Isoniazid and Rifampicin Resistance-Conferring Mutations in Mycobacterium tuberculosis Isolates from South Africa
Abstract
1. Introduction
2. Methods
2.1. Study Protocol
2.2. Databases and Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction
2.5. Meta-Analysis
3. Results
3.1. Search Results
3.2. Characteristics of Studies Included
3.3. Prevalence of any Rifampicin (RIF) or Isoniazid (INH) Resistance in Mycobacterium tuberculosis Isolates
3.4. Frequency of rpoB, katG and inhA promoter Mutations
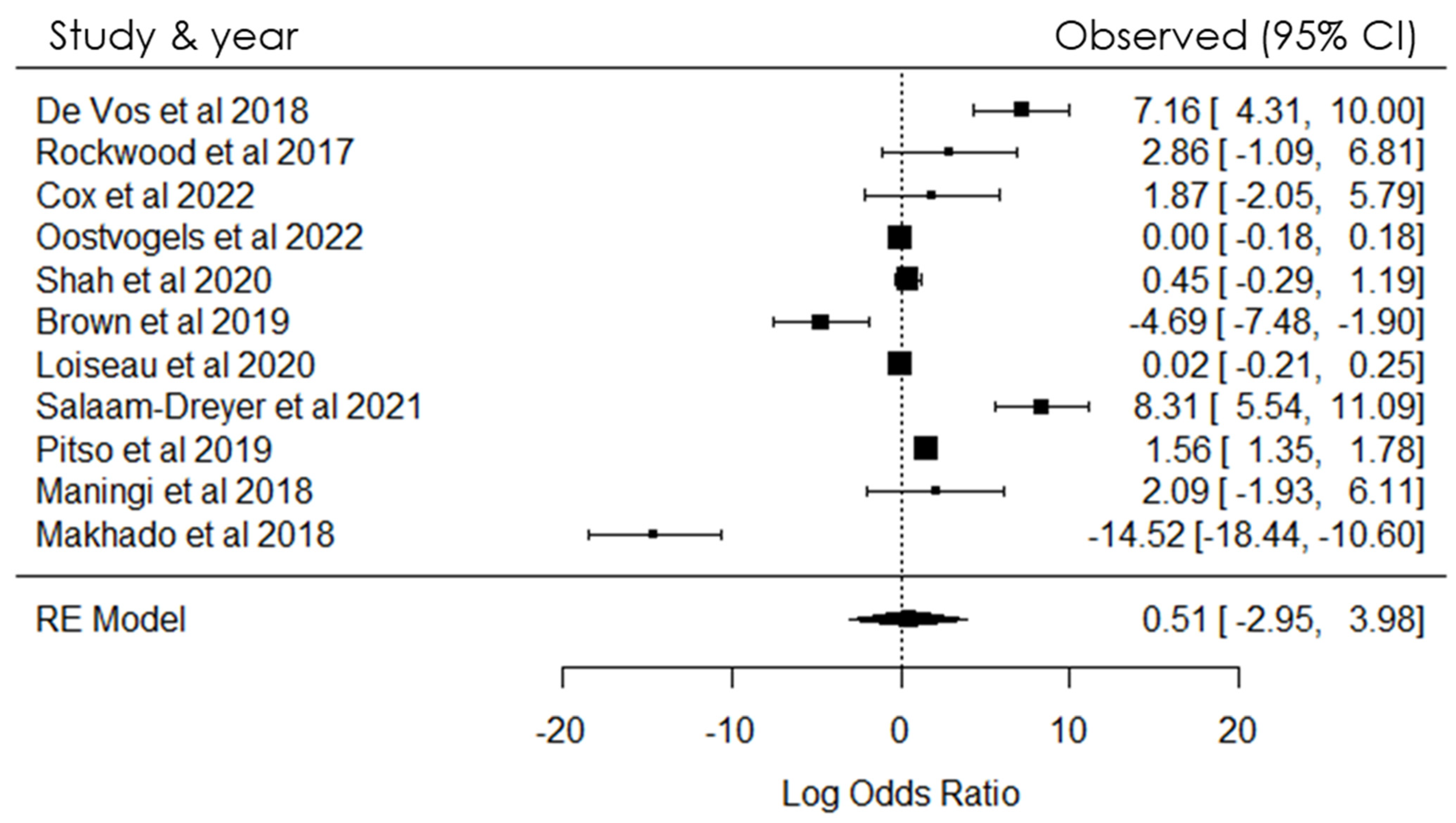
4. Meta-Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Tuberculosis Report 2020; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 16 April 2021).
