Association of CYP2C19, CYP2D6 and CYP3A4 Genetic Variants on Primaquine Hemolysis in G6PD-Deficient Patients
Abstract
:1. Introduction
2. Materials and Methods
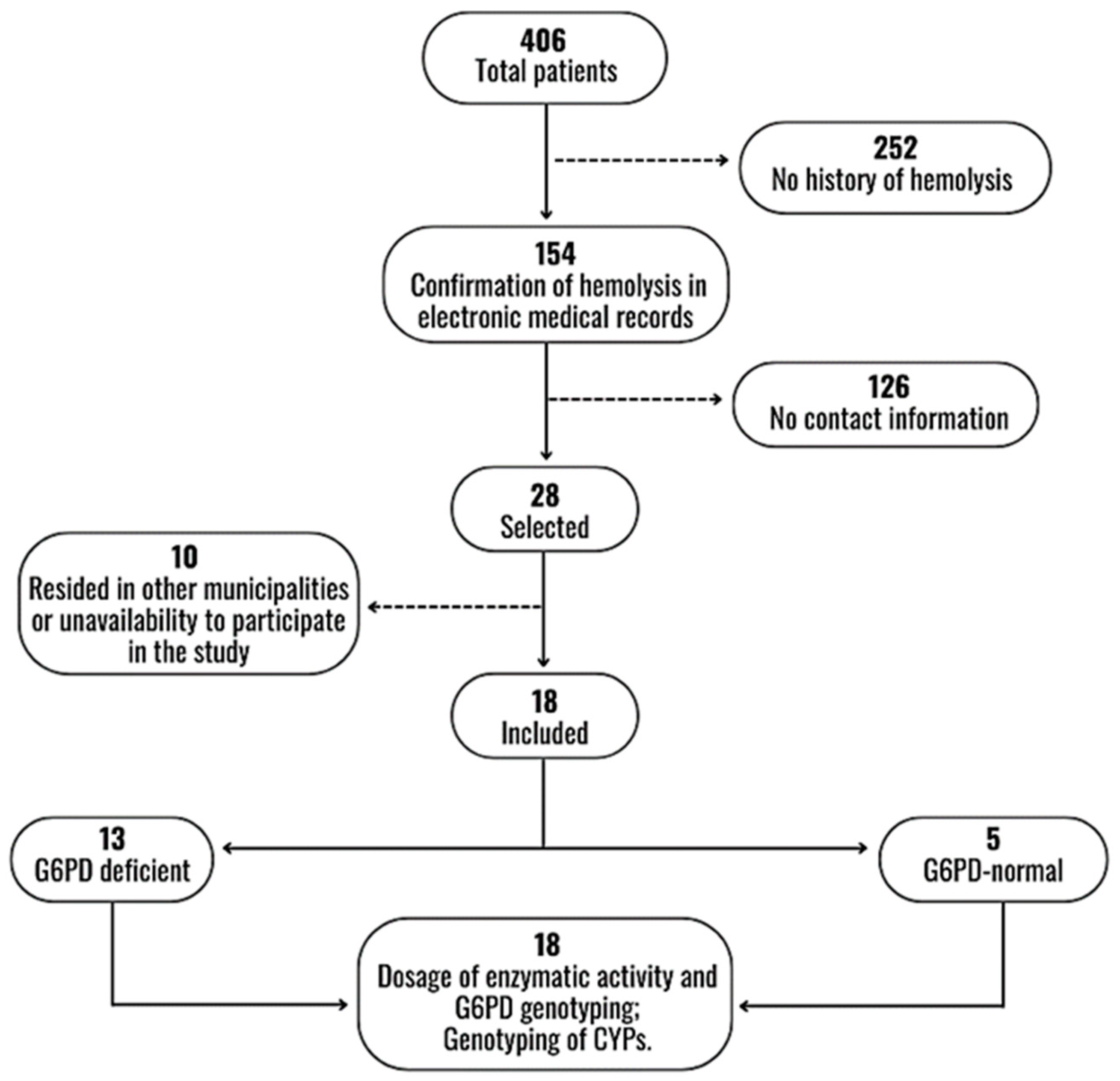
2.1. Study Subjects
2.2. G6PD Phenotyping
2.3. Genotyping of G6PD and CYPs
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Laboratory Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- do Nascimento, T.L.; Vasconcelos, S.P.; Peres, Y.; de Oliveira, M.J.S.; Taminato, M.; de Souza, K.M.J. Prevalence of malaria relapse: Systematic review with meta-analysis. Rev. Lat. Am. Enfermagem. 2019, 27, e3111. [Google Scholar] [CrossRef] [PubMed]
- Brasil, P.; Zalis, M.G.; de Pina-Costa, A.; Siqueira, A.M.; Júnior, C.B.; Silva, S.; Areas, A.L.L.; Pelajo-Machado, P.M.; de Alvarenga, D.A.M.; da Silva Santelli, A.C.F. Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: A molecular epidemiological investigation. Lancet Glob. Health 2017, 5, e1038–e1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luzzatto, L.; Ally, M.; Notaro, R. Glucose-6-Phosphate Dehydrogenase Deficiency. Blood 2020, 10, 1225–1240. [Google Scholar] [CrossRef]
- Krudsood, S.; Tangpukdee, N.; Wilairatana, P.; Phophak, N.; Baird, J.K.; Brittenham, G.M.; Looareesuwan, S. High-dose primaquine regimens against relapse of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 2008, 78, 736–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, J.K. 8-Aminoquinoline Therapy for Latent Malaria. Clin. Microbiol. 2019, 32, e00011-19. [Google Scholar] [CrossRef]
- Luzzatto, L.; Nannelli, C.; Notaro, R. Glucose-6-phosphate dehydrogenase deficiency. Hematol. Oncol. Clin. N. Am. 2016, 30, 373–393. [Google Scholar] [CrossRef]
- Brito-sousa, J.D.; Santos, T.C.; Avalos, S.; Fontecha, G.; Melo, G.C.; Val, F.; Siqueira, A.M.; Alecrim, G.C.; Bassat, Q.; Lacerda, M.V.G. Clinical Spectrum of Primaquine- induced Hemolysis in Glucose-6- Phosphate Dehydrogenase Deficiency: A 9-Year Hospitalization-based Study From the Brazilian Amazon. Clin. Infect. Dis. 2019, 69, 1440–1442. [Google Scholar] [CrossRef]
- Monteiro, W.M.; Moura-Neto, J.P.; Recht, J.; Bassat, Q.; Lacerda, M.V.G. Fatal Primaquine-Induced Hemolysis in a Patient with Plasmodium vivax Malaria and G6PD A(-) Variant in the Brazilian Amazon. Clin. Infect. Dis. 2016, 62, 1188. [Google Scholar] [CrossRef] [Green Version]
