Hepatitis C Virus Down-Regulates the Expression of Ribonucleotide Reductases to Promote Its Replication
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical/Drugs
2.2. Cell Culture and Viruses
2.3. Quantitation of Intracellular Nucleoside Triphosphate (NTP) and Deoxynucleoside Triphosphate (dNTP) Pools
2.4. RNA Extraction and Real-Time Reverse Transcriptase–Polymerase Chain Reaction (Real-Time RT-PCR)
2.5. Plasmid Construction and DNA Transfection
2.6. Western Blotting Analysis
2.7. The shRNA Knockdown and Stably Over-Expressed Experiments
2.8. Luciferase Assay
3. Results
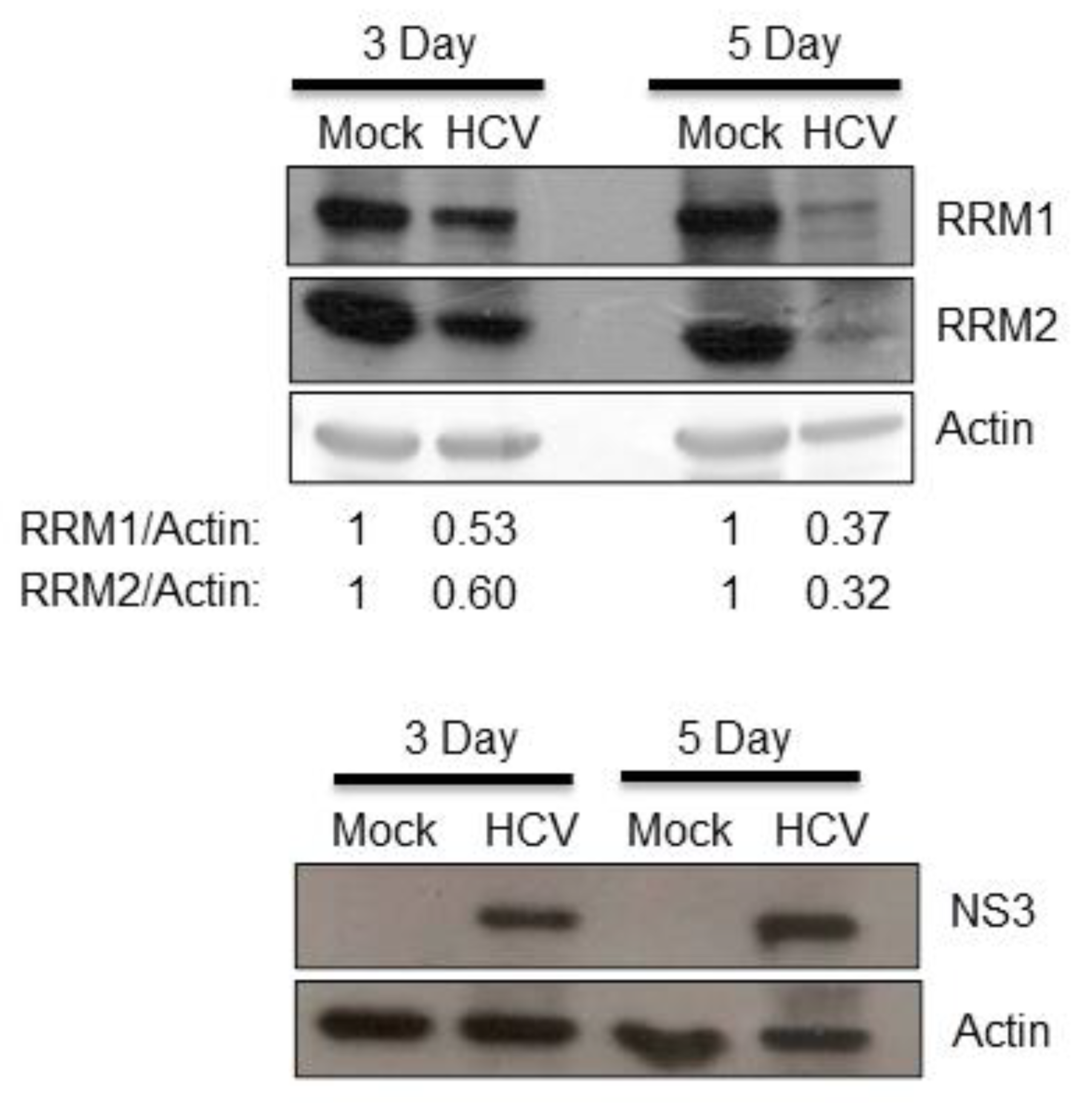
3.1. HCV Suppressed the Expression of RRM1 and RRM2 and Up-Regulated the Intracellular NTP Level
3.2. Reduced Expression of RRMs Led to a Higher Intracellular NTP/dNTP Ratio
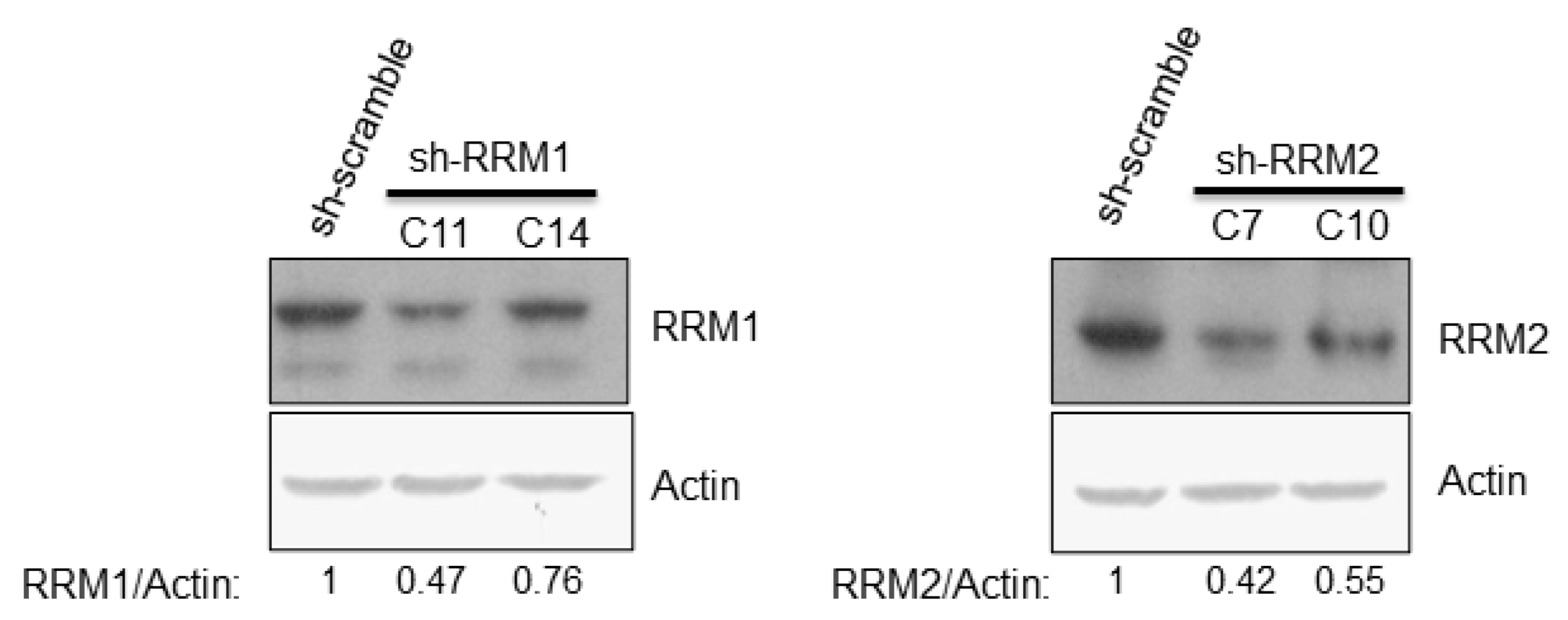
3.3. Reduced Expression of RRMs Led to Enhancement of HCV Replication
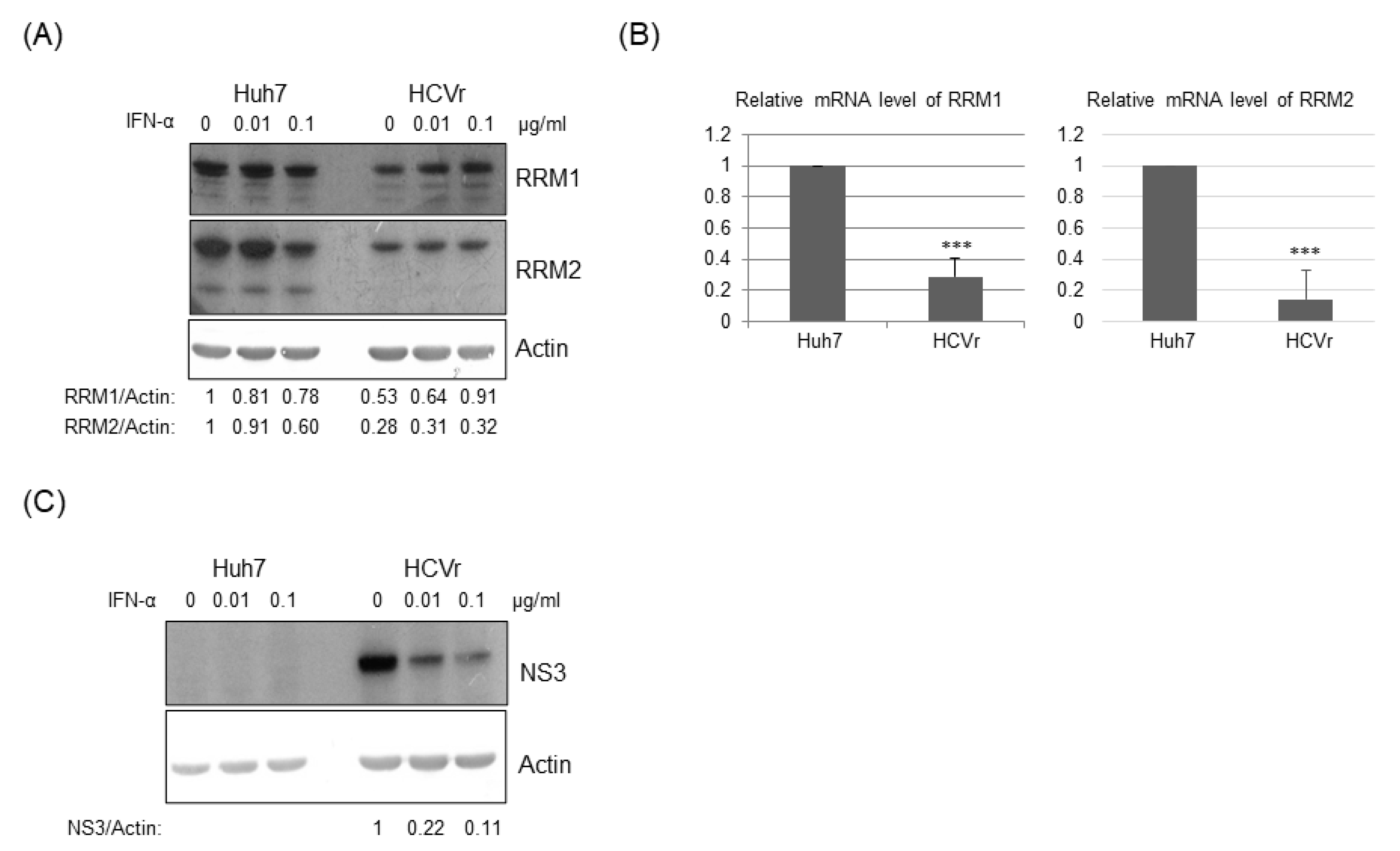
3.4. Inhibition of RRMs’ Activity Facilitated HCV Replication
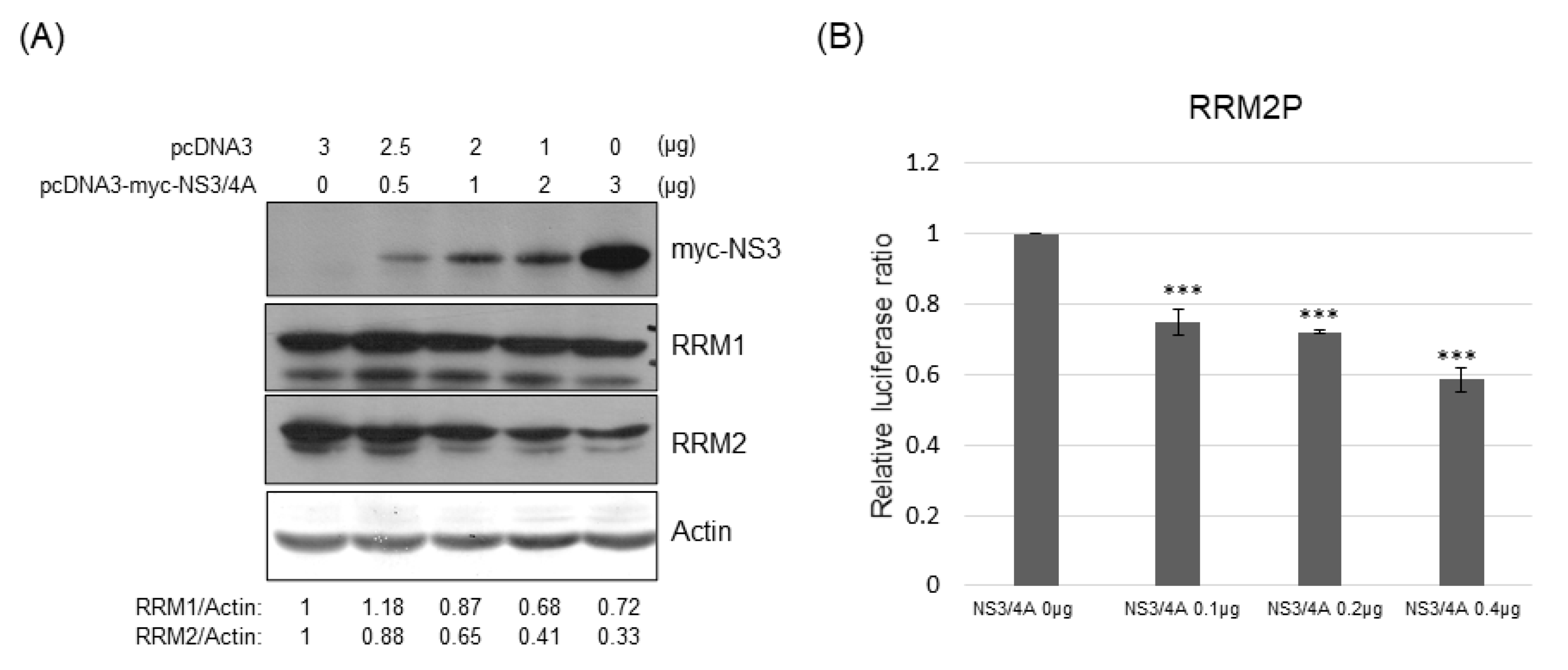
3.5. RRM Expression Was Suppressed by HCV Viral Proteins NS5A and/or NS3/4A
3.6. Transcriptomic Data from NCBI GEO Database
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khatun, M.; Ray, R.; Ray, R.B. Hepatitis C virus associated hepatocellular carcinoma. Adv. Cancer Res. 2021, 149, 103–142. [Google Scholar] [PubMed]
- Li, H.C.; Lo, S.Y. Hepatitis C virus: Virology, diagnosis and treatment. World J. Hepatol. 2015, 7, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Li, H.C.; Yang, C.H.; Lo, S.Y. Cellular factors involved in the hepatitis C virus life cycle. World J. Gastroenterol. 2021, 27, 4555–4581. [Google Scholar] [CrossRef] [PubMed]