- World Health Organization (WHO). Global Tuberculosis Report 2019; WHO: Geneva, Switzerland, 2019; Available online: https://www.who.int/publications/i/item/9789241565714 (accessed on 16 April 2021).
- Abebe, G.; Paasch, F.; Apers, L.; Rigouts, L.; Colebunders, R. Tuberculosis drug resistance testing by molecular methods: Opportunities and challenges in resource-limited settings. J. Microbiol. Methods 2022, 84, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Cox, H.; Goig, G.A.; Salaam-Dreyer, Z.; Dippenaar, A.; Reuter, A.; Mohr-Holland, E.; Daniels, J.; Cudahy, P.G.; Nicol, M.P.; Borrell, S.; et al. Whole-genome sequencing has the potential to improve treatment for rifampicin-resistant tuberculosis in high-burden settings: A retrospective cohort study. J. Clin. Microbiol. 2022, 60, e0236221. [Google Scholar] [CrossRef] [PubMed]
- Oostvogels, S.; Ley, S.D.; Heupink, T.H.; Dippenaar, A.; Streicher, E.M.; De Vos, E.; Meehan, C.J.; Dheda, K.; Warren, R.; Van Rie, A. Transmission, distribution and drug resistance-conferring mutations of extensively drug-resistant tuberculosis in the Western Cape Province, South Africa. Microb. Genom. 2022, 8, 000815. [Google Scholar] [CrossRef] [PubMed]
- Laurenzo, D.; Mousa, S.A. Mechanisms of drug resistance in Mycobacterium tuberculosis and current status of rapid molecular diagnostic testing. Acta Trop. 2011, 119, 5–10. [Google Scholar] [CrossRef] [PubMed]
- de Vos, M.; Derendinger, B.; Dolby, T.; Simpson, J.; van Helden, P.D.; Rice, J.E.; Wangh, L.J.; Theron, G.; Warren, R.M. Diagnostic accuracy and utility of FluoroType MTBDR, a new molecular assay for multidrug-resistant tuberculosis. J. Clin. Microbiol. 2018, 56, e00531-18. [Google Scholar] [CrossRef]
- Rockwood, N.; Sirgel, F.; Streicher, E.; Warren, R.; Meintjes, G.; Wilkinson, R.J. Low frequency of acquired isoniazid and rifampicin resistance in rifampicin-susceptible pulmonary tuberculosis in a setting of high HIV-1 infection and tuberculosis coprevalence. J. Infect. Dis. 2017, 216, 632–640. [Google Scholar] [CrossRef]
- Shah, M.; Paradis, S.; Betz, J.; Beylis, N.; Bharadwaj, R.; Caceres, T.; Gotuzzo, E.; Joloba, M.; Mave, V.; Nakiyingi, L.; et al. Multicenter study of the accuracy of the BD MAX multidrug-resistant tuberculosis assay for detection of Mycobacterium tuberculosis complex and mutations associated with resistance to rifampin and isoniazid. Clin. Infect. Dis. 2020, 71, 1161–1167. [Google Scholar] [CrossRef]
- Brown, T.S.; Challagundla, L.; Baugh, E.H.; Omar, S.V.; Mustaev, A.; Auld, S.C.; Shah, N.S.; Kreiswirth, B.N.; Brust, J.C.; Nelson, K.N.; et al. Pre-detection history of extensively drug-resistant tuberculosis in KwaZulu-Natal, South Africa. Proc. Natl. Acad. Sci. USA 2019, 116, 23284–23291. [Google Scholar] [CrossRef]