- Howes, R.E.; Piel, F.B.; Patil, A.P.; Nyangiri, O.A.; Gething, P.W.; Dewi, M.; Hogg, M.M.; Battle, K.E.; Padilla, C.D.; Baird, J.K.; et al. G6PD Deficiency Prevalence and Estimates of Affected Populations in Malaria Endemic Countries: A Geostatistical Model-Based Map. PLoS Med. 2012, 9, e1001339. [Google Scholar] [CrossRef] [Green Version]
- Santana, M.S.; Monteiroa, W.M.; Siqueiraa, A.M.; Costa, M.F.; Sampaio, V.; Lacerdaa, M.V.; Alecrim, M.G. Glucose-6-phosphate dehydrogenase deficient variants are associated with reduced susceptibility to malaria in the brazilian amazon. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 301–306. [Google Scholar] [CrossRef]
- Oliveira, R.A.G.; Oshiro, M.; Hirata, M.H.; Hirata, R.D.C.; Ribeiro, G.S.; Medeiros, T.M.D.; Barretto, O.C.D.O. A novel point mutation in a class IV glucose-6-phosphate dehydrogenase variant (G6PD São Paulo) and polymorphic G6PD variants in São Paulo State, Brazil. Genet. Mol. Biol. 2009, 32, 251–254. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.; Neto, D.M.; Dourado, M.V.; Galvão, M.; Gonçalves, M.S. A novel c. 197T→A variant among Brazilian neonates with glucose-6-phosphate dehydrogenase deficiency. Genet. Mol. Biol. 2008, 31, 33–35. [Google Scholar]
- Monteiro, W.M.; Val, F.F.A.; Siqueira, A.M.; Franca, G.P.; Sampaio, V.S.; Melo, G.C.; Almeida, A.C.G.; Brito, M.A.M.; Peixoto, H.M.; Fuller, D.; et al. G6PD deficiency in Latin America: Systematic review on prevalence and variants. Mem. Inst. Oswaldo Cruz. 2014, 109, 553–568. [Google Scholar] [CrossRef]
- Dombrowski, J.G.; Souza, R.M.; Curry, J.; Hinton, L.; Silva, N.R.M.; Grignard, L.; Gonçalves, L.A.; Gomes, A.R.; Epiphanio, S.; Drakeley, C.; et al. G6PD deficiency alleles in a malaria-endemic region in the Western Brazilian Amazon. Malar. J. 2017, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Recht, J.; Ashley, E.A.; White, N.J. Use of primaquine and glucose-6-phosphate dehydrogenase deficiency testing: Divergent policies and practices in malaria endemic countries. PLoS Negl. Trop. Dis. 2018, 12, e0006230. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, J.R.; Brito-sousa, J.D.; Gomes, C.; Melo, M.M.; Costa, M.R.F.; Barbosa, L.R.A.; Ramos, R.N.; Silva-Neto, A.V.; da Silva Balieiro, P.C.; Figueiredo, E.F.G.; et al. Articles Prevalence of glucose 6-phosphate dehydrogenase de fi ciency in highly malaria-endemic municipalities in the Brazilian Amazon: A region-wide screening study. Lancet Reg. Health—Am. 2022, 12, 100273. [Google Scholar]
- Marcsisin, S.R.; Reichard, G.; Pybus, B.S. Primaquine pharmacology in the context of CYP 2D6 pharmacogenomics: Current state of the art. Pharmacol. Ther. 2016, 161, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bahar, M.A.; Setiawan, D.; Hak, E.; Wilffert, B. Pharmacogenomics of drug–drug interaction and drug–drug–gene interaction: A systematic review on CYP2C9, CYP2C19 and CYP2D6. Pharmacogenom. J. 2017, 18, 701–739. [Google Scholar] [CrossRef]
- Friedrich, D.C.; Genro, J.P.; Sortica, V.A.; Suarez-Kurtz, G.; De Moraes, M.E.; Pena, S.D.J.; Santos, A.K.R.D.; Romano-Silva, M.A.; Hutz, M.H. Distribution of CYP2D6 alleles and phenotypes in the Brazilian population. PLoS ONE 2014, 9, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Chamnanphon, M.; Gaedigk, A.; Puangpetch, A.; Pasomsub, E.; Chantratita, W.; Longley, R.J.; Sattabongkot, J.; Chariyavilaskul, P.; Sukasem, C. Pharmacogene variation in Thai Plasmodium vivax relapse patients treated with a combination of primaquine and chloroquine. Pharmgenom. Pers. Med. 2020, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, J.L.M.S.Y.; Almeida, A.C.G.; Barbosa LRAS, E.L.; Rodrigues, M.G.A.R.-S.F.; Sampaio, V.S.S.A.; Lacerda, M.V.G.; Monteiro, W.M.M.G. Influence of CYP2D6, CYP3A4 and CYP2C19 Genotypes on Recurrence of Plasmodium vivax. Front. Trop. Dis. 2022, 3, 845451. [Google Scholar] [CrossRef]
- Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Imunização e Doenças Transmissíveis. In Guia de Tratamento da Malária no Brasil, Malaria’s treatment in Brazil guide, 1st ed.; Ministério da Saúde, Organizador: Brasília, Brazil, 2020; 76p. [Google Scholar]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; World Health Organization: Geneva, Switzerland, 2011; pp. 1–6. [Google Scholar]
- STANDARDTM. G6PD Test. 2019. Available online: www.sdbiosensor.com (accessed on 26 June 2023).
