Molecular Characterization of Rotavirus C from Rescued Sloth Bears, India: Evidence of Zooanthroponotic Transmission
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. RNA Extraction
2.3. RT-PCR for Diagnosis of Rotavirus C
2.4. Cloning and Sequencing
2.5. NCBI GenBank Submission
2.6. Related Sequences Search and Phylogenetic Analysis
2.7. Structure Prediction and Alignment
2.8. Selection Pressure Analysis
3. Results and Discussion
3.1. RVC Occurrence in Indian Sloth Bears
3.2. Genetic Analysis of VP6 Gene from Sloth-Bear RVC Isolates, UP-SB37, and UP-SB21
3.2.1. Sequence Analysis
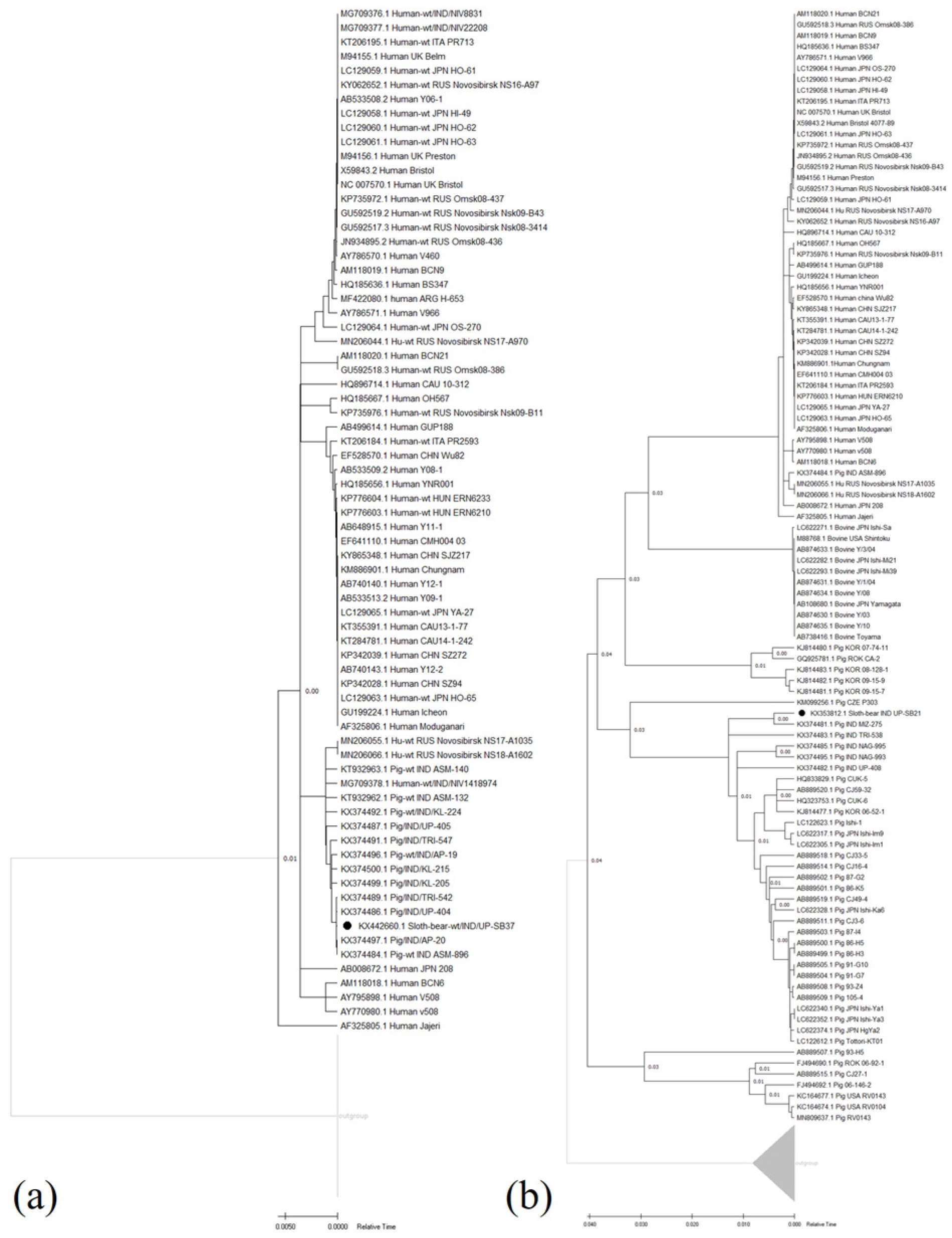
3.2.2. Genotyping and Phylogenetic Analyses
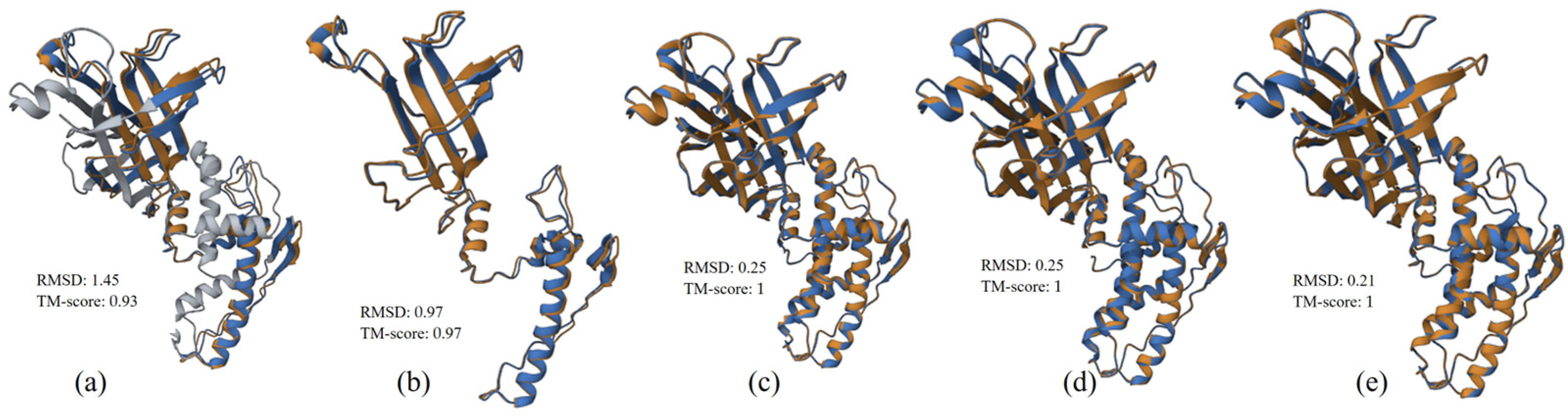
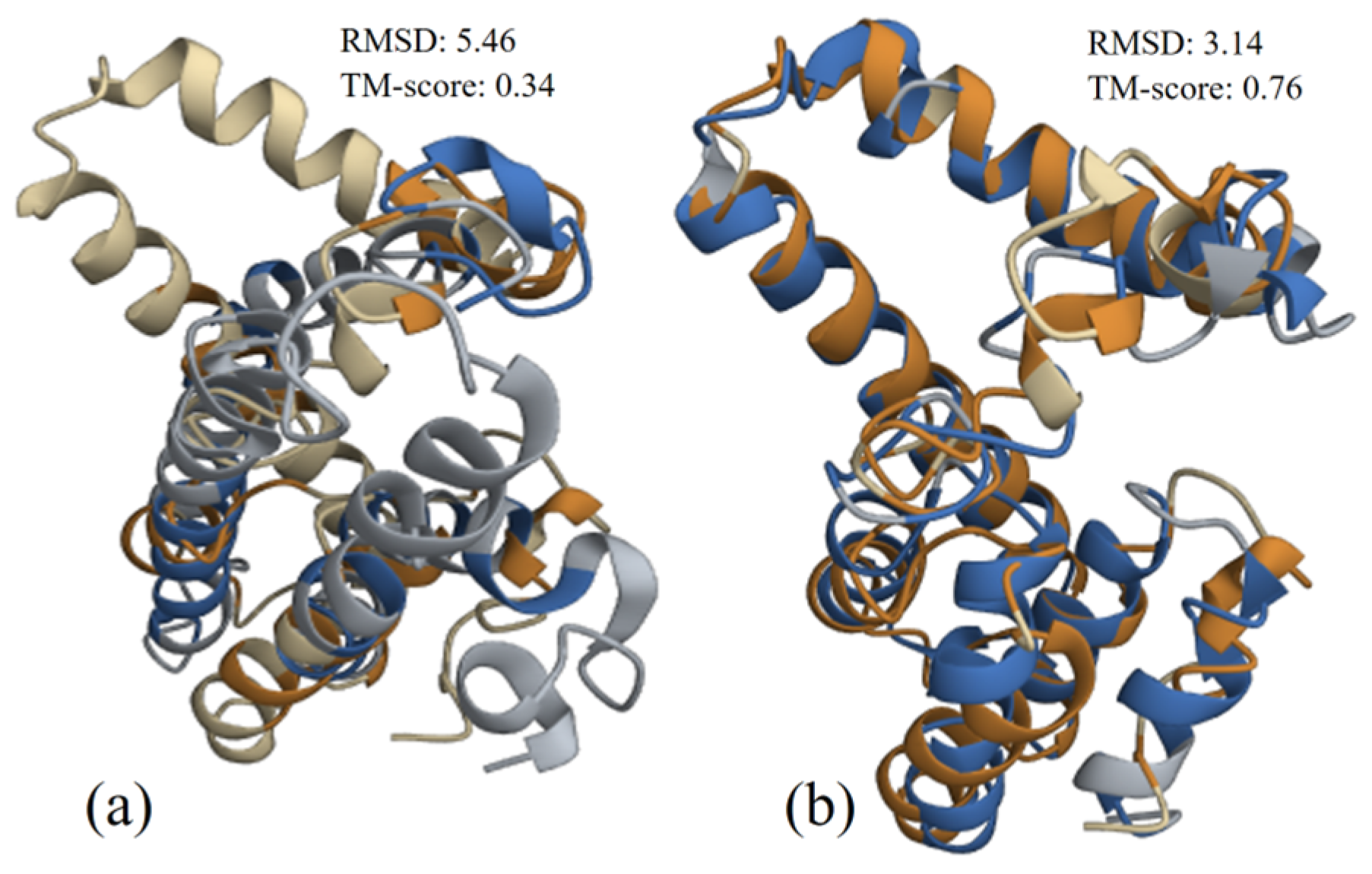
3.2.3. Structure Alignment and Analysis
3.2.4. Selection Pressure Analysis
3.3. Genetic Analysis of VP7 Gene from Sloth-Bear RVC Isolates, UP-SB37, and UP-SB19
3.3.1. Sequence Analysis
3.3.2. Genotyping and Phylogenetic Analyses
3.3.3. Structure Alignment and Analysis
3.3.4. Selection Pressure Analysis
3.4. Genetic Analysis of NSP4 Gene from Sloth-Bear RVC Strain UP-SB37
3.4.1. Sequence Analysis
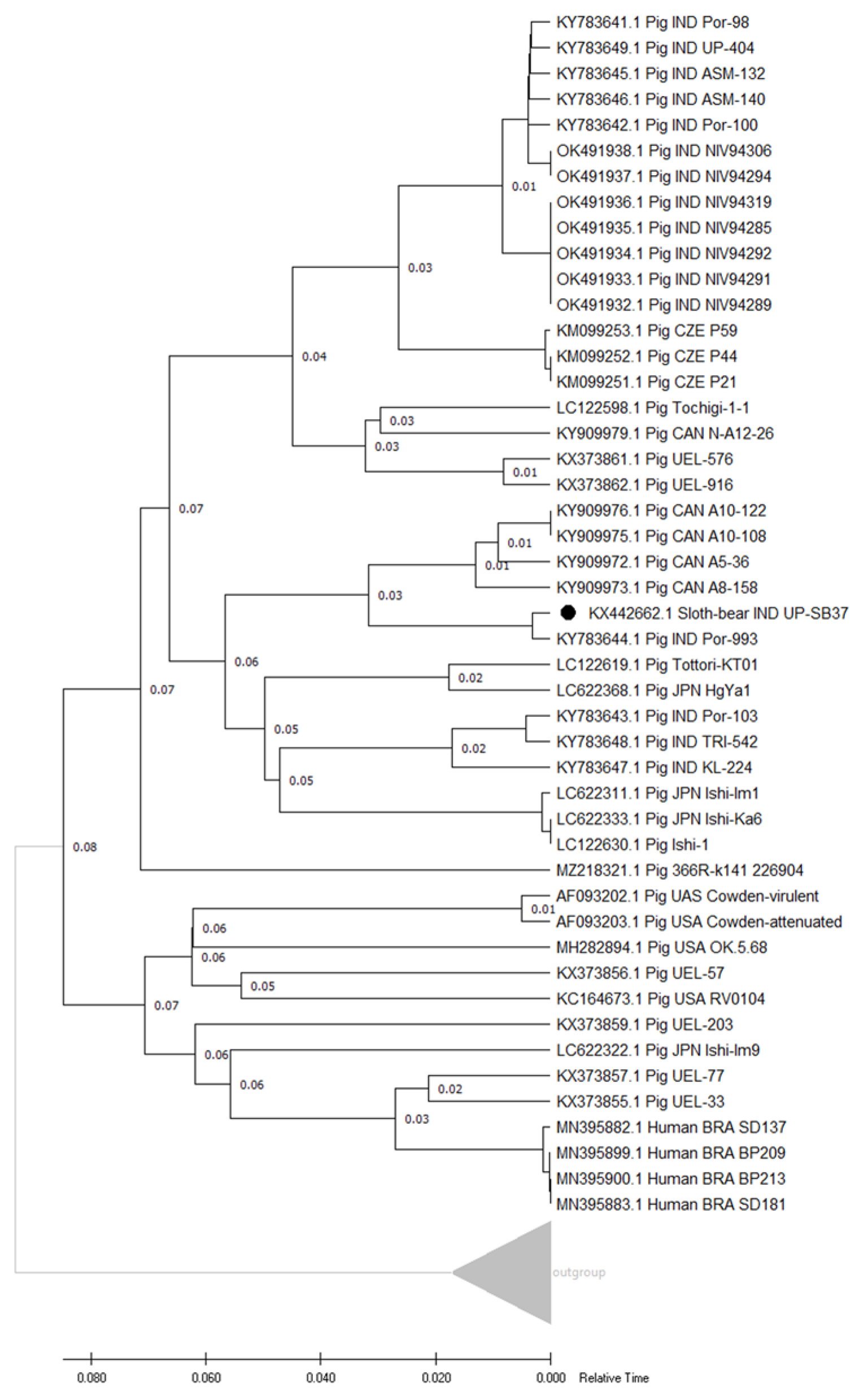
3.4.2. Genotype and Phylogenetic Analyses
3.4.3. Structure Alignment and Analysis
3.4.4. Selection Pressure Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadiq, A.; Bostan, N.; Yinda, K.C.; Naseem, S.; Sattar, S. Rotavirus: Genetics, pathogenesis and vaccine advances. Rev. Med. Virol. 2018, 28, e2003. [Google Scholar] [CrossRef]
- Estes, M.; Greenberg, H. Rotaviruses. In Fields Virology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1347–1401. [Google Scholar]
- Matthijnssens, J.; Otto, P.H.; Ciarlet, M.; Desselberger, U.; Van Ranst, M.; Johne, R. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch. Virol. 2012, 157, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Johne, R.; Tausch, S.H.; Grützke, J.; Falkenhagen, A.; Patzina-Mehling, C.; Beer, M.; Höper, D.; Ulrich, R.G. Distantly related rotaviruses in common shrews, Germany, 2004–2014. Emerg. Infect. Dis. 2019, 25, 2310. [Google Scholar] [CrossRef] [PubMed]