- Herrick, J.; Sclavi, B. Ribonucleotide reductase and the regulation of DNA replication: An old story and an ancient heritage. Mol. Microbiol. 2007, 63, 22–34. [Google Scholar] [CrossRef]
- Long, M.J.C.; Ly, P.; Aye, Y. Still no Rest for the Reductases: Ribonucleotide Reductase (RNR) Structure and Function: An Update. Subcell. Biochem. 2022, 99, 155–197. [Google Scholar]
- Ruskoski, T.B.; Boal, A.K. The periodic table of ribonucleotide reductases. J. Biol. Chem. 2021, 297, 101137. [Google Scholar] [CrossRef]
- Gammon, D.B.; Gowrishankar, B.; Duraffour, S.; Andrei, G.; Upton, C.; Evans, D.H. Vaccinia virus-encoded ribonucleotide reductase subunits are differentially required for replication and pathogenesis. PLoS Pathog. 2010, 6, e1000984. [Google Scholar] [CrossRef]
- Lembo, D.; Brune, W. Tinkering with a viral ribonucleotide reductase. Trends Biochem. Sci. 2009, 34, 25–32. [Google Scholar] [CrossRef]
- Wnuk, S.F.; Robins, M.J. Ribonucleotide reductase inhibitors as anti-herpes agents. Antivir. Res. 2006, 71, 122–126. [Google Scholar] [CrossRef]
- Anacker, D.C.; Aloor, H.L.; Shepard, C.N.; Lenzi, G.M.; Johnson, B.A.; Kim, B.; Moody, C.A. HPV31 utilizes the ATR-Chk1 pathway to maintain elevated RRM2 levels and a replication-competent environment in differentiating Keratinocytes. Virology 2016, 499, 383–396. [Google Scholar] [CrossRef]