- LTDA ED. G6PD STRIP ECO Teste—TR.0054. 2019, pp. 6–8. Available online: www.ecodiagnostica.com.br (accessed on 26 June 2023).
- Caudle, K.E.; Sangkuhl, K.; Whirl-carrillo, M.; Swen, J.J.; Haidar, C.E.; Klein, T.E.; Gammal, R.S.; Relling, M.V.; Scott, S.A.; Hertz, D.L.; et al. Standardizing CYP2D6 Genotype to Phenotype Translation: Consensus Recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin. Transl. Sci. 2020, 13, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Nofziger, C.; Turner, A.J.; Sangkuhl, K.; Whirl-Carrillo, M.; Agúndez, J.A.G.; Black, J.L.; Dunnenberger, H.M.; Ruano, G.; Kennedy, M.A.; Phillips, M.S.; et al. PharmVar GeneFocus: CYP2D6. Clin. Pharmacol. Ther. 2020, 107, 154–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaedigk, A.; Sangkuhl, K.; Whirl-carrillo, M.; Klein, T.; Leeder, J.S. Open Prediction of CYP2D6 phenotype from genotype across world populations. Am. Coll. Med. Genet. Genom. 2017, 19, 69–76. [Google Scholar]
- Lo, E.; Zhong, D.; Raya, B.; Pestana, K.; Koepfli, C.; Lee, M.-C.; Yewhalaw, D.; Yan, G. Prevalence and distribution of G6PD deficiency: Implication for the use of primaquine in malaria treatment in Ethiopia. Malar. J. 2019, 18, 340. [Google Scholar] [CrossRef]
- Olvany, J.M.; Williams, S.M.; Zimmerman, P.A. Global perspectives on CYP2D6 associations with primaquine metabolism and Plasmodium vivax radical cure. Front. Pharmacol. 2022, 13, 4803. [Google Scholar] [CrossRef]
- Howes, R.E.; Battle, K.E.; Satyagraha, A.W.; Baird, J.K.; Hay, S.I. G6PD Deficiency. Global Distribution, Genetic Variants and Primaquine Therapy. Adv. Parasitol. 2013, 81, 133–201. [Google Scholar] [CrossRef]
- Chu, C.S.; Bancone, G.; Nosten, F.; White, N.J.; Luzzatto, L. Primaquine-induced haemolysis in females heterozygous for G6PD deficiency. Malar. J. 2018, 17, 101. [Google Scholar] [CrossRef]