- Johne, R.; Schilling-Loeffler, K.; Ulrich, R.G.; Tausch, S.H. Whole genome sequence analysis of a prototype strain of the novel putative rotavirus species L. Viruses 2022, 14, 462. [Google Scholar] [CrossRef]
- Bhat, S.; Kattoor, J.J.; Malik, Y.S.; Sircar, S.; Deol, P.; Rawat, V.; Rakholia, R.; Ghosh, S.; Vlasova, A.N.; Nadia, T. Species C rotaviruses in children with diarrhea in India, 2010–2013: A potentially neglected cause of acute gastroenteritis. Pathogens 2018, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Soma, J.; Tsunemitsu, H.; Miyamoto, T.; Suzuki, G.; Sasaki, T.; Suzuki, T. Whole-genome analysis of two bovine rotavirus C strains: Shintoku and Toyama. J. Gen. Virol. 2013, 94, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Chepngeno, J.; Diaz, A.; Paim, F.C.; Saif, L.J.; Vlasova, A.N. Rotavirus C: Prevalence in suckling piglets and development of virus-like particles to assess the influence of maternal immunity on the disease development. Vet. Res. 2019, 50, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bányai, K.; Estes, M.K.; Martella, V.; Parashar, U.D. Viral gastroenteritis. Lancet 2018, 392, 175–186. [Google Scholar] [CrossRef]
- Saif, L. Animal rotaviruses. In Viral Infections of the Gastrointestinal Tract, 2nd ed.; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- Conner, M.E.; Darlington, R. Rotavirus infection in foals. Am. J. Vet. Res. 1980, 41, 1699–1703. [Google Scholar]
- Martella, V.; Decaro, N.; Buonavoglia, C. Enteric viral infections in lambs or kids. Vet. Microbiol. 2015, 181, 154–160. [Google Scholar] [CrossRef]
- Rahman, M.; Banik, S.; Faruque, A.S.; Taniguchi, K.; Sack, D.A.; Van Ranst, M.; Azim, T. Detection and characterization of human group C rotaviruses in Bangladesh. J. Clin. Microbiol. 2005, 43, 4460–4465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, D.F.; Kisielius, J.J.; Ueda, M.; Gabbay, Y.B.; Carmona, R.C.; Timenetsky, M.d.C.S.; Mascarenhas, J.D.; Takimoto, S.; Tanaka, H. An outbreak of group C rotavirus gastroenteritis among adults living in Valentim Gentil, Sao Paulo State, Brazil. J. Diarrhoeal Dis. Res. 1998, 16, 59–65. [Google Scholar] [PubMed]
