, A.; Schülke, S. Immune metabolism of IL-4-activated B cells and Th2 cells in the context of allergic diseases. Front. Immunol. 2021, 12, 790658. [Google Scholar] [CrossRef] [PubMed]
- Santaguida, M.; Nardo, S.; Del Duca, S.; Lococo, E.; Virili, C.; Gargano, L.; Lenti, L.; Centanni, M. Increased interleukin-4-positive lymphocytes in patients with Hashimoto’s thyroiditis and concurrent non-endocrine autoimmune disorders. Clin. Exp. Immunol. 2011, 165, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.; Ghoreschi, K. The role of IL-4 in psoriasis. Expert Rev. Clin. Immunol. 2017, 13, 171–173. [Google Scholar] [CrossRef] [Green Version]
- Tamgue, O.; Gcanga, L.; Ozturk, M.; Whitehead, L.; Pillay, S.; Jacobs, R.; Roy, S.; Schmeier, S.; Davids, M.; Medvedeva, Y.A.; et al. Differential targeting of c-Maf, Bach-1, and Elmo-1 by microRNA-143 and microRNA-365 promotes the intracellular growth of Mycobacterium tuberculosis in alternatively IL-4/IL-13 activated macrophages. Front. Immunol. 2019, 10, 421. [Google Scholar] [CrossRef]
- Chi, G.; Feng, X.-X.; Ru, Y.-X.; Xiong, T.; Gao, Y.; Wang, H.; Luo, Z.-L.; Mo, R.; Guo, F.; He, Y.-P.; et al. TLR2/4 ligand-amplified liver inflammation promotes initiation of autoimmune hepatitis due to sustained IL-6/IL-12/IL-4/IL-25 expression. Mol. Immunol. 2018, 99, 171–181. [Google Scholar] [CrossRef]
- Mikoś, H.; Mikoś, M.; Obara-Moszyńska, M.; Niedziela, M. The role of the immune system and cytokines involved in the pathogenesis of autoimmune thyroid disease (AITD). Endokrynol. Pol. 2014, 65, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Srirangan, S.; Choy, E.H. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2010, 2, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, S.; Mancino, R.; Bergami, A.; Mori, F.; Castelli, M.; De Chiara, V.; Studer, V.; Mataluni, G.; Sancesario, G.; Parisi, V.; et al. Potential role of IL-13 in neuroprotection and cortical excitability regulation in multiple sclerosis. Mult. Scler. J. 2011, 17, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Yuan, J.; Wang, H.; Zhang, J.; Li, S.; Zhang, H.; Liu, Y.; Yin, Y.; Zhang, X. Streptococcus pneumoniae PepO promotes host anti-infection defense via autophagy in a Toll-like receptor 2/4 dependent manner. Virulence 2020, 11, 270–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Jin, C.; Ding, Z.; Zhu, Y.; He, Q.; Zhang, X.; Ai, R.; Yin, Y.; He, Y. TLR4 regulates ROS and autophagy to control neutrophil extracellular traps formation against Streptococcus pneumoniae in acute otitis media. Pediatr. Res. 2021, 89, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Caillat-Zucman, S. Molecular mechanisms of HLA association with autoimmune diseases. Tissue Antigens 2009, 73, 1–8. [Google Scholar] [CrossRef]