- Rodrigues-Soares, F.; Kehdy, F.S.G.; Sampaio-Coelho, J.; Andrade, P.X.C.; Céspedes-Garro, C.; Zolini, C.; Aquino, M.M.; Barreto, M.L.; Horta, B.L.; Lima-Costa, M.F.; et al. Genetic structure of pharmacogenetic biomarkers in Brazil inferred from a systematic review and population-based cohorts: A RIBEF/EPIGEN-Brazil initiative. Pharmacogenom. J. 2018, 18, 749–759. [Google Scholar] [CrossRef]
- Stewart, A.G.A.; Zimmerman, P.A.; Mccarthy, J.S. Genetic Variation of G6PD and CYP2D6: Clinical Implications on the Use of Primaquine for Elimination of Plasmodium vivax. Front. Pharmacol. 2021, 12, 784909. [Google Scholar] [CrossRef]
- Chamchoy, K.; Sudsumrit, S.T.T.; Krudsood, S.; Patrapuvich, R.B.U. Cytochrome P450 2D6 (CYP2D6) and glucose6-phosphate dehydrogenase (G6PD) genetic variations in Thai vivax malaria patients: Implications for 8-aminoquinoline radical cure. PLoS Negl. Trop. Dis. 2022, 6, e0010986. [Google Scholar] [CrossRef]
- Avalos, S.; Mejia, R.E.; Banegas, E.; Salinas, C.; Gutierrez, L.; Fajardo, M.; Galo, S.; Pinto, A.; Mejia, A. G6PD deficiency, primaquine treatment, and risk of haemolysis in malaria-infected patients. Malar. J. 2018, 17, 1–11. [Google Scholar] [CrossRef]
- World Health Organization. Testing for G6PD Deficiency for Safe Use of Primaquine in Radical Cure of P. vivax and P. ovale Malaria; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
| Total | G6PDd Median 2.5 IU/g Hb (IQR = 2–3.1) | G6PDn Median 8.4 IU/g Hb (IQR = 7.8–9.7) | p Value | |
|---|---|---|---|---|
| Age, years, mean (SD) | 25.4 (18.2) | 22.1 (14.4) | 34 (25.7) | 0.2269 |
| Sex | ||||
| Female, n/N (%) | 5 (27.8) | 4 (30.8) | 1 (20.0) | 0.567 |
| Male, n/N (%) | 13 (72.2) | 9 (69.2) | 4 (80.0) | |
| Hospitalization, days, median (IQR) | 4 (3–7) | 4 (3–14) | 4 (4–5) | 0.6175 |
| Level of anemia, n/N (%) | ||||
| +/2 | 2 (13.3) | 1 (8.3) | 1 (33.3) | 0.446 |
| + | 3 (20.0) | 2 (16.7) | 1 (33.3) | |
| ++ | 8 (53.3) | 7 (58.3) | 1 (33.3) | |
| +++ | 2 (13.3) | 2 (16.7) | - | |
