Quantitative Risk Assessment of Oocyst Versus Bradyzoite Foodborne Transmission of Toxoplasma gondii in Brazil
Abstract
1. Introduction
2. Materials and Methods
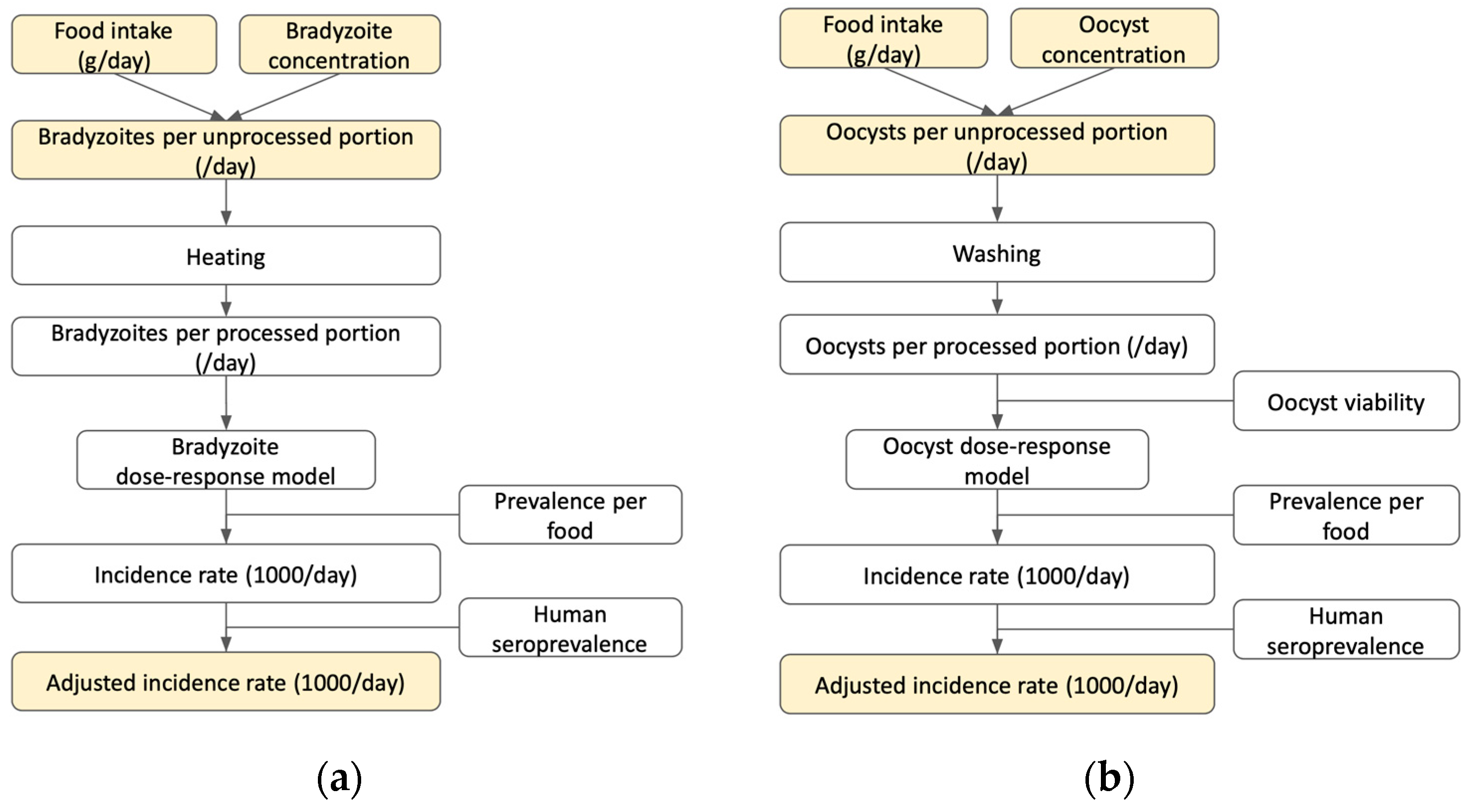
2.1. Hazard Identification
2.2. Exposure Assessment
2.2.1. Food Intake Quantity (g/day)
Bradyzoite Exposure—Meat
Oocyst Exposure—Fresh Produce
Oocyst Exposure—Seafood
2.2.2. Parasite Concentration
2.2.3. Parasites per Unprocessed Portion
2.2.4. Parasites per Processed Portion
2.3. Dose–Response Assessment
2.4. Risk Characterization
2.4.1. Prevalence of Contamination by Food Product
2.4.2. Adjusted Incidence Rate
2.5. Data Analysis
2.6. Sensitivity and Scenario Analysis
3. Results
3.1. Sensitivity Analysis
3.2. Scenario Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, J.P. The History of Toxoplasma gondii—The First 100 Years. J. Eukaryot. Microbiol. 2008, 55, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Dubey, J.P. Toxoplasma gondii: Transmission, diagnosis and prevention. Clin. Microbiol. Infect. 2002, 8, 634–640. [Google Scholar] [CrossRef]
- Elbez-Rubinstein, A.; Ajzenberg, D.; Dardé, M.L.; Cohen, R.; Dumètre, A.; Yera, H.; Gondon, E.; Janaud, J.C.; Thulliez, P. Congenital toxoplasmosis and reinfection during pregnancy: Case report, strain characterization, experimental model of reinfection, and review. J. Infect. Dis. 2009, 199, 280–285. [Google Scholar] [CrossRef]
- Wang, Z.D.; Liu, H.H.; Ma, Z.X.; Ma, H.Y.; Li, Z.Y.; Yang, Z.B.; Zhu, X.Q.; Xu, B.; Wei, F.; Liu, Q. Toxoplasma gondii infection in immunocompromised patients: A systematic review and meta-analysis. Front. Microbiol. 2017, 8, 389. [Google Scholar] [CrossRef]
- Torgerson, P.R.; Mastroiacovo, P. The global burden of congenital toxoplasmosis: A systematic review. Bull. World Health Organ. 2013, 91, 501–508. [Google Scholar] [CrossRef] [PubMed]
- IBGE (Brazilian Institute of Geography and Statistics). Estimates of Resident Population for Municipalities and Federation Units. 2021. Available online: https://www.ibge.gov.br/en/statistics/social/population/18448-estimates-of-resident-population-for-municipalities-and-federation-units.html?=&t=resultados (accessed on 7 February 2023).