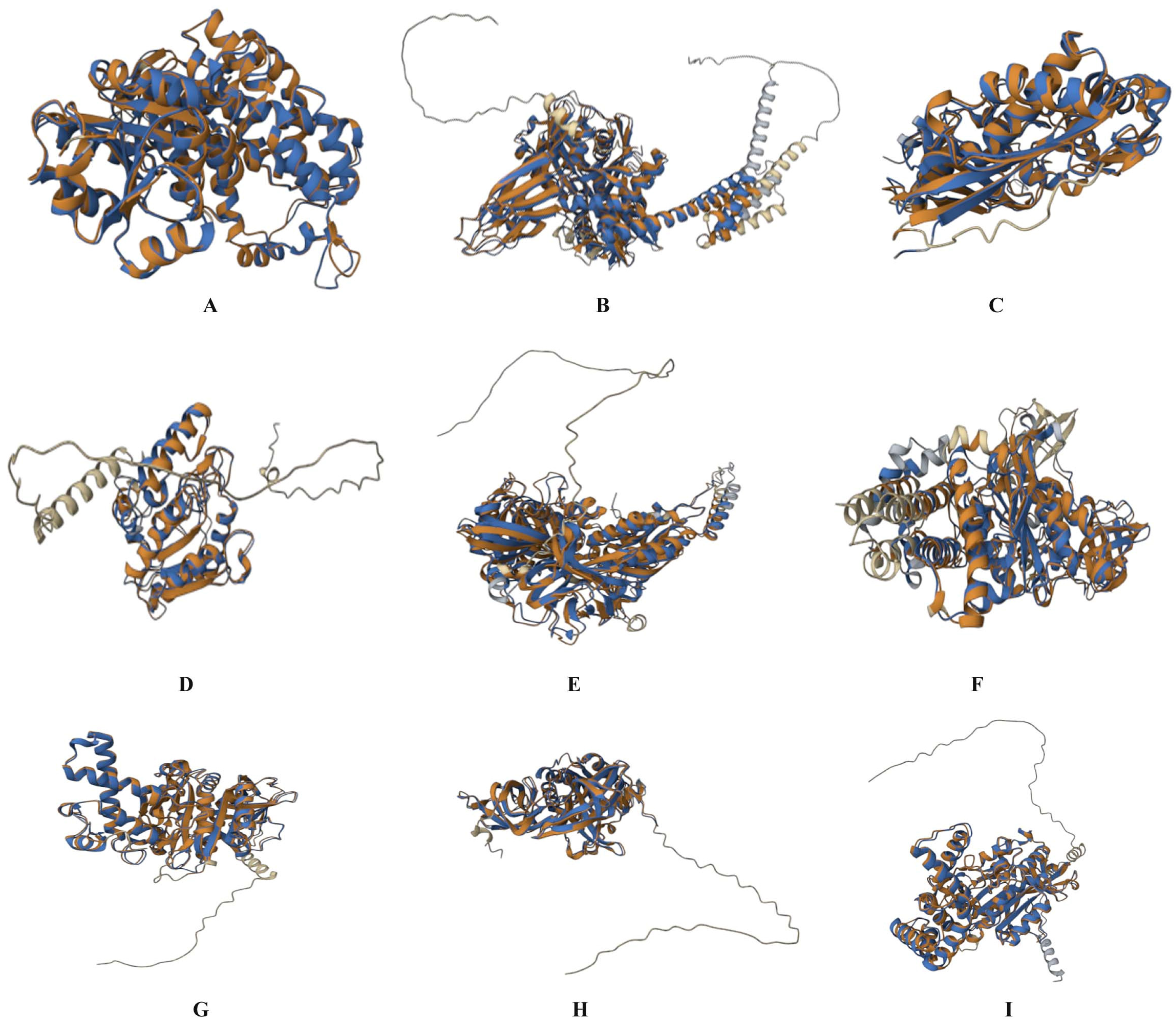
| Serial No. | Name | UniProt ID of Human Homolog | NCBI Accession of Bacterial Homolog | Bacterial Protein Structure AlphaFold ID | No. of Similar Peptides (Length ≥ 10) | Molecular Mimic Region (Length ≥ 10) | Superposed Protein RMSD (iPBA) | TM-Align RMSD |
|---|---|---|---|---|---|---|---|---|
| 1 | 6-phosphogluconate dehydrogenase | P52209.3 | ARD34052.1 | J1NT41 | 3 | LIQAQRDYFGAHTY SKIISYAQGF VKMVHNGIEYGDMQLI | 0.79 | 0.91 |
| 2 | Chaperone protein DnaK | P38646 | ARD34182.1 | C1CQ18 | 5 | PQIEVTFDID KSQVFSTAAD LLDVTPLSLGIET EPTAAALAYGLDK AVITVPAYFND | 1.36 | 2.6 |
| 3 | Methionine adenosyltransferase | Q00266 | ARD34414.1 | B2INE5 | 4 | GAGDQGLMFG GRFVIGGPQGD TGRKIIVDTYGG HGGGAFSGKD | 0.86 | 1.24 |
| 4 | Uracil-DNA glycosylase | P13051 | ARD34802.1 | C1CRP5 | 2 | VKVVILGQDPYHGP AHPSPLSVYRGF | 1.35 | 1.73 |
| 5 | GTP-binding protein LepA | Q8N442 | ARD34823.1 | B1IC02 | 2 | DHGKSTLADR LIDTPGHVDF LVKMDILLNG | 2.13 | 2.76 |
| 6 | V-type ATP synthase alpha chain | P38606 | ARD34956.1 | B1ICT1 | 0 | - | 1.53 | 3.12 |
| 7 | V-type ATP synthase beta chain | P21281 | ARD34955.1 | B1ICC8 | 3 | EALREVSAAR DDITHPIPDLTGYITEGQI PPINVLPSLSRL | 0.84 | 1.04 |
| 8 | Translation elongation factor Tu | P49411 | ARD35089.1 | C1CSB0 | 3 | GTIGHVDHGKTTLTAAIT PGHADYVKNMITG DGPMPQTREH | 0.81 | 0.81 |
| 9 | ATP synthase F1, alpha subunit | P25705 | ARD35106.1 | B1ICT1 | 6 | LVPIGRGQRELIIGDRQTGKT YDDLSKQAVAYR SLLLRRPPGREA PGDVFYLHSRLLER TNVISITDGQIFL FGSDLDAATQ | 0.74 | 1.04 |
| 10 | ATP synthase F1, beta subunit | P06576 | ARD35104.1 | B8ZLA9 | 7 | GLFGGAGVGKTVLI SVFAGVGERTREGNDLY GQMNEPPGAR EGQDVLLFIDNIFRFTQAGSEVSALLGR PSAVGYQPTLAT LGIYPAVDPL LQDIIAILGMDELS | 0.72 | 0.88 |
| 11 | UDP-glucose 4-epimerase | Q14376 | ARD35197.1 | A5MBZ8 | 2 | VIHFAGLKAVGES DYDTEDGTGVRDYIHVVDLAKGH | 0.84 | 0.99 |
| 12 | 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase | P15259 | ARD35240.1 | C1C8P5 | 1 | WRLNERHYGGLTG | 1.03 | 1.03 |
| 13 | chaperonin GroL | P10809 | ARD35488.1 | J1NT41 | 2 | AGDGTTTATVL DALNATRAAVEEGIV | 1.81 | 3.81 |
| Serial No. | Protein Homolog | PHAROS | PATHDIP | Literature | |||
|---|---|---|---|---|---|---|---|
| Autoimmunity Pathway | Infection Pathway | Autoimmune Pathway | Infection Pathway | Autoimmune Pathway | Infection Pathway | ||