| Level of jaundice, n/N (%) | ||||
| +/2 | - | - | - | 0.345 |
| + | 2 (18.2) | 1 (11.1) | 1 (50.0) | |
| ++ | 9 (81.8) | 8 (88.9) | 1 (50.0) | |
| +++ | - | - | - | |
| Erythrocyte (millions/ mm3), mean (SD) | 2.7 (1.1) | 2.6 (0.97) | 3.6 (1.0) | 0.0655 |
| Ht (%), mean (SD) | 24.3 (7.2) | 22.4 (6.3) | 29.1 (7.7) | 0.0716 |
| Hb (g/dL), mean (SD) | 8.3 (2.1) | 7.7 (1.8) | 9.7 (2.4) | 0.0661 |
| Platelets (mm3), median (IQR) | 81,420 (65,160–315,000) | 194,000 (70,150–353,000) | 71,000 (47,570–73,290) | 0.1263 |
| TB (mg/dL), median (IQR) | 2.3 (1.15–4) | 2.4 (1.15–4) | 1.9 (1.2–7.2) | 0.9516 |
| DB (mg/dL), median (IQR) | 0.5 (0.4–1.1) | 0.5 (0.4–1.1) | 0.8 (0.4–4) | 0.6668 |
| IB (mg/dL), median (IQR) | 1.4 (0.8–3.2) | 1.6 (0.8–3.2) | 1.2 (0.8–3.2) | >0.999 |
| SGOT (U/L), median (IQR) | 58.5 (35–101) | 62 (45–101) | 35 (33–56) | 0.2565 |
| SGPT (U/L), median (IQR) | 52 (36–66) | 59 (46–66) | 36 (30–37) | 0.1674 |
| LDH (U/L), median (IQR) | 1337.5 (772–2119) | 1424 (859–2422) | 601 (414–1613) | 0.1391 |
| Glucose (mg/dL), mean (SD) | 119.6 (28.6) | 116.9 (28.6) | 147 (-) | - |
| Creatinine (mg/dL), median (IQR) | 0.95 (0.5–2.2) | 1.0 (0.7–1.6) | 0.5 (0.4–2.2) | 0.3743 |
| Urea (mg/dL), median (IQR) | 42 (27–69) | 43 (28–75) | 36 (27–49) | 0.5873 |
| Hemoglobinuria, n/N (%) | 9 (81.8) | 8 (80.0) | 1 (100.0) | 0.818 |
| Proteinuria, n/N (%) | 7 (63.6) | 6 (60.0) | 1 (100.0) | 0.636 |
| Bilirubinuria, n/N (%) | 3 (27.3) | 3 (30.0) | 0 (0.0) | 0.727 |
| Transfusion (RBCC), n/N (%) | 9 (56.3) | 9 (69.2) | 0 (0.0) | 0.062 |
| AKI, n/N (%) | 2 (12.5) | 2 (15.4) | 0 (0.0) | 0.650 |
| Dialysis, n/N (%) | 2 (12.5) | 2 (15.4) | 0 (0.0) | 0.650 |
| Gene | Allele | Total | G6PDd | G6PDn | p Value | |||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |||
| CYP2C19 Predicted CYP2C19 phenotype | *1 | 28 | 77.8 | 19 | 73.1 | 9 | 90.0 | 0.269 |
| *2 | 4 | 11.1 | 4 | 15.4 | 0 | 0.0 | 0.254 | |