- Cohen, D.; Adamovich, Y.; Reuven, N.; Shaul, Y. Hepatitis B virus activates deoxynucleotide synthesis in nondividing hepatocytes by targeting the R2 gene. Hepatology 2010, 51, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Ricardo-Lax, I.; Ramanan, V.; Michailidis, E.; Shamia, T.; Reuven, N.; Rice, C.M.; Shlomai, A.; Shaul, Y. Hepatitis B virus induces RNR-R2 expression via DNA damage response activation. J. Hepatol. 2015, 63, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Fritzer-Szekeres, M.; Novotny, L.; Vachalkova, A.; Findenig, G.; Elford, H.L.; Szekeres, T. Iron binding capacity of didox (3,4-dihydroxybenzohydroxamic acid) and amidox (3,4-dihydroxybenzamidoxime) new inhibitors of the enzyme ribonucleotide reductase. Life Sci. 1997, 61, 2231–2237. [Google Scholar] [CrossRef]
- Rauko, P.; Romanova, D.; Miadokova, E.; Macakova, K.; Novotny, L.; Elford, H.L.; Szekeres, T. DNA-protective activity of new ribonucleotide reductase inhibitors. Anticancer Res. 1997, 17, 3437–3440. [Google Scholar] [PubMed]
- Musialek, M.W.; Rybaczek, D. Hydroxyurea-The Good, the Bad and the Ugly. Genes 2021, 12, 1096. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Li, H.C.; Ku, T.S.; Wu, P.C.; Yeh, Y.J.; Cheng, J.C.; Lin, T.Y.; Lo, S.Y. Hepatitis C virus down-regulates SERPINE1/PAI-1 expression to facilitate its replication. J. Gen. Virol. 2017, 98, 2274–2286. [Google Scholar] [CrossRef]
- Choi, J.; Lee, K.J.; Zheng, Y.; Yamaga, A.K.; Lai, M.M.; Ou, J.H. Reactive oxygen species suppress hepatitis C virus RNA replication in human hepatoma cells. Hepatology 2004, 39, 81–89. [Google Scholar] [CrossRef]
- Lindenbach, B.D.; Evans, M.J.; Syder, A.J.; Wolk, B.; Tellinghuisen, T.L.; Liu, C.C.; Maruyama, T.; Hynes, R.O.; Burton, D.R.; McKeating, J.A.; et al. Complete replication of hepatitis C virus in cell culture. Science 2005, 309, 623–626. [Google Scholar] [CrossRef]