- Joshi, M.; Jare, V.; Gopalkrishna, V. Group C rotavirus infection in patients with acute gastroenteritis in outbreaks in western India between 2006 and 2014. Epidemiol. Infect. 2017, 145, 310–315. [Google Scholar] [CrossRef]
- Saif, L.J.; Bohl, E.H.; Theil, K.W.; Cross, R.F.; House, J.A. Rotavirus-like, calicivirus-like, and 23-nm virus-like particles associated with diarrhea in young pigs. J. Clin. Microbiol. 1980, 12, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.; Nielsen, P.; Ward, L.; Saif, L. Dual infection of gnotobiotic calves with bovine strains of group A and porcine-like group C rotaviruses influences pathogenesis of the group C rotavirus. J. Virol. 1999, 73, 9284–9293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabbay, Y.B.; Borges, A.A.; Oliveira, D.S.; Linhares, A.C.; Mascarenhas, J.D.; Barardi, C.R.; Simões, C.M.; Wang, Y.; Glass, R.I.; Jiang, B. Evidence for zoonotic transmission of group C rotaviruses among children in Belém, Brazil. J. Med. Virol. 2008, 80, 1666–1674. [Google Scholar] [CrossRef]
- Costa, F.B.; Flores, P.S.; Amorim, A.R.; Mendes, G.d.S.; Santos, N. Porcine rotavirus C strains carrying human-like NSP4 and NSP5. Zoonoses Public Health 2020, 67, 849–861. [Google Scholar] [CrossRef]
- Torres-Medina, A. Isolation of an atypical rotavirus causing diarrhea in neonatal ferrets. Lab. Anim. Sci. 1987, 37, 167–171. [Google Scholar]
- Otto, P.; Schulze, P.; Herbst, W. Demonstration of group C rotaviruses in fecal samples of diarrheic dogs in Germany. Arch. Virol. 1999, 144, 2467–2473. [Google Scholar] [CrossRef]
- Collins, P.; Martella, V.; O’shea, H. Detection and characterization of group C rotaviruses in asymptomatic piglets in Ireland. J. Clin. Microbiol. 2008, 46, 2973–2979. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.-J.; Matthijnssens, J.; Kim, D.-S.; Kim, J.-Y.; Alfajaro, M.M.; Park, J.-G.; Hosmillo, M.; Son, K.-Y.; Soliman, M.; Baek, Y.-B. Genetic diversity of the VP7, VP4 and VP6 genes of Korean porcine group C rotaviruses. Vet. Microbiol. 2015, 176, 61–69. [Google Scholar] [CrossRef]