| 1 | 6-phosphogluconate dehydrogenase | Diabetes mellitus | - | Huntington’s disease, Rheumatoid arthritis, Parkinson’s disease, Alzheimer’s disease, Non-alcoholic steatohepatitis | Vibrio infection, HIV, Influenz, Tuberculosis, Human papillomavirus infection | Nephritogenic autoimmunity [36] | - |
| 2 | Chaperone protein DnaK | Autoimmune disease, Parkinson’s disease | Perinatal necrotizing enterocolitis, HIV, Tuberculosis | - | - | Guillain–Barré syndrome, Multiple sclerosis, Systemic lupus erythematosus [37,38] | - |
| 3 | Methionine adenosyltransferase | Type 2 diabetes mellitus, demyelinating diseases, MODY, Psoriasis, Fatty liver, or Non-alcoholic steatohepatitis | - | - | - | - | Herpes simplex type 1 [39], Poxvirus [40] |
| 4 | Uracil-DNA glycosylase | - | - | - | - | - | - |
| 5 | GTP-binding protein LepA | - | - | - | - | - | - |
| 6 | V-type ATP synthase alpha chain | Psoriasis | - | Alzheimer, Parkinson’s disease, Huntington’s disease, Rheumatoid arthritis | HPV, Vibrio cholerae | - | Rabies [41] |
| 7 | V-type ATP synthase beta chain | IgA glomerulonephritis | - | Huntington’s disease, Rheumatoid arthritis | HPV | - | - |
| 8 | Translation–elongation factor Tu | - | - | Huntington’s disease, Parkinson’s disease | HCV, HBV, Legionellosis, E. coli, V. cholerae | Sjogren’s syndrome [42], Crohn’s disease [43] | Streptococcus pneumoniae [44], Other microbes pathogenesis [45] |
| 9 | ATP synthase F1, alpha subunit | Alzheimer’s disease | - | - | - | Sjogren’s syndrome, Crohn’s disease [43] | - |
| 10 | ATP synthase F1, beta subunit | - | - | Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, Non-alcoholic fatty acid liver diseases | Epstein–Barr virus infection, HBV, HCV, HPV, Measles, Legionellosis, E. Coli | Autoimmune myocarditis [46] | - |
| 11 | UDP-glucose 4-epimerase | Psoriasis, interstitial cystitis | Tinea corporis, Tinea pedis | - | - | - | Haemophilus influenzae [47] |
| 12 | 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase | Juvenile dermatomyositis | - | - | - | - | Papillomavirus infection [48] |
| 13 | chaperonin GroL | Allergic rhinitis | Tuberculosis, HIV | - | - | Type-1 diabetes, Juvenile chronic arthritis, Atherosclerosis, Crohn disease, Rheumatoid arthritis, Systemic lupus erythematosus, Sjogren’s syndrome, Hashimoto thyroiditis, and Myasthenia gravis [49], Autism [50] | P. aeruginosa and S. aureus [51] |
| Serial No. | Antigenic Score | Sequence | Length | Immunogenicity Score | SVM Score for Toxicity | Hydrophobicity | Hydropathicity | Hydrophilicity | Charge | Mol wt | Allergenicity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.5685 | PQIEVTFDID | 10 | 0.43 | −1.33 | −0.04 | −0.03 | 0.12 | −3.00 | 1176.43 | Non-allergen |