- Yeh, Y.J.; Tseng, C.P.; Hsu, S.D.; Huang, H.Y.; Lai, M.M.C.; Huang, H.D.; Cheng, J.C. Dual Effects of Let-7b in the Early Stage of Hepatitis C Virus Infection. J. Virol. 2021, 95, e01800-20. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Z.; Liu, S.; Xie, Z.; Aimiuwu, J.; Pang, J.; Klisovic, R.; Blum, W.; Grever, M.R.; Marcucci, G.; et al. A LC-MS/MS method for the analysis of intracellular nucleoside triphosphate levels. Pharm. Res. 2009, 26, 1504–1515. [Google Scholar] [CrossRef]
- The dMIQE Group; Huggett, J.F. The Digital MIQE Guidelines Update: Minimum Information for Publication of Quantitative Digital PCR Experiments for 2020. Clin. Chem. 2020, 66, 1012–1029. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Li, H.C.; Shiu, Y.L.; Ku, T.S.; Wang, C.W.; Tu, Y.S.; Chen, H.L.; Wu, C.H.; Lo, S.Y. Influenza A virus upregulates PRPF8 gene expression to increase virus production. Arch. Virol. 2017, 162, 1223–1235. [Google Scholar] [CrossRef]
- Traut, T.W. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 1994, 140, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.; Wiegand, R.; Wang, J.; Bepler, G.; Li, J. Quantitative analysis of intracellular nucleoside triphosphates and other polar metabolites using ion pair reversed-phase liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 1006, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Levine, M. Characterization of the promoter of the human ribonucleotide reductase R2 gene. Biochem. Biophys. Res. Commun. 2000, 267, 651–657. [Google Scholar] [CrossRef]