- Martella, V.; Bányai, K.; Lorusso, E.; Bellacicco, A.L.; Decaro, N.; Camero, M.; Bozzo, G.; Moschidou, P.; Arista, S.; Pezzotti, G. Prevalence of group C rotaviruses in weaning and post-weaning pigs with enteritis. Vet. Microbiol. 2007, 123, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marthaler, D.; Rossow, K.; Culhane, M.; Collins, J.; Goyal, S.; Ciarlet, M.; Matthijnssens, J. Identification, phylogenetic analysis and classification of porcine group C rotavirus VP7 sequences from the United States and Canada. Virology 2013, 446, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Cunliffe, N.A.; Dove, W.; Jiang, B.; Thinwda Cert, B.D.; Broadhead, R.L.; Molyneux, M.E.; Hart, C.A. Detection of group C rotavirus in children with acute gastroenteritis in Blantyre, Malawi. Pediatr. Infect. Dis. J. 2001, 20, 1088–1090. [Google Scholar] [CrossRef]
- Phan, T.G.; Nishimura, S.; Okame, M.; Nguyen, T.A.; Khamrin, P.; Okitsu, S.; Maneekarn, N.; Ushijima, H. Virus diversity and an outbreak of group C rotavirus among infants and children with diarrhea in Maizuru city, Japan during 2002–2003. J. Med. Virol. 2004, 74, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Abid, I.; Guix, S.; Aouni, M.; Pintó, R.; Bosch, A. Detection and characterization of human group C rotavirus in the pediatric population of Barcelona, Spain. J. Clin. Virol. 2007, 38, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-J.; Park, S.-I.; Hosmillo, M.; Shin, D.-J.; Chun, Y.-H.; Kim, H.-J.; Kwon, H.-J.; Kang, S.-Y.; Woo, S.-K.; Park, S.-J. Detection and molecular characterization of porcine group C rotaviruses in South Korea. Vet. Microbiol. 2009, 138, 217–224. [Google Scholar] [CrossRef]
- Yamamoto, D.; Ghosh, S.; Kuzuya, M.; Wang, Y.-H.; Zhou, X.; Chawla-Sarkar, M.; Paul, S.K.; Ishino, M.; Kobayashi, N. Whole-genome characterization of human group C rotaviruses: Identification of two lineages in the VP3 gene. J. Gen. Virol. 2011, 92, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Kattoor, J.J.; Saurabh, S.; Malik, Y.S.; Sircar, S.; Dhama, K.; Ghosh, S.; Bányai, K.; Kobayashi, N.; Singh, R.K. Unexpected detection of porcine rotavirus C strains carrying human origin VP6 gene. Vet. Q. 2017, 37, 252–261. [Google Scholar] [CrossRef] [Green Version]