| *17 | 4 | 11.1 | 3 | 11.5 | 1 | 10.0 | 0.695 | |
| gNM | 10 | 55.6 | 6 | 46.1 | 4 | 80.0 | 0.225 | |
| gIM | 4 | 22.2 | 4 | 30.8 | 0 | 0.0 | 0.234 | |
| gRM | 4 | 22.2 | 3 | 23.1 | 1 | 20.0 | 0.701 | |
| CYP2D6 | *1 | 14 | 77.8 | 11 | 84.6 | 3 | 60.0 | 0.299 |
| *2 | 7 | 38.9 | 7 | 53.8 | 0 | 0.0 | 0.054 | |
| *4 | 3 | 16.7 | 1 | 7.7 | 2 | 40.0 | 0.172 | |
| *5 | 1 | 5.6 | 0 | 0.0 | 1 | 20.0 | 0.278 | |
| *17 | 3 | 16.7 | 2 | 15.4 | 1 | 20.0 | 0.650 | |
| *29 | 1 | 5.6 | 1 | 7.7 | 0 | 0.0 | 0.722 | |
| *34 | 5 | 27.8 | 3 | 23.1 | 2 | 40.0 | 0.433 | |
| *35 | 1 | 5.6 | 0 | 0.0 | 1 | 20.0 | 0.278 | |
| Predicted CYP2D6 phenotype | *1xN | 1 | 5.6 | 1 | 7.7 | 0 | 0.0 | 0.722 |
| gNM | 13 | 72.2 | 11 | 84.6 | 2 | 40.0 | 0.099 | |
| gIM | 4 | 22.2 | 1 | 7.7 | 3 | 60.0 | 0.044 | |
| gUM | 1 | 5.6 | 1 | 7.7 | 0 | 0.0 | 0.722 | |
| CYP3A4 | *1 | 31 | 86.1 | 24 | 92.3 | 7 | 70.0 | 0.119 |
| *1B | 5 | 13.9 | 2 | 7.7 | 3 | 30.0 | 0.119 | |
| A376G | 2 | 11.1 | 2 | 15.4 | 0 | 0.0 | - | |
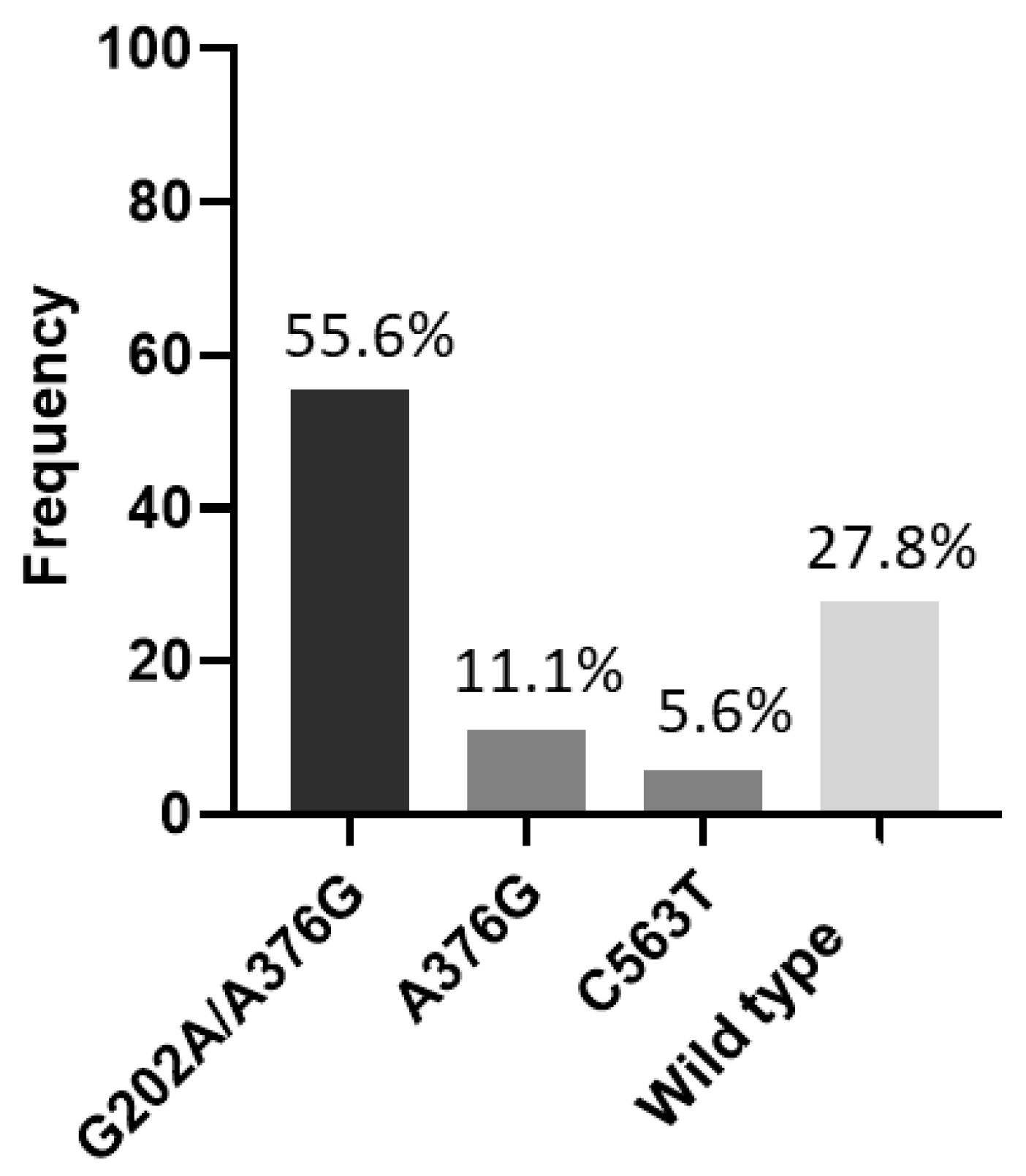
| G6PD | G202A/A376G | 10 | 55.6 | 10 | 76.9 | 0 | 0.0 | - |
| C563T | 1 | 5.6 | 1 | 7.7 | 0 | 0.0 | - | |
| Wild type | 5 | 27.8 | 0 | 0.0 | 5 | 100.0 | - | |
| ID | Variant G6PD | Dosage of G6PD (IU/g Hb) | CYP2C19 | CYP2D6 | CYP3A4 | |||
|---|---|---|---|---|---|---|---|---|
| Genotype | Phenotype | Genotype | Phenotype | CNV | Genotype | |||
| HPQ01 | G202A/A376G | 2.5 (G6PDd) | *1/*1 | gNM | *1/*2 | gNM | 2 | *1/*1B |
| HPQ02 | G202A/A376G | 5.0 (G6PDd) | *1/*17 | gRM | *1/*29 | gNM | 2 | *1/*1B |
| HPQ03 | G202A/A376G | 3.1 (G6PDd) | *1/*2 | gIM | *1/*34 | gNM | 3 | *1/*1 |
| HPQ04 | G202A/A376G | 2.3 (G6PDd) | *1/*1 | gNM | *1/*34 | gNM | 2 | *1/*1 |
| HPQ05 | A376G | 1.7 (G6PDd) | *1/*1 | gNM | *1/*2 | gNM | 2 | *1/*1 |
| HPQ06 | C563T | 1.8 (G6PDd) | *1/*1 | gNM | *1/*34 | gNM | 2 | *1/*1 |
| HPQ07 | G202A/A376G | 2.6 (G6PDd) | *1/*1 | gNM | *2/*4 | gIM | 2 | *1/*1 |