- Parker, N.J.; Begley, C.G.; Fox, R.M. Human R1 subunit of ribonucleotide reductase (RRM1): 5’ flanking region of the gene. Genomics 1994, 19, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.J.; Begley, C.G.; Fox, R.M. Human gene for the large subunit of ribonucleotide reductase (RRM1): Functional analysis of the promoter. Genomics 1995, 27, 280–285. [Google Scholar] [CrossRef]
- Zhou, B.; Yen, Y. Characterization of the human ribonucleotide reductase M2 subunit gene; genomic structure and promoter analyses. Cytogenet. Cell Genet. 2001, 95, 52–59. [Google Scholar] [CrossRef]
- Li, H.C.; Ma, H.C.; Yang, C.H.; Lo, S.Y. Production and pathogenicity of hepatitis C virus core gene products. World J. Gastroenterol. 2014, 20, 7104–7122. [Google Scholar] [CrossRef]
- Tegtmeyer, B.; Vieyres, G.; Todt, D.; Lauber, C.; Ginkel, C.; Engelmann, M.; Herrmann, M.; Pfaller, C.K.; Vondran, F.W.R.; Broering, R.; et al. Initial HCV infection of adult hepatocytes triggers a temporally structured transcriptional program containing diverse pro- and anti-viral elements. J. Virol. 2021, 95, e00245-21. [Google Scholar] [CrossRef]
- Boldanova, T.; Suslov, A.; Heim, M.H.; Necsulea, A. Transcriptional response to hepatitis C virus infection and interferon-alpha treatment in the human liver. EMBO Mol. Med. 2017, 9, 816–834. [Google Scholar] [CrossRef] [PubMed]