- Dubey, J.P.; Lago, E.G.; Gennari, S.M.; Su, C.; Jones, J.L. Toxoplasmosis in humans and animals in Brazil: High prevalence, high burden of disease, and epidemiology. Parasitology 2012, 139, 1375–1424. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Murata, F.H.; Cerqueira-Cézar, C.K.; Kwok, O.C.; Villena, I. Congenital toxoplasmosis in humans: An update of worldwide rate of congenital infections. Parasitology 2021, 148, 1406–1416. [Google Scholar] [CrossRef]
- Glasner, P.D.; Silveira, C.; Kruszon-Moran, D.; Martins, M.C.; Burnier, M.; Silveira, S.; Camargo, M.E.; Nussenblatt, R.B.; Kaslow, R.A.; Belfort, R. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. Am. J. Ophthalmol. 1992, 114, 136–144. [Google Scholar] [CrossRef]
- Cook, A.J.C.; Holliman, R.; Gilbert, R.E.; Buffolano, W.; Zufferey, J.; Petersen, E.; Jenum, P.A.; Foulon, W.; Semprini, A.E.; Dunn, D.T. Sources of Toxoplasma infection in pregnant women: European multicentre case-control study. Commentary: Congenital toxoplasmosis—Further thought for food. BMJ 2000, 321, 142–147. [Google Scholar] [CrossRef]
- Pinto-Ferreira, F.; Caldart, E.T.; Pasquali, A.K.S.; Mitsuka-Breganó, R.; Freire, R.L.; Navarro, I.T. Patterns of transmission and sources of infection in outbreaks of human toxoplasmosis. Emerg. Infect. Dis. 2019, 25, 2177–2182. [Google Scholar] [CrossRef]
- Minuzzi, C.E.; Fernandes, F.D.; Portella, L.P.; Bräunig, P.; Sturza, D.A.F.; Giacomini, L.; Salvagni, E.; Ribeiro, J.D.S.; Silva, C.R.; Difante, C.M.; et al. Contaminated water confirmed as source of infection by bioassay in an outbreak of toxoplasmosis in South Brazil. Transbound. Emerg. Dis. 2021, 68, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Bahia-Oliveira, L.M.G.; Jones, J.L.; Azevedo-Silva, J.; Alves, C.C.F.; Oréfice, F.; Addiss, D.G. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro State, Brazil. Emerg. Infect. Dis. 2003, 9, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bowie, W.R.; King, A.S.; Werker, D.H.; Isaac-Renton, J.L.; Bell, A.; Eng, S.B.; Marion, S.A. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet 1997, 350, 173–177. [Google Scholar] [CrossRef]
- Schumacher, A.C.; Elbadawi, L.I.; DeSalvo, T.; Straily, A.; Ajzenberg, D.; Letzer, D.; Moldenhauer, E.; Handly, T.L.; Hill, D.; Dardé, M.L.; et al. Toxoplasmosis outbreak associated with Toxoplasma gondii-contaminated venison—High attack rate, unusual clinical presentation, and atypical genotype. Clin. Infect. Dis. 2021, 72, 1557–1565. [Google Scholar] [CrossRef]
- Dubey, J.P. Outbreaks of clinical toxoplasmosis in humans: Five decades of personal experience, perspectives and lessons learned. Parasites Vectors 2021, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Pires, S. Attributing Toxoplasma gondii Infections to Sources: Current Knowledge and Addressing Data Gaps. Engormix. 2021. Available online: https://en.engormix.com/pig-industry/articles/attributing-toxoplasma-gondii-infections-t47578.htm (accessed on 27 October 2022).