- Mathesh, K.; Thankappan, S.; Deneke, Y.; Vamadevan, B.; Siddappa, C.M.; Sharma, A.K.; Selvaraj, I.; Sha, A.; Kumar, A. A multipronged approach for the detection of leptospirosis in captive sloth bears (Melursus ursinus) in Agra and Bannerghatta sloth bear rescue centers in India. J. Vet. Med. Sci. 2021, 83, 1059–1067. [Google Scholar] [CrossRef]
- Sanchez-Fauquier, A.; Roman, E.; Colomina, J.; Wilhelmi, I.; Glass, R.; Jiang, B. First detection of group C rotavirus in children with acute diarrhea in Spain. Arch. Virol. 2003, 148, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.; Clarke, I.; Freitas, R.; Gabbay, Y.; Lambden, P. The correct sequence of the porcine group C/Cowden rotavirus major inner capsid protein shows close homology with human isolates from Brazil and the UK. Virology 1992, 190, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal omega. Curr. Protoc. Bioinform. 2014, 48, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat Methods 2015, 12, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Skolnick, J. TM-align: A protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 2005, 33, 2302–2309. [Google Scholar] [CrossRef]
- Pond, S.L.; Frost, S.D.; Muse, S.V. HyPhy: Hypothesis testing using phylogenies. Bioinformatics 2005, 21, 676–679. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Hasebe, A. A provisional complete genome-based genotyping system for rotavirus species C from terrestrial mammals. J. Gen. Virol. 2017, 98, 2647–2662. [Google Scholar] [CrossRef]
- Tiku, V.R.; Jiang, B.; Kumar, P.; Aneja, S.; Bagga, A.; Bhan, M.K.; Ray, P. First study conducted in Northern India that identifies group C rotavirus as the etiological agent of severe diarrhea in children in Delhi. Virol. J. 2017, 14, 100. [Google Scholar] [CrossRef] [Green Version]
- Moutelíková, R.; Prodělalová, J.; Dufková, L. Diversity of VP7, VP4, VP6, NSP2, NSP4, and NSP5 genes of porcine rotavirus C: Phylogenetic analysis and description of potential new VP7, VP4, VP6, and NSP4 genotypes. Arch. Virol. 2015, 160, 1715–1727. [Google Scholar] [CrossRef]