- Bhave, S.; Elford, H.; McVoy, M.A. Ribonucleotide reductase inhibitors hydroxyurea, didox, and trimidox inhibit human cytomegalovirus replication in vitro and synergize with ganciclovir. Antivir. Res. 2013, 100, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, C.; Oakley, O.; Piper, J.; Hughes, N.K.; Phillips, J.; Birch, N.J.; Elford, H.L.; Gallicchio, V.S. Effective use of ribonucleotide reductase inhibitors (Didox and Trimidox) alone or in combination with didanosine (ddI) to suppress disease progression and increase survival in murine acquired immunodeficiency syndrome (MAIDS). Cell. Mol. Biol. 1997, 43, 1019–1029. [Google Scholar]
- Liu, X.; Xu, Z.; Hou, C.; Wang, M.; Chen, X.; Lin, Q.; Song, R.; Lou, M.; Zhu, L.; Qiu, Y.; et al. Inhibition of hepatitis B virus replication by targeting ribonucleotide reductase M2 protein. Biochem. Pharmacol. 2016, 103, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, Z.; Tian, J.; Liu, Q.; Dong, J.; Guo, L.; Hai, B.; Liu, X.; Yao, H.; Chen, Z.; et al. Pterostilbene inhibits hepatocellular carcinoma proliferation and HBV replication by targeting ribonucleotide reductase M2 protein. Am. J. Cancer Res. 2021, 11, 2975–2989. [Google Scholar] [PubMed]
- Moradpour, D.; Penin, F. Hepatitis C virus proteins: From structure to function. Curr. Top. Microbiol. Immunol. 2013, 369, 113–142. [Google Scholar]
- Kitab, B.; Satoh, M.; Ohmori, Y.; Munakata, T.; Sudoh, M.; Kohara, M.; Tsukiyama-Kohara, K. Ribonucleotide reductase M2 promotes RNA replication of hepatitis C virus by protecting NS5B protein from hPLIC1-dependent proteasomal degradation. J. Biol. Chem. 2019, 294, 5759–5773. [Google Scholar] [CrossRef]



| Primer Sequences for the Cloning of Expressing Plasmids | |
|---|---|
| EGFP-S2 | 5′- CTAGCTAGCATGGTGAGCAAGGGCGAGGA-3′ |