- Loiseau, C.; Brites, D.; Reinhard, M.; Zürcher, K.; Borrell, S.; Ballif, M.; Fenner, L.; Cox, H.; Rutaihwa, L.K.; Wilkinson, R.J.; et al. HIV coinfection is associated with low-fitness rpoB variants in rifampicin-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2020, 64, e00782-20. [Google Scholar] [CrossRef]
- Salaam-Dreyer, Z.; Streicher, E.M.; Sirgel, F.A.; Menardo, F.; Borrell, S.; Reinhard, M.; Doetsch, A.; Cudahy, P.G.; Mohr-Holland, E.; Daniels, J.; et al. Rifampicin-monoresistant tuberculosis is not the same as multidrug-resistant tuberculosis: A descriptive study from Khayelitsha, South Africa. Antimicrob. Agents Chemoth. 2021, 65, e0036421. [Google Scholar] [CrossRef]
- Pitso, L.; Potgieter, S.; Van der Spoel van Dijk, A. Prevalence of isoniazid resistance-conferring mutations associated with multidrug-resistant tuberculosis in Free State Province, South Africa. S. Afr. Med. J. 2019, 109, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Maningi, N.E.; Daum, L.T.; Rodriguez, J.D.; Said, H.M.; Peters, R.P.; Sekyere, J.O.; Fischer, G.W.; Chambers, J.P.; Fourie, P.B. Multi-and extensively drug resistant Mycobacterium tuberculosis in South Africa: A molecular analysis of historical isolates. J. Clin. Microbiol. 2018, 56, e01214-17. [Google Scholar] [CrossRef] [PubMed]
- Makhado, N.A.; Matabane, E.; Faccin, M.; Pinçon, C.; Jouet, A.; Boutachkourt, F.; Goeminne, L.; Gaudin, C.; Maphalala, G.; Beckert, P.; et al. Outbreak of multidrug-resistant tuberculosis in South Africa undetected by WHO-endorsed commercial tests: An observational study. Lancet Infect. Dis. 2018, 18, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Marahatta, S.B.; Gautam, S.; Dhital, S.; Pote, N.; Jha, A.K.; Mahato, R.; Mishra, S.; Poudel, B.H.; Ramasoota, P.; Kaewkungwal, J.; et al. katG (SER 315 THR) gene mutation in isoniazid-resistant Mycobacterium tuberculosis. Kathmandu Univ. Med. J. 2011, 9, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Sekyere, J.; Reta, M.A.; Maningi, N.E.; Fourie, P.B. Antibiotic resistance of Mycobacterium tuberculosis complex in Africa: A systematic review of cur- rent reports of molecular epidemiology, mechanisms and diagnostics. J. Infect. 2019, 79, 550–571. [Google Scholar] [CrossRef]
- Alagappan, C.; Shivekar, S.S.; Brammacharry, U.; Kapalamurthy, V.R.C.; Sakkaravarthy, A.; Subashkumar, R.; Muthaiah, M. Prevalence of mutations in genes as- sociated with isoniazid resistance in Mycobacterium tuberculosis isolates from re-treated smear-positive pulmonary tuberculosis patients: A meta-analysis. J. Glob. Antimicrob. Res. 2018, 14, 253–259. [Google Scholar] [CrossRef]
- Kigozi, E.; Kasule, G.W.; Musisi, K.; Lukoye, D.; Kyobe, S.; Katabazi, F.A.; Wampande, E.M.; Joloba, M.L.; Kateete, D.P. Prevalence and patterns of rifampicin and isoniazid resistance-conferring mutations in Mycobacterium tuberculosis isolates from Uganda. PLoS ONE 2015, 13, e0198091. [Google Scholar] [CrossRef]