- Amimo, J.O.; Vlasova, A.N.; Saif, L.J. Prevalence and genetic heterogeneity of porcine group C rotaviruses in nursing and weaned piglets in Ohio, USA and identification of a potential new VP4 genotype. Vet. Microbiol. 2013, 164, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Hasebe, A.; Miyazaki, A.; Tsunemitsu, H. Phylogenetic characterization of VP6 gene (inner capsid) of porcine rotavirus C collected in Japan. Infect. Genet. Evol. 2014, 26, 223–227. [Google Scholar] [CrossRef]
- Ball, L.A. The universal taxonomy of viruses in theory and practice. In Virus Taxonomy; Elsevier: Amsterdam, The Netherlands, 2005; pp. 3–8. [Google Scholar]
- Tuanthap, S.; Phupolphan, C.; Luengyosluechakul, S.; Duang-In, A.; Theamboonlers, A.; Wattanaphansak, S.; Vongpunsawad, S.; Amonsin, A.; Poovorawan, Y. Porcine rotavirus C in pigs with gastroenteritis on Thai swine farms, 2011–2016. PeerJ 2018, 6, e4724. [Google Scholar] [CrossRef] [Green Version]
- Doan, Y.H.; Haga, K.; Fujimoto, A.; Fujii, Y.; Takai-Todaka, R.; Oka, T.; Kimura, H.; Yoshizumi, S.; Shigemoto, N.; Okamoto-Nakagawa, R.; et al. Genetic analysis of human rotavirus C: The appearance of Indian-Bangladeshi strain in Far East Asian countries. Infect. Genet. Evol. 2016, 41, 160–173. [Google Scholar] [CrossRef]
- Niira, K.; Ito, M.; Masuda, T.; Saitou, T.; Abe, T.; Komoto, S.; Sato, M.; Yamasato, H.; Kishimoto, M.; Naoi, Y.; et al. Whole genome sequences of Japanese porcine species C rotaviruses reveal a high diversity of genotypes of individual genes and will contribute to a comprehensive, generally accepted classification system. Infect. Genet. Evol. 2016, 44, 106–113. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Amimo, J.O.; Saif, L.J. Porcine Rotaviruses: Epidemiology, Immune Responses and Control Strategies. Viruses 2017, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Collins, P.J.; Martella, V.; Sleator, R.D.; Fanning, S.; O’Shea, H. Detection and characterisation of group A rotavirus in asymptomatic piglets in southern Ireland. Arch. Virol. 2010, 155, 1247–1259. [Google Scholar] [CrossRef]