| EGFP-AS3 | 5′- GCTCTAGACTTGTACAGCTCGTCCAT-3′ |
| Core-S | 5′-CGGAATTCATGAGCACGAATCCTAA-3′ |
| Core-AS3 | 5′GCTCTAGAGGCTGAAGCGGGCACAGT-3′ |
| NS3/4A-S | 5′-CGGGATCCGCGCCCATCACGGCG -3′ |
| NS3/4A-AS | 5′-GCTCTAGACTATTAGCACTCTTCCATCTC -3′ |
| NS4B-F | 5′-CGGAATTCATGTCTCAGCACTTACCGTAC -3′ |
| NS4B-AS | 5′-GCTCTAGATTAGCATGGAGTGGTACA-3′ |
| NS5A-S | 5′-CGGAATTCATGTCCGGTTCCTGGCTAAG -3′ |
| NS5A-AS3 | 5′-GCTCTAGAGCAGCACACGACATCTTC -3′ |
| RRM1P1000-S | 5′-GGGGTACCACCATGCCTGGCTACT-3′ |
| RRM1P+300-AS | 5′-GAAGATCTATCCAAGACTGGACTGCG-3′ |
| RRM2P1510-S | 5′-CTAGCTAGCTTCCTGGAGATGGATGCTTTA-3′ |
| RRM2P+50-AS | 5′-GAAGATCTTGGCTGCGCCTTGC-3′ |
| Primer Sequences for the Real-Time RT-PCR Analysis | |
| QRRM1-F | 5′-ACCGCCCACAACTTTCTAG-3′ |
| QRRM1-R | 5′- CCAGTAGCCCGAATACAACTC-3′ |
| QRRM2-F | 5′- AAGGACATTCAGCACTGGG-3′ |
| QRRM2-R | 5′- AGCGGGCTTCTGTAATCTG-3′ |
| Qbetaactin-F | 5′-CATCGAGCACGGCATCGTCA-3′ |
| Qbetaactin-R | 5′-TAGCACAGCCTGGATAGCAAC-3′ |
| HCV-RTF | 5′-AGCGTCTAGCCATGGCGT-3′ |
| HCV-RTR | 5′-CAAGCACCCTATCAGGCAGT-3′ |
| Huh7 | HCVr | p-Value | |
|---|---|---|---|
| ATP/dGTP | 356.9 ± 5.8 | 421.2 ± 10.7 | 0.0003 |
| UTP | 181.5 ± 12.5 | 179.6 ± 4.4 | 0.4068 |
| CTP | 67.4 ± 3.1 | 82.1 ± 8.4 | 0.0238 |
| GTP | 252.1 ± 5.9 | 233 ± 8.4 | 0.0161 |
| dATP | 2.8 ± 0.5 | 6.8 ± 0.6 | 0.0004 |
| dTTP | 11.5 ± 2.7 | 9.7 ± 2.4 | 0.2180 |
| dCTP | 1.4 ± 0 | 1.7 ± 0.5 | 0.1879 |
| sh-Scramble | sh-RRM1 C11 | p-Value | |
|---|---|---|---|
| ATP/dGTP | 354.2 ± 14.1 | 531.5 ± 59.8 | 0.003754 |
| UTP | 145.8 ± 13.4 | 247.4 ± 36.2 | 0.005197 |
| CTP | 41.8 ± 4.7 | 21.3 ± 2 | 0.00113 |