- Rodwell, T.C.; Valafar, F.; Douglas, J.; Qian, L.; Garfein, R.S.; Chawla, A.; Torres, J.; Zadorozhny, V.; Kim, M.S.; Hoshide, M.; et al. Predicting extensively drug-resistant Mycobacterium tuberculosis phenotypes with genetic mutations. J. Clin. Microbiol. 2014, 52, 781–789. [Google Scholar] [CrossRef]
- Seifert, M.; Catanzaro, D.; Catanzaro, A.; Rodwell, T.C. Genetic mutations associated with isoniazid resistance in Mycobacterium tuberculosis: A systematic review. PLoS ONE 2015, 10, e0119628. [Google Scholar] [CrossRef]
- Rando-Segura, A.; Aznar, M.L.; Moreno, M.M.; Espasa, S.M.; Sulleiro, I.E.; Bocanegra, G.C.; Gil, O.E.; Nindia, E.A.; Escartin, H.C.; Zacarias, A.; et al. Molecular characterization of rpoB gene mutations in isolates from tuberculosis patients in Cubal, Republic of Angola. BMC Infect. Dis. 2021, 21, 1056. [Google Scholar] [CrossRef]
- Saravanan, M.; Niguse, S.; Abdulkader, M.; Tsegay, E.; Hailekiros, H.; Gebrekidan, A.; Araya, T.; Pugazhendhi, A. Review on emergence of drug-resistant tuberculosis (MDR & XDR-TB) and its molecular diagnosis in Ethiopia. Microb. Pathog. 2018, 117, 237–242. [Google Scholar] [PubMed]
- Eddabra, R.; Neffa, M. Mutations associated with rifampicin resistance in Mycobacterium tuberculosis isolates from Moroccan patients: Systematic review. Interdiscip. Perspect. Infect. Dis. 2020, 2020, 5185896. [Google Scholar] [CrossRef] [PubMed]
- Reta, M.A.; Alemnew, B.; Abate, B.B.; Fourie, P.B. Prevalence of drug resistance-conferring mutations associated with isoniazid- and rifampicin-resistant Mycobacterium tuberculosis in Ethiopia a systematic review and metaanalysis. J. Glob. Antimicrob. Resist. 2021, 26, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Seid, A.; Berhane, N.; Nureddin, S. Frequency of rpoB, katG, and inhA Gene Polymorphisms Associated with Multidrug-Resistant Mycobacterium tuberculosis Complex Isolates among Ethiopian TB Patients: A Systematic Review. Interdiscip. Perspect. Infect. Dis. 2022, 2022, 1967675. [Google Scholar] [CrossRef]
- Isakova, J.; Sovkhozova, N.; Vinnikov, D.; Goncharova, Z.; Talaibekova, E.; Aldasheva, N.; Aldashev, A. Mutations of rpoB, katG, inhA and ahp genes in rifampicin and isoniazid-resistant Mycobacterium tuberculosis in Kyrgyz Republic. BMC Microbiol. 2018, 18, 22. [Google Scholar] [CrossRef]
- Minh, N.N.; Van Bac, N.; Son, N.T.; Lien, V.T.K.; Ha, C.H.; Cuong, N.H.; Mai, C.T.N.; Le, T.H. Molecular characteristics of rifampicin-and isoniazid-resistant Mycobacterium tuberculosis strains isolated in Vietnam. J. Clin. Microbiol. 2012, 50, 598–601. [Google Scholar] [CrossRef][Green Version]
- Adikaram, C.P.; Perera, J.; Wijesundera, S.S. Geographical profile of rpoB gene mutations in rifampicin resistant Mycobacterium tuberculosis isolates in Sri Lanka. Microbiol. Drug Res. 2012, 18, 525–530. [Google Scholar] [CrossRef]