- Lachapelle, V.; Sohal, J.S.; Lambert, M.C.; Brassard, J.; Fravalo, P.; Letellier, A.; L’Homme, Y. Genetic diversity of group A rotavirus in swine in Canada. Arch. Virol. 2014, 159, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Fukai, K.; Takahashi, T.; Tajima, K.; Koike, S.; Iwane, K.; Inoue, K. Molecular characterization of a novel bovine group A rotavirus. Vet. Microbiol. 2007, 123, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Nagai, M.; Yamasato, H.; Tsuchiaka, S.; Okazaki, S.; Katayama, Y.; Oba, M.; Nishiura, N.; Sassa, Y.; Omatsu, T. Identification of novel bovine group A rotavirus G15P [14] strain from epizootic diarrhea of adult cows by de novo sequencing using a next-generation sequencer. Vet. Microbiol. 2014, 171, 66–73. [Google Scholar] [CrossRef]
- Sato, M.; Nakagomi, T.; Tajima, K.; Ezura, K.; Akashi, H.; Nakagomi, O. Isolation of serotype G8, P6[1] bovine rotavirus from adult cattle with diarrhea. J. Clin. Microbiol. 1997, 35, 1266–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhirakovskaia, E.; Tikunov, A.; Klemesheva, V.; Loginovskikh, N.; Netesov, S.; Tikunova, N. First genetic characterization of rotavirus C in Russia. Infect. Genet. Evol. 2016, 39, 1–8. [Google Scholar] [CrossRef]
- Luchs, A.; Morillo, S.G.; de Oliveira, C.M.; Timenetsky Mdo, C. Monitoring of group C rotavirus in children with acute gastroenteritis in Brazil: An emergent epidemiological issue after rotavirus vaccine? J. Med. Virol. 2011, 83, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Kumazaki, M.; Usuku, S. Epidemiological and genetic analysis of human group C rotaviruses isolated from outbreaks of acute gastroenteritis in Yokohama, Japan, between 2006 and 2012. Arch. Virol. 2014, 159, 761–771. [Google Scholar] [CrossRef]


| Primers | Diagnostic, Genotyping, and Sequencing Primer | Annealing Temperature (°C) | Amplicon Size (bp) | References |
|---|---|---|---|---|
| RVC VP6-F & RVC VP6-R | 5′-GGCTTTAAAAATCTCATTCA-3′ | 48 | 1353 bp | [34] |
| 3′-AGCCACATAGTTCACATTTC-5′ | ||||
| RVC-VP6-PF & BMJ44 | 5′ARTCHGTTCTATGYGATTC3′ | 48 | 335 | [31,33] |