| 2 | 0.8794 | LLDVTPLSLGIET | 13 | 0.10 | −1.30 | 0.12 | 0.98 | −0.38 | −2.00 | 1370.81 | Allergen |
| 3 | 0.5923 | GAGDQGLMFG | 10 | −0.14 | −1.07 | 0.09 | 0.17 | −0.29 | −1.00 | 952.20 | Allergen |
| 4 | 1.2285 | HGGGAFSGKD | 10 | −0.06 | −1.31 | −0.10 | −0.84 | 0.28 | 0.50 | 932.10 | Allergen |
| 5 | 0.5672 | GTIGHVDHGKTTLTAAIT | 18 | 0.28 | −1.54 | −0.00 | 0.12 | −0.27 | 1.00 | 1793.29 | Allergen |
| 6 | 0.5607 | DGPMPQTREH | 10 | −0.17 | −0.50 | −0.41 | −2.06 | 0.70 | −0.50 | 1167.40 | Allergen |
| 7 | 0.7650 | SLLLRRPPGREA | 12 | 0.20 | −0.59 | −0.36 | −0.68 | 0.53 | 2.00 | 1364.78 | Allergen |
| 8 | 1.0099 | FGSDLDAATQ | 10 | 0.07 | −1.42 | −0.08 | −0.22 | 0.08 | −2.00 | 1024.18 | Non-allergen |
| 9 | 1.0376 | PSAVGYQPTLAT | 12 | 0.01 | −1.47 | 0.03 | 0.08 | −0.58 | 0.00 | 1204.51 | Allergen |
| 10 | 0.5485 | LGIYPAVDPL | 10 | 0.12 | −1.22 | 0.19 | 0.97 | −0.67 | −1.00 | 1057.40 | Non-allergen |
| 11 | 0.9488 | DYDTEDGTGVRDYIHVVDLAKGH | 23 | 0.50 | −0.62 | −0.20 | −0.80 | 0.39 | −3.00 | 2576.07 | Non-allergen |
| 12 | 0.7924 | WRLNERHYGGLTG | 13 | 0.24 | −1.09 | −0.26 | −1.21 | −0.08 | 1.50 | 1558.92 | Allergen |
| 13 | 1.9333 | AGDGTTTATVL | 11 | 0.25 | −0.75 | 0.04 | 0.41 | −0.26 | −1.00 | 1006.23 | Allergen |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashraqi, M.M.; Alzamami, A.; Alturki, N.A.; Alshamrani, S.; Alshahrani, M.M.; Almasoudi, H.H.; Basharat, Z. Molecular Mimicry Mapping in Streptococcus pneumoniae: Cues for Autoimmune Disorders and Implications for Immune Defense Activation. Pathogens 2023, 12, 857. https://doi.org/10.3390/pathogens12070857
Mashraqi MM, Alzamami A, Alturki NA, Alshamrani S, Alshahrani MM, Almasoudi HH, Basharat Z. Molecular Mimicry Mapping in Streptococcus pneumoniae: Cues for Autoimmune Disorders and Implications for Immune Defense Activation. Pathogens. 2023; 12(7):857. https://doi.org/10.3390/pathogens12070857
Chicago/Turabian StyleMashraqi, Mutaib M., Ahmad Alzamami, Norah A. Alturki, Saleh Alshamrani, Mousa M. Alshahrani, Hassan H. Almasoudi, and Zarrin Basharat. 2023. "Molecular Mimicry Mapping in Streptococcus pneumoniae: Cues for Autoimmune Disorders and Implications for Immune Defense Activation" Pathogens 12, no. 7: 857. https://doi.org/10.3390/pathogens12070857
APA StyleMashraqi, M. M., Alzamami, A., Alturki, N. A., Alshamrani, S., Alshahrani, M. M., Almasoudi, H. H., & Basharat, Z. (2023). Molecular Mimicry Mapping in Streptococcus pneumoniae: Cues for Autoimmune Disorders and Implications for Immune Defense Activation. Pathogens, 12(7), 857. https://doi.org/10.3390/pathogens12070857