- Hald, T.; Aspinall, W.; Devleesschauwer, B.; Cooke, R.; Corrigan, T.; Havelaar, A.H.; Gibb, H.J.; Torgerson, P.R.; Kirk, M.D.; Angulo, F.J.; et al. World Health Organization estimates of the relative contributions of food to the burden of disease due to selected foodborne hazards: A structured expert elicitation. PLoS ONE 2016, 11, e0145839. [Google Scholar] [CrossRef]
- Belluco, S.; Patuzzi, I.; Ricci, A. Bovine meat versus pork in Toxoplasma gondii transmission in Italy: A quantitative risk assessment model. Int. J. Food Microbiol. 2018, 269, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Crotta, M.; Limon, G.; Blake, D.P.; Guitian, J. Knowledge gaps in host-parasite interaction preclude accurate assessment of meat-borne exposure to Toxoplasma gondii. Int. J. Food Microbiol. 2017, 261, 95–101. [Google Scholar] [CrossRef]
- Guo, M.; Lambertini, E.; Buchanan, R.L.; Dubey, J.P.; Hill, D.E.; Gamble, H.R.; Jones, J.L.; Pradhan, A.K. Quantifying the risk of human Toxoplasma gondii infection due to consumption of fresh pork in the United States. Food Control 2017, 73, 1210–1222. [Google Scholar] [CrossRef]
- Opsteegh, M.; Prickaerts, S.; Frankena, K.; Evers, E.G. A quantitative microbial risk assessment for meatborne Toxoplasma gondii infection in The Netherlands. Int. J. Food Microbiol. 2011, 150, 103–114. [Google Scholar] [CrossRef]
- Deng, H.; Swart, A.; Wu, Y.; Li, X.; Li, J.; Liu, M.; Opsteegh, M.; van der Giessen, J.W. Quantitative risk assessment of meat-borne Toxoplasma gondii infection in the mainland of China. Microb. Risk Anal. 2020, 14, 100090. [Google Scholar] [CrossRef]
- Condoleo, R.; Rinaldi, L.; Sette, S.; Mezher, Z. Risk assessment of human toxoplasmosis associated with the consumption of pork meat in Italy. Risk Anal. Off. Publ. Soc. Risk Anal. 2018, 38, 1202–1222. [Google Scholar] [CrossRef]
- López Ureña, N.; Chaudhry, U.; Calero Bernal, R.; Cano Alsua, S.; Messina, D.; Evangelista, F.; Betson, M.; Lalle, M.; Jokelainen, P.; Ortega Mora, L.M.; et al. Contamination of soil, water, fresh produce, and bivalve mollusks with Toxoplasma gondii oocysts: A systematic review. Microorganisms 2022, 10, 517. [Google Scholar] [CrossRef]
- Plummer, M.; Stukalov, A.; Denwood, M. rjags: Bayesian Graphical Models Using MCMC. 2022. Available online: https://CRAN.R-project.org/package=rjags (accessed on 1 November 2022).
- IBGE (Brazilian Institute of Geography and Statistics). POF 2017-2018. Consumer Expenditure Survey—POF. 2018. Available online: https://www.ibge.gov.br/en/statistics/social/population/25610-pof-2017-2018-pof-en.html?=&t=notas-tecnicas (accessed on 1 July 2021).
- OECD. Meat Consumption (Indicator); OECD: Paris, France, 2022. [Google Scholar] [CrossRef]
- Ekman, C.C.J.; do Valle Chiossi, M.F.; Meireles, L.R.; de Andrade, H.F., Jr.; Figueiredo, W.M.; Marciano, M.A.M.; de Albuquerque Luna, E.J. Case-control study of an outbreak of acute toxoplasmosis in an industrial plant in the state of São Paulo, Brazil. Rev. Inst. Med. Trop. 2012, 54, 239–244. [Google Scholar] [CrossRef]
- dos Anjos Pinheiro Bogoevich, M.R.; Freire, A.B.C.; Barbosa, D.R.L.; de Cássia Tork da Silva, L.; Pinheiro, A.F.; Silveira da Costa, S.; de Paula Ramos, F.L.; Bichara, C.N.C.; Lima, L.J.B.; da Silva, A.V.; et al. Surto de Toxoplasmose Aguda no Município de Ponta de Pedras, Arquipélago do Marajó, Estado do Pará, Brasil: Características Clínicas, Laboratoriais e Epidemiológicas. 2016. Available online: https://patua.iec.gov.br/handle/iec/3012 (accessed on 1 March 2023).