| HPQ08 | G202A/A376G | 3.0 (G6PDd) | *1/*2 | gIM | *2/*17 | gNM | 2 | *1/*1 |
| HPQ09 | G202A/A376G | 1.7 (G6PDd) | *1/*1 | gNM | *1/*2 | gNM | 2 | *1/*1 |
| HPQ10 | A376G | 2.0(G6PDd) | *1/*17 | gRM | *1/*17 | gNM | 2 | *1/*1 |
| HPQ11 | G202A/A376G | 2.2 (G6PDd) | *1/*17 | gRM | *1/*1x3 | gUM | 3 | *1/*1 |
| HPQ12 | G202A/A376G | 6.0 (G6PDd) | *1/*2 | gIM | *1/*2 | gNM | 1 | *1/*1 |
| HPQ13 | G202A/A376G | 5.7 (G6PDd) | *1/*2 | gIM | *1/*2 | gNM | 2 | *1/*1 |
| HPQ14 | Wild type | 7.2 (G6PDn) | *1/*17 | gRM | *1/*34 | gNM | 2 | *1/*1 |
| HPQ15 | Wild type | 7.8 (G6PDn) | *1/*1 | gNM | *1/*4 | gIM | 2 | *1/*1 |
| HPQ16 | Wild type | 10.5 (G6PDn) | *1/*1 | gNM | *1/*34 | gNM | 1 | *1/*1B |
| HPQ17 | Wild type | 9.7 (G6PDn) | *1/*1 | gNM | *4/*17 | gIM | 2 | *1/*1B |
| HPQ18 | Wild type | 8.4 (G6PDn) | *1/*1 | gNM | *5/*35 | gIM | 1 | *1/*1B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macêdo, M.M.; Almeida, A.C.G.; Silva, G.S.; Oliveira, A.C.; Mwangi, V.I.; Shuan, A.C.; Barbosa, L.R.A.; Rodrigues-Soares, F.; Melo, G.C. Association of CYP2C19, CYP2D6 and CYP3A4 Genetic Variants on Primaquine Hemolysis in G6PD-Deficient Patients. Pathogens 2023, 12, 895. https://doi.org/10.3390/pathogens12070895
Macêdo MM, Almeida ACG, Silva GS, Oliveira AC, Mwangi VI, Shuan AC, Barbosa LRA, Rodrigues-Soares F, Melo GC. Association of CYP2C19, CYP2D6 and CYP3A4 Genetic Variants on Primaquine Hemolysis in G6PD-Deficient Patients. Pathogens. 2023; 12(7):895. https://doi.org/10.3390/pathogens12070895
Chicago/Turabian StyleMacêdo, Marielle M., Anne C. G. Almeida, Gabrielly S. Silva, Amanda C. Oliveira, Victor I. Mwangi, Ana C. Shuan, Laila R. A. Barbosa, Fernanda Rodrigues-Soares, and Gisely C. Melo. 2023. "Association of CYP2C19, CYP2D6 and CYP3A4 Genetic Variants on Primaquine Hemolysis in G6PD-Deficient Patients" Pathogens 12, no. 7: 895. https://doi.org/10.3390/pathogens12070895