| Author(s) | Study Region | Study Period | Type of patients (EXTRA or PTB) | Study design | Molecular Diagnostic Method(s) | Participants (n) | All Positive Cases (n) | Total Isolates with DST Performed (n) | Any Drug Resistance (n) | Any INH or RIF Resistance (n) | MDR-TB (n) | Anti-TB Drug Resistance or Mechanisms rpoB and KatG (n) | Frequency of Gene Mutations (n) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INH | RIF | katG | inhA | katG + inhA | rpoB + katG | ||||||||||||
| Western cape (capetown) | 2018 | PTB | - | Flouro Type MTBDR and MTBDRplus | 448/51,623 (0.9%) | 244/43,580 (0.5%) | 448/650 (69%) | - | 13/2995 (0.4%) | 35/3460 (1%) | 163/2909 (5.6%) | rpoB: L511P (1), D516V (1), H526L (1), H526N (1) and L533P (1) | - | - | - | - |
| Western cape (Khayelitsha) | March 2013–2014 | PTB | Prospective cohort study | GeneXpert MTB/RIF | 306/51,623 (0.6%) | 306/43,580 (1%) | - | - | 17/2995 (0.5%) | - | rpoB: S531L (2) KatG: S315T (2) | - | 13/2457 (0.5%) | - | - | |
| Western cape (Khayelitsha) | 2008–2017 | Extra | Retrospective Cohort Study | Whole-genome sequencing (Illumina HiSeq 2500) | 1274/51,623 (2.5%) | 1274/43,580 (3%) | - | - | 196/2995 (6.5%) | - | 12/2909 (0.4%) | - | - | - | 196/404 (48.5%) | - |
| Western cape | 2006–2017 | PTB | Prospective study | MTBDRplus line probe assay Xpert MTB/RIF | 748/51,623 (1.4%) | 461/43,580 (1%) | - | 461/3637 (12.7%) | 461/2995 (15%) | 461/3460 (12%) | 197/2909 (7%) | - | - | - | - | - |
| Cape-town, Pune, Kampala, Lima | 2017–2018 | PTB | Prospective/multicenter study | Xpert MTB/RIF BD MAX MDR-TB assay | 1053/5,1623 (1%) | 984/43,580 (2.2%) | 202/650 (31%) | 232/3637 (6.4%) | 27/2995 (1%) | 10/3460 (0.3%) | - | D435Y (2), D435F (1), L430P (2), L452P (1) | 16/885 (2%) | 4/2457 (0.2%) | - | - |
| KZN (uMkhanyakude district, Ugu and Uthukela district) | 2011–2014 | PTB | Prospective study | Whole-genome sequencing | 404/51,623 (1%) | 318/43,580 (1%) | - | 318/3637 (8.7%) | 54/2995 (2%) | 54/2909 (2%) | KatG: S315T (1) | - | - | - | - | |
| Peru (Lima), Thailand (Bangkok), South Africa (Cape-Town), Kenya (Eldoret), Côte d’Ivoire (Abidjan), Botswana, DRC, Nigeria (Abuja), Tanzania (Bagamoyo) | - | PTB | Multicenter study | Whole-genome sequencing | 312/51,623 (1%) | 312/43,580 (1%) | - | 312/3637 (8.6%) | 276/2995 (9%) | 282/3460 (8%) | 246/2909 (8%) | rpoB S450L (282) KatG: S315T (250) | 282/885 (32%) | 276/2457 (11%) | - | - |
| Cape town | 2008–2017 | PTB | Descriptive study | Xpert MTB/RIF | 2161/51,623 (4%) | 1119/43,580 (2.5%) | - | 1119/3637 (31%) | 2041/3460 (59% | 899/2909 (31%) | rpoB L430P (32), rpoB S450L (73) | - | 2041/2457 (83%) | - | - | |