- Marino, A.M.F.; Giunta, R.P.; Salvaggio, A.; Castello, A.; Alfonzetti, T.; Barbagallo, A.; Aparo, A.; Scalzo, F.; Reale, S.; Buffolano, W.; et al. Toxoplasma gondii in edible fishes captured in the Mediterranean basin. Zoonoses Public Health 2019, 66, 826–834. [Google Scholar] [CrossRef]
- Opsteegh, M.; Langelaar, M.; Sprong, H.; den Hartog, L.; De Craeye, S.; Bokken, G.; Ajzenberg, D.; Kijlstra, A.; van Der Giessen, J. Direct detection and genotyping of Toxoplasma gondii in meat samples using magnetic capture and PCR. Int. J. Food Microbiol. 2010, 139, 193–201. [Google Scholar] [CrossRef]
- Otieno, J. From the Classical Beta Distribution to Generalized Beta Distributions. Master’s Thesis, University of Nairobi, Nairobi, Kenya, 2008. [Google Scholar]
- Marques, C.S.; Sousa, S.; Castro, A.; da Costa, J.M.C. Detection of Toxoplasma gondii oocysts in fresh vegetables and berry fruits. Parasites Vectors 2020, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Temesgen, T.T.; Robertson, L.J.; Stigum, V.M.; Tysnes, K.R. Removal of parasite transmission stages from berries using washing procedures suitable for consumers. Foods 2021, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Mishra, A.; Buchanan, R.L.; Dubey, J.P.; Hill, D.E.; Gamble, H.R.; Jones, J.L.; Du, X.; Pradhan, A.K. Development of dose-response models to predict the relationship for human Toxoplasma gondii infection associated with meat consumption. Risk Anal. 2016, 36, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Bonačić Marinović, A.A.; Opsteegh, M.; Deng, H.; Suijkerbuijk, A.W.M.; van Gils, P.F.; van der Giessen, J. Prospects of toxoplasmosis control by cat vaccination. Epidemics 2020, 30, 100380. [Google Scholar] [CrossRef]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Yang, Y.R. Public health significance of Toxoplasma gondii infections in cattle: 2009–2020. J. Parasitol. 2020, 106, 772–788. [Google Scholar] [CrossRef]
- Dubey, J.P.; Cerqueira-Cézar, C.K.; Murata, F.H.A.; Kwok, O.C.H.; Hill, D.; Yang, Y.; Su, C. All about Toxoplasma gondii infections in pigs: 2009–2020. Vet. Parasitol. 2020, 288, 109185. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Pena, H.F.J.; Cerqueira-Cézar, C.K.; Murata, F.H.A.; Kwok, O.C.H.; Yang, Y.R.; Gennari, S.M.; Su, C. Epidemiologic significance of Toxoplasma gondii infections in chickens (Gallus domesticus): The past decade. Parasitology 2020, 147, 1263–1289. [Google Scholar] [CrossRef] [PubMed]
- Marchioro, A.A.; Tiyo, B.T.; Colli, C.M.; de Souza, C.Z.; Garcia, J.L.; Gomes, M.L.; Falavigna-Guilherme, A.L. First detection of Toxoplasma gondii DNA in the fresh leafs of vegetables in South America. Vector Borne Zoonotic Dis. 2016, 16, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.P.; Caldart, E.T.; Freire, R.L.; Mitsuka-Breganó, R.; de Freitas, F.M.; Miura, A.C.; Marenze, M.; Martins, F.D.C.; Urbano, M.R.; Seifert, A.L.; et al. The effect of water source and soil supplementation on parasite contamination in organic vegetable gardens. Rev. Bras. Parasitol. Vet. 2018, 27, 327–337. [Google Scholar] [CrossRef]
- Pinto-Ferreira, F.; Mitsuka-Breganó, R.; Monica, T.C.; Martins, F.D.C.; de Matos, R.L.N.; Mareze, M.; de Souza Lima Nino, B.; Narciso, S.G.; Freire, R.L.; Navarro, I.T. Investigation and environmental analysis of samples from outbreak of toxoplasmosis at research institution in Londrina, Paraná, Brazil, 2016. Braz. J. Vet. Parasitol. 2019, 28, 518–521. [Google Scholar] [CrossRef]
- Perim, L.V.; Custódio, N.C.C.; de Castro Vieira Lima, V.; da Igreja, J.A.S.L.; de Sousa Mendes Moreira Alves, D.; Storchilo, H.R.; Gomes, A.R.; de Castro, A.M.; da Costa, W.L.G.; Rezende, H.H.A. Occurrence of parasites in salads in restaurants in Aparecida de Goiânia, Goiás, Brazil. Rev. Patol. Trop. J. Trop. Pathol. 2020, 49. [Google Scholar] [CrossRef]
- Ortiz Pineda, C.; Temesgen, T.T.; Robertson, L.J. Multiplex quantitative PCR analysis of strawberries from Bogotá, Colombia, for contamination with three parasites. J. Food Prot. 2020, 83, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Delignette-Muller, M.L.; Dutang, C.; Pouillot, R.; Denis, J.B.; Siberchicot, A. fitdistrplus: Help to Fit of a Parametric Distribution to Non-Censored or Censored Data. 2022. Available online: https://CRAN.R-project.org/package=fitdistrplus (accessed on 7 March 2023).
- Kim, M.; Rueda, L.; Packman, A.; Moore, J.; Wuertz, S.; Shapiro, K. Molecular detection and viability discrimination of zoonotic protozoan pathogens in oyster and seawater. Int. J. Food Microbiol. 2023; submitted for publication. [Google Scholar]
- Esmerini, P.O.; Gennari, S.M.; Pena, H.F.J. Analysis of marine bivalve shellfish from the fish market in Santos city, São Paulo state, Brazil, for Toxoplasma gondii. Vet. Parasitol. 2010, 170, 8–13. [Google Scholar] [CrossRef]
- de Melo, C.M.R.; Divilov, K.; Schoolfield, B.; Langdon, C. Selection of group and individual traits of Pacific oysters (Crassostrea gigas) on the West Coast, US. Aquaculture 2019, 512, 734389. [Google Scholar] [CrossRef]
- Mirza Alizadeh, A.; Jazaeri, S.; Shemshadi, B.; Hashempour-Baltork, F.; Sarlak, Z.; Pilevar, Z.; Hosseini, H. A review on inactivation methods of Toxoplasma gondii in foods. Pathog. Glob. Health 2018, 112, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.; Nosaka, K.; Hunter, M.; Wang, W. Global status of Toxoplasma gondii infection: Systematic review and prevalence snapshots. Trop. Biomed. 2019, 36, 898–925. [Google Scholar]
- Plummer, M.; Best, N.; Cowles, K.; Vines, K.; Sarkar, D.; Bates, D.; Almond, R.; Magnusson, A. Coda: Output Analysis and Diagnostics for MCMC. 2020. Available online: https://CRAN.R-project.org/package=coda (accessed on 1 November 2022).