| 3′AGCCACATAGTTCACATTTC5′ | ||||
| RVC-VP6-PF & RVC-VP6-PR | 5′TCATACTGGGGCATTGGAAC3′ | 48 | 584 | [31] |
| 3′GCCAAGTGTTTGATTATTAGG5′ | ||||
| GrC VP7-20F & GrC VP7-1062R | 5′GCTGTCTGACAAACTGGTC3′ | 48 | 1241 | [13] |
| 3′GCCACATGATCTTGTTTACGC5′ | ||||
| RVC-NSP4-CDS-FP & RVC-NSP4-CDS-RP | 5′CTCTACGAAGCAATGGAGTTCATCAA3′ | 48 | 477 | [31] |
| 3′AGCGCAGAAGATTCATAGACA5′ |
| Genes | Significant Codon Sites (p ≤ 0.01) | Total Substitutions/Sites | dN/dS * | Selection Pressure | dN/dS with Porcine | dN/dS with Human |
|---|---|---|---|---|---|---|
| NSP4 UP-SB37 | 66 | 2.673 | 0.2878 | Purifying selection | 0.2907 | 0.2133 |
| VP6 UP-SB21 | 179 | 3.192 | 3.6590 | Positive selection | 3.7696 | 0.0648 |
| VP6 UP-SB37 | 253 | 2.515 | 0.0393 | Purifying selection | 1.718 | 0.0340 |
| VP7 UP-SB37 & UP-SB19 | 79 | 2.291 | 5.4405 | Positive selection | 5.3210 | 4.7483 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, Y.S.; Ansari, M.I.; Karikalan, M.; Sircar, S.; Selvaraj, I.; Ghosh, S.; Singh, K. Molecular Characterization of Rotavirus C from Rescued Sloth Bears, India: Evidence of Zooanthroponotic Transmission. Pathogens 2023, 12, 934. https://doi.org/10.3390/pathogens12070934
Malik YS, Ansari MI, Karikalan M, Sircar S, Selvaraj I, Ghosh S, Singh K. Molecular Characterization of Rotavirus C from Rescued Sloth Bears, India: Evidence of Zooanthroponotic Transmission. Pathogens. 2023; 12(7):934. https://doi.org/10.3390/pathogens12070934
Chicago/Turabian StyleMalik, Yashpal Singh, Mohd Ikram Ansari, Mathesh Karikalan, Shubhankar Sircar, Ilayaraja Selvaraj, Souvik Ghosh, and Kalpana Singh. 2023. "Molecular Characterization of Rotavirus C from Rescued Sloth Bears, India: Evidence of Zooanthroponotic Transmission" Pathogens 12, no. 7: 934. https://doi.org/10.3390/pathogens12070934
APA StyleMalik, Y. S., Ansari, M. I., Karikalan, M., Sircar, S., Selvaraj, I., Ghosh, S., & Singh, K. (2023). Molecular Characterization of Rotavirus C from Rescued Sloth Bears, India: Evidence of Zooanthroponotic Transmission. Pathogens, 12(7), 934. https://doi.org/10.3390/pathogens12070934