| GTP | 165.6 ± 4.9 | 281.1 ± 34 | 0.002163 |
| dATP | 4.8 ± 1 | 2.1 ± 0.3 | 0.006417 |
| dTTP | 8.9 ± 1.8 | 16.6 ± 1.6 | 0.002676 |
| dCTP | 1.7 ± 0.3 | 0.6 ± 0.2 | 0.003065 |
| sh-scramble | sh-RRM2 C10 | p-Value | |
|---|---|---|---|
| ATP/dGTP | 354.2 ± 14.1 | 714.9 ± 118.5 | 0.003178 |
| UTP | 145.8 ± 13.4 | 354.8 ± 108.2 | 0.014691 |
| CTP | 41.8 ± 4.7 | 25.7 ± 4.2 | 0.005665 |
| GTP | 165.6 ± 4.9 | 598.6 ± 35.9 | 0.000016 |
| dATP | 4.8 ± 1 | 3.1 ± 0.5 | 0.032387 |
| dTTP | 8.9 ± 1.8 | 5.7 ± 0.8 | 0.02581 |
| dCTP | 1.7 ± 0.3 | 1.7 ± 0.4 | 0.423585 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-H.; Wu, C.-H.; Lo, S.-Y.; Lua, A.-C.; Chan, Y.-R.; Li, H.-C. Hepatitis C Virus Down-Regulates the Expression of Ribonucleotide Reductases to Promote Its Replication. Pathogens 2023, 12, 892. https://doi.org/10.3390/pathogens12070892
Yang C-H, Wu C-H, Lo S-Y, Lua A-C, Chan Y-R, Li H-C. Hepatitis C Virus Down-Regulates the Expression of Ribonucleotide Reductases to Promote Its Replication. Pathogens. 2023; 12(7):892. https://doi.org/10.3390/pathogens12070892
Chicago/Turabian StyleYang, Chee-Hing, Cheng-Hao Wu, Shih-Yen Lo, Ahai-Chang Lua, Yu-Ru Chan, and Hui-Chun Li. 2023. "Hepatitis C Virus Down-Regulates the Expression of Ribonucleotide Reductases to Promote Its Replication" Pathogens 12, no. 7: 892. https://doi.org/10.3390/pathogens12070892
APA StyleYang, C.-H., Wu, C.-H., Lo, S.-Y., Lua, A.-C., Chan, Y.-R., & Li, H.-C. (2023). Hepatitis C Virus Down-Regulates the Expression of Ribonucleotide Reductases to Promote Its Replication. Pathogens, 12(7), 892. https://doi.org/10.3390/pathogens12070892