- Kapperud, G.; Jenum, P.A.; Stray-Pedersen, B.; Melby, K.K.; Eskild, A.; Eng, J. Risk factors for Toxoplasma gondii infection in pregnancy: Results of a prospective case-control study in Norway. Am. J. Epidemiol. 1996, 144, 405–412. [Google Scholar] [CrossRef]
- Carellos, E.V.M.; de Andrade, G.M.Q.; Vasconcelos-Santos, D.V.; Januário, J.N.; Romanelli, R.M.C.; Abreu, M.N.S.; da Silva, F.M.; Loures, I.R.C.; de Andrade, J.Q.; Caiaffa, W.T.; et al. Adverse socioeconomic conditions and oocyst-related factors are associated with congenital toxoplasmosis in a population-based study in Minas Gerais, Brazil. PLoS ONE 2014, 9, e88588. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.S.; Cabrera, L.A.A.; Lima, S.V.M.A.; dos Santos, A.D.; Oliveira, L.M.G.B.; de Oliveira, R.C.; de Sousa Menezes, J.; de Figueiredo, J.A.; de Moura Lane, V.F.; de Lima Júnior, F.E.F.; et al. Temporal trend, spatial analysis and spatiotemporal clusters of infant mortality associated with congenital toxoplasmosis in Brazil: Time series from 2000 to 2020. Trop Med. Int. Health 2023, 28, 476–485. [Google Scholar] [CrossRef]
- Alemu, G.; Nega, M.; Alemu, M. Parasitic contamination of fruits and vegetables collected from local markets of Bahir Dar City, northwest Ethiopia. Res. Rep. Trop. Med. 2020, 11, 17–25. [Google Scholar] [CrossRef]
- Tefera, T.; Biruksew, A.; Mekonnen, Z.; Eshetu, T. Parasitic contamination of fruits and vegetables collected from selected local markets of Jimma Town, Southwest Ethiopia. Int. Sch. Res. Not. 2014, 2014, 382715. [Google Scholar] [CrossRef]
- Tefera, T.; Tysnes, K.R.; Utaaker, K.S.; Robertson, L.J. Parasite contamination of berries: Risk, occurrence, and approaches for mitigation. Food Waterborne Parasitol. 2018, 10, 23–38. [Google Scholar] [CrossRef]
- Dubey, J.P.; Ferreira, L.R.; Martins, J.; Mcleod, R. Oral oocyst-induced mouse model of toxoplasmosis: Effect of infection with Toxoplasma gondii strains of different genotypes, dose, and mouse strains (transgenic, out-bred, in-bred) on pathogenesis and mortality. Parasitology 2012, 139, 1–13. [Google Scholar] [CrossRef]
- Lélu, M.; Villena, I.; Dardé, M.L.; Aubert, D.; Geers, R.; Dupuis, E.; Marnef, F.; Poulle, M.L.; Gotteland, C.; Dumètre, A.; et al. Quantitative estimation of the viability of Toxoplasma gondii oocysts in soil. Appl. Environ. Microbiol. 2012, 78, 5127–5132. [Google Scholar] [CrossRef] [PubMed]
- Marciano, M.A.M.; Silva, R.A.; Barbosa, M.L.; Ferreira, A.R.S.; Pereira-Chioccola, V.L. Determination of the viability of Toxoplasma gondii oocysts by PCR real-time after treatment with propidium monoazide. Rev. Inst. Med. Trop. 2020, 62, e84. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Exel, K.E.; Swart, A.; Bonačić Marinović, A.A.; Dam-Deisz, C.; van der Giessen, J.W.B.; Opsteegh, M. Digging into Toxoplasma gondii infections via soil: A quantitative microbial risk assessment approach. Sci. Total Environ. 2021, 755, 143232. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, H.V.; D’ávila, S.; Bastos, R.R.; Cyrino, C.D.; de Lima Detoni, M.; Garcia, J.L.; das Neves, L.B.; Nicolau, J.L.; Amendoeira, M.R.R. Seroprevalence and risk factors of toxoplasmosis in cattle from extensive and semi-intensive rearing systems at Zona da Mata, Minas Gerais state, Southern Brazil. Parasites Vectors 2013, 6, 191. [Google Scholar] [CrossRef]
- Guimarães, A.M.; Bruhn, F.R.P.; da Rocha, C.M.B.M.; de Araújo, T.H.; Mesquita, C.A.M. Seroepidemiology of Toxoplasma gondii in dairy cows in southeastern Brazil: Seropositive cows on all farms investigated. Acta Parasitol. 2020, 65, 628–635. [Google Scholar] [CrossRef]