| Free state (Fezile- Dabi, Lejweleputswa, Mangaung, Thabo Mofutsanyana, Xhariep) | 2014–2016 | PTB | Retrospective study | GenoType MTBDRplus | 6648/51,623 (13%) | 918/43,580 (2%) | - | 918/3637 (25.2%) | 123/2995 (4%) | 587/3460 (17%) | 918/2909 (31%) | - | 587/885 (66%) | 123/2457 (5%) | 208/404 (51%) | - |
| Western cape and Gauteng | 1993–1995 | - | - | Hain line probe assay (LPA) Illumina Miseq whole-genome sequencing (WGS) GenoType version 2 MTBDRplus assay (Hain Lifescience, Germany). | 625/51,623 (1.2%) | - | 5/2995 (0.2%) | 44/3460 (1.3%) | 171/2909 (6%) | rpoB (Thr480Ala (1), Gln253Arg (1), Val249Met (1), Val251Tyr (1), Val251Phe (2) KatG: (Trp477STOP (1), Gln88STOP (1), Trp198STOP (1), Trp412STOP (1) | - | - | - | - | ||
| Gauteng, Mpumalanga, North west and Limpopo | 2013–2016 | - | Multiplex allele-specific PCR Deeplex-MycTB Whole-genome sequencing (Sanger sequencing) | 37,644/51,623 (73%) | 37,644/43,580 (86%) | 277/3637 (7.6%) | 1823/2995 (61%) | - | 249/2909 (8%) | rpoB; IIe491phe (35) KatG: S315T (249) | - | - | - | - | ||
| Total | - | - | - | - | - | 51,623/51,623 (100%) | 43,580/51,623 (84%) | 650/51,623 (1.2%) | 3637/51,623 (7%) | 2995/51,623 (6%) | 3460/51,623 (7%) | 2909/51,623 (6%) | rpoB: L511P (1), L533P (1), L430P (34)L452P (1), D516(1), D435Y (2), D435P (1), H526L (1), H526N (1), S531L (2), S450L (355), Thr480Ala (1), Gln 253 Arg (1), Val 249 Met (1), Val 251 Tyr (1), Val 251Phe (2), Ile 491 Phe (35) Total rpoB (441) KatG: S315T (502), Trp477STOP (1), Gln88STOP (1), Trp198STOP (1), Trp412STOP (1) Total KatG (506) | 885/51,623 (2%) | 2457/51,623 (5%) | 404/51,623 (1%) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traoré, A.N.; Rikhotso, M.C.; Mphaphuli, M.A.; Patel, S.M.; Mahamud, H.A.; Kachienga, L.O.; Kabue, J.-P.; Potgieter, N. Isoniazid and Rifampicin Resistance-Conferring Mutations in Mycobacterium tuberculosis Isolates from South Africa. Pathogens 2023, 12, 1015. https://doi.org/10.3390/pathogens12081015
Traoré AN, Rikhotso MC, Mphaphuli MA, Patel SM, Mahamud HA, Kachienga LO, Kabue J-P, Potgieter N. Isoniazid and Rifampicin Resistance-Conferring Mutations in Mycobacterium tuberculosis Isolates from South Africa. Pathogens. 2023; 12(8):1015. https://doi.org/10.3390/pathogens12081015
Chicago/Turabian StyleTraoré, Afsatou Ndama, Mpumelelo Casper Rikhotso, Marry Avheani Mphaphuli, Sana Mustakahmed Patel, Hafsa Ali Mahamud, Leonard Owino Kachienga, Jean-Pierre Kabue, and Natasha Potgieter. 2023. "Isoniazid and Rifampicin Resistance-Conferring Mutations in Mycobacterium tuberculosis Isolates from South Africa" Pathogens 12, no. 8: 1015. https://doi.org/10.3390/pathogens12081015
APA StyleTraoré, A. N., Rikhotso, M. C., Mphaphuli, M. A., Patel, S. M., Mahamud, H. A., Kachienga, L. O., Kabue, J.-P., & Potgieter, N. (2023). Isoniazid and Rifampicin Resistance-Conferring Mutations in Mycobacterium tuberculosis Isolates from South Africa. Pathogens, 12(8), 1015. https://doi.org/10.3390/pathogens12081015