- Cabral Monica, T.; Evers, F.; de Souza Lima Nino, B.; Pinto-Ferreira, F.; Breganó, J.W.; Ragassi Urbano, M.; Rubinsky-Elefant, G.; Freire, R.L.; Navarro, I.T.; Mitsuka-Breganó, R. Socioeconomic factors associated with infection by Toxoplasma gondii and Toxocara canis in children. Transbound. Emerg. Dis. 2022, 69, 1589–1595. [Google Scholar] [CrossRef]
- Jones, J.L.; Dargelas, V.; Roberts, J.; Press, C.; Remington, J.S.; Montoya, J.G. Risk factors for Toxoplasma gondii infection in the United States. Clin. Infect. Dis. 2009, 49, 878–884. [Google Scholar] [CrossRef]
- de Lima Bessa, G.; de Almeida Vitor, R.W.; dos Santos Martins-Duarte, E. Toxoplasma gondii in South America: A differentiated pattern of spread, population structure and clinical manifestations. Parasitol. Res. 2021, 120, 3065–3076. [Google Scholar] [CrossRef]
- Khan, A.; Jordan, C.; Muccioli, C.; Vallochi, A.L.; Rizzo, L.V.; Belfort, R.; Vitor, R.W.; Silveira, C.; Sibley, L.D. Genetic divergence of Toxoplasma gondii strains associated with ocular toxoplasmosis, Brazil. Emerg. Infect. Dis. 2006, 12, 942–949. [Google Scholar] [CrossRef]
- Galal, L.; Hamidović, A.; Dardé, M.L.; Mercier, M. Diversity of Toxoplasma gondii strains at the global level and its determinants. Food Waterborne Parasitol. 2019, 15, e00052. [Google Scholar] [CrossRef]
- Araujo, F.; Slifer, T.; Kim, S. Chronic infection with Toxoplasma gondii does not prevent acute disease or colonization of the brain with tissue cysts following reinfection with different strains of the parasite. J. Parasitol. 1997, 83, 521–522. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, T.V.; Guy, E.C.; Roberts, K.E.; Joynson, D.H.M.; Hyde, J.E.; Sims, P.F.G. Molecular evidence for multiple Toxoplasma gondii infections in individual patients in England and Wales: Public health implications. Int. J. Parasitol. 2003, 33, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Val, A.L.; de M. Bicudo, C.E.; de C. Bicudo, D.; Pujoni, D.G.F.; Rosado, F.S.; de Souza Nogueira, I.; Hespanhol, I.; Cirilo, J.A.; Tundisi, J.G.; Val, P.; et al. Water quality in Brazil. Water Qual. Am. 2019, 103. Available online: https://www.researchgate.net/profile/Martin-Forde-2/publication/331839428_Water_and_Health/links/5c8f9e88299bf14e7e842611/Water-and-Health.pdf#page=104 (accessed on 26 May 2023).
- Pataca, L.C.M.; Pedrosa, M.A.F.; Zolnikov, T.R.; Mol, M.P.G. Water quality index and sanitary and socioeconomic indicators in Minas Gerais, Brazil. Environ. Monit. Assess. 2020, 192, 476. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.A.F.; Navarro, I.T.; Ruffolo, B.B.; Bugni, F.M.; de Castro, M.V.; Freire, R.L. Toxoplasma gondii in fresh pork sausage and seroprevalence in butchers from factories in Londrina, Paraná State, Brazil. Rev. Inst. Med. Trop. 2005, 47, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Herrero, L.; Gracia, M.J.; Pérez-Arquillué, C.; Lázaro, R.; Herrera, A.; Bayarri, S. Toxoplasma gondii in raw and dry-cured ham: The influence of the curing process. Food Microbiol. 2017, 65, 213–220. [Google Scholar] [CrossRef] [PubMed]
- USDA ER. Weights, Measures, and Conversion Factors for Agricultural Commodities and Their Products; USDA: Washington, DC, USA, 1992. Available online: http://www.ers.usda.gov/publications/pub-details/?pubid=41881 (accessed on 7 February 2023).
- Central Intelligence Agency. Brazil. In The World Factbook; Central Intelligence Agency: Washington, DC, USA, 2023. Available online: https://www.cia.gov/the-world-factbook/countries/brazil (accessed on 7 February 2023).
- Baptista, R.C.; Rodrigues, H.; Sant’Ana, A.S. Consumption, knowledge, and food safety practices of Brazilian seafood consumers. Food Res. Int. 2020, 132, 109084. [Google Scholar] [CrossRef]

| Variable | Equation/Distribution/Value | References |
|---|---|---|
| Exposure assessment bradyzoite | ||
| N1: n log10-transformed bradyzoites/100 g unprocessed meat | N1∼Beta general (shape 1 = 6.5, shape 2 = 5.7, min = 0, max = 6.8) N1 transformed (N1.1) = Beta(0.955, 0.838) × 6.8 | [23] |
| N2: n bradyzoite consumed/meat type | N2∼Poisson (λ = Cmeat × (10N1×6.8/100)) | [23] |
| C: food consumption amount (g) | Table S1 | IBGE [28] |
| N3: n bradyzoites after home cooking per consumed portion | N3 = N2 × RF(T) | [23] |
| RF(T): temperature reduction factor | RF(T) = D(T)/D(T0) | [23] |
| D(T): dose of bradyzoites | D(T) = −ln(1 − P0(T))/r0 | [23] |
| P0(T): probability of infection in mice | P0(T) = 1/(1 + e^−(44.181−0.834×T) | [24] |
| r0: T. gondii infection probability of a single bradyzoite in mice | r0 = 0.011 | [24] |
| T: temperature C | T~Laplace (m = location, s = dispersion) Pork m = 71.11, s = 9.88 Beef and sheep m= 71.11, s = 9.82 Poultry m = 75.56, s = 9.31 | [23] |
| T0: temperature before cooking (C) | T0 = 25 | [23] |
| Exposure assessment oocyst | ||
| N4: n oocysts/g unprocessed produce | N4~Beta (shape 1 = 0.105, shape 2 = 0.702) | Fitted data from Marques et al. [35] |
| N5: n oocysts consumed/produce type | N5∼Poisson (λ = Cproduce × N4 × 180) | [23] |
| N6: n oocysts after washing W(T): washing | N6 × W(T) W(T)~Beta (1, 0.57) | Distribution fit from Temesgen et al. 2021 [36] |
| Dose–response | ||
| P1: probability of human infection (per meat type in one day) | P1 = 1 − e(−r1 × N3) | [37] |
| r1: probability of single bradyzoite initiating T. gondii infection in humans | r1 = 0.001535 | [37] |
| P2: probability of human infection (per produce type in one day) | P2 = 1 − e(−r2 × N6) | [38] |
| r2: probability of single oocyst initiating T. gondii infection in humans | r2 = 0.46 | [38] |
| Risk characterization | ||
| P3: probability of infection through consumption of food products in human population (/meat or produce type per day) | P3 = P1 or P2 × Pfood × (1 − Phuman) | [23] |
| Pfood: prevalence per food type. Overall averaged prevalences for the entirety of Brazil were used due to the low number of available studies. | Pfood: Supplemental Table S2 | [39,40,41,42,43,44,45,46] |
| Phuman: seroprevalence of human population | Phuman: Unif (0.215, 0.974) | [8] |
| Scenarios | Definitions |
|---|---|
| Baseline model | Baseline consumption of food products averaged over all five regions of Brazil to compare against other scenarios |
| (1) Lower bradyzoite concentration in beef | Bradyzoite concentration for beef lowered to 1/100 that of other meat species to reflect lower estimated tissue cyst burden in cattle |
| (2) Improved washing efficiency | Washing oocysts off of produce increased by 25% from baseline |
| (3) Decreased oocyst viability | Oocyst viability reduced by 50% (25% viability overall) |
| (4) Shellfish | Oyster consumption added as another source of oocyst foodborne transmission due to its growing popularity. Production data were obtained for the South of Brazil and converted to consumption quantity (g) (Table S3). |
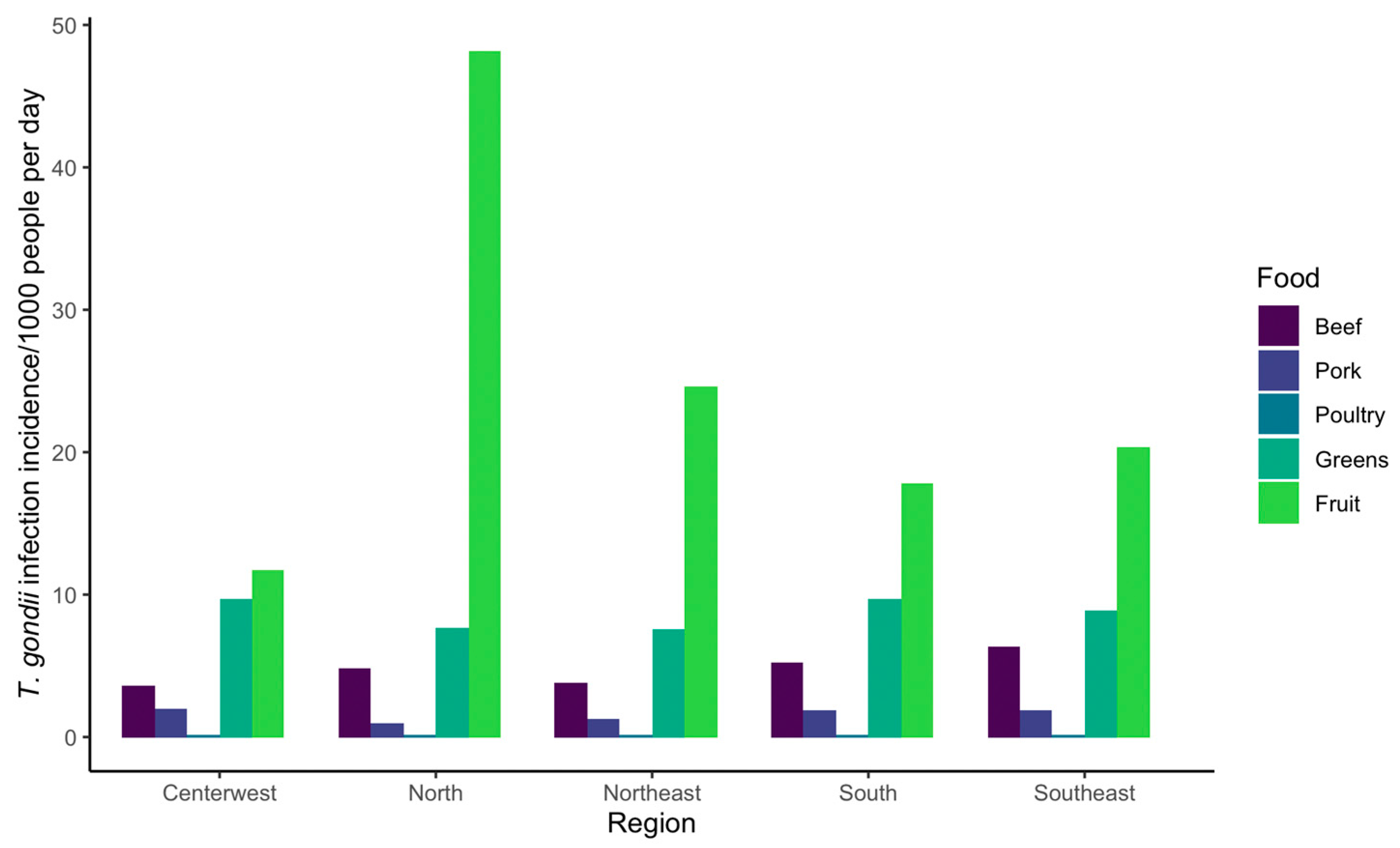
| North | Northeast | Center-West | Southeast | South | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Food | Mean (95% CI) | % | Mean (95% CI) | % | Mean (95% CI) | % | Mean (95% CI) | % | Mean (95% CI) | % |
| Beef | 4.81 (1 × 10−6, 46.1) | 7.8 | 3.78 (0, 48) | 10.1 | 6.32 (4 × 10−8, 59.4) | 16.9 | 5.19 (0, 53.4) | 14.9 | 3.58 (8 × 10−7, 39.6) | 13.2 |
| Pork | 0.97 (0, 12.7) | 1.6 | 1.28 (0, 16.3) | 3.4 | 1.91 (0, 20.3) | 5.1 | 1.91 (0, 20.7) | 5.5 | 2.03 (0, 23.6) | 7.5 |
| Poultry | 0.13 (1 × 10−7, 1.3) | 0.2 | 0.17 (0, 1.8) | 0.5 | 0.11 (0, 1.1) | 0.3 | 0.15 (0, 1.36) | 0.4 | 0.11 (0, 1.1) | 0.4 |
| Greens | 7.69 (0, 60.2) | 12.5 | 7.58 (0, 57.4) | 20.3 | 8.86 (0, 71.9) | 23.6 | 9.68 (0, 70.4) | 27.9 | 9.67 (0, 80.3) | 35.6 |
| Fruit | 48.13 (0, 314.2) | 77.9 | 24.62 (0, 259.5) | 65.7 | 20.31 (0, 179.5) | 54.1 | 17.85 (0, 198.1) | 51.3 | 11.76 (0, 118.1) | 43.3 |
| Total | 61.73 | 37.43 | 37.51 | 34.78 | 27.15 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; VanWormer, E.; Martínez-López, B.; Bahia-Oliveira, L.M.G.; DaMatta, R.A.; Rodrigues, P.S.; Shapiro, K. Quantitative Risk Assessment of Oocyst Versus Bradyzoite Foodborne Transmission of Toxoplasma gondii in Brazil. Pathogens 2023, 12, 870. https://doi.org/10.3390/pathogens12070870
Zhu S, VanWormer E, Martínez-López B, Bahia-Oliveira LMG, DaMatta RA, Rodrigues PS, Shapiro K. Quantitative Risk Assessment of Oocyst Versus Bradyzoite Foodborne Transmission of Toxoplasma gondii in Brazil. Pathogens. 2023; 12(7):870. https://doi.org/10.3390/pathogens12070870
Chicago/Turabian StyleZhu, Sophie, Elizabeth VanWormer, Beatriz Martínez-López, Lílian Maria Garcia Bahia-Oliveira, Renato Augusto DaMatta, Pedro Souto Rodrigues, and Karen Shapiro. 2023. "Quantitative Risk Assessment of Oocyst Versus Bradyzoite Foodborne Transmission of Toxoplasma gondii in Brazil" Pathogens 12, no. 7: 870. https://doi.org/10.3390/pathogens12070870
APA StyleZhu, S., VanWormer, E., Martínez-López, B., Bahia-Oliveira, L. M. G., DaMatta, R. A., Rodrigues, P. S., & Shapiro, K. (2023). Quantitative Risk Assessment of Oocyst Versus Bradyzoite Foodborne Transmission of Toxoplasma gondii in Brazil. Pathogens, 12(7), 870. https://doi.org/10.3390/pathogens12070870








