Epilepsy Due to Solitary Calcified Cysticercus Granuloma
Abstract
1. Introduction
2. Solitary Cysticercus Granuloma
3. SCG—Natural Evolution—Seizure Relapse
4. SCG: Risk Factors for Calcification
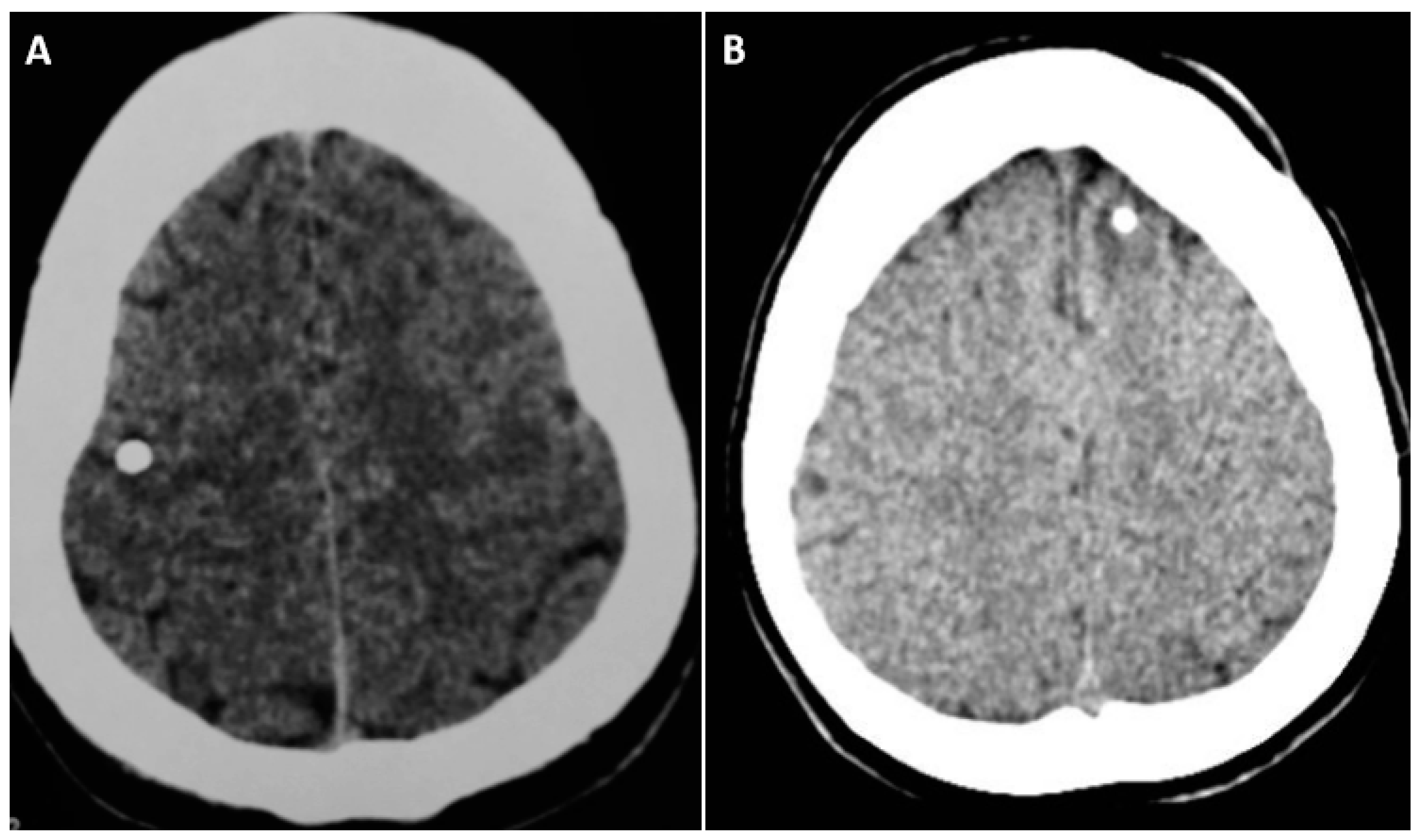
5. Calcified NCC: Epileptogenesis
6. Epilepsy due to Solitary Calcified NCC: Epidemiology
7. Epilepsy due to Solitary Calcified NCC: Clinical Characteristic
8. Anti-Seizure Medication
9. Neurocysticercosis: Epilepsy Surgery
10. Conclusions and Feature Research
11. Highlights
- Solitary calcified NCC is the most common type of NCC in India compared to South American countries
- Focal–onset with or without impaired consciousness and focal-onset to bilateral tonic-clonic seizures are the most common seizure type
- The calcified cysticercus cyst is probably the epileptogenic focus and the mechanisms involved in the pathogenesis are not well understood.
- Seizure remission rate is about 80%, more often with single anti-seizure medication in people with epilepsy due to solitary calcified NCC
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Garcia, H.H.; Del Brutto, O.H. for The Cysticercosis Working Group in Peru. Neurocystiecosis: Update concepts about an old disease. Lancet Neurol. 2005, 4, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Chandy, M.J.; Rajshekhar, V. Focal epilepsy in India [letter]. J. Neurol. Neurosurg. Psychiatry 1988, 51, 1234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Escobar, A.; Weidenheim, K. The pathology of neurocysticercosis. In Taenia solium Cysticercosis: From Basics to Clinical Science; Singh, G., Prabhakar, S., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 289–305. [Google Scholar]
- Singh, G.; Rajshekhar, V.; Murthy, J.M.K.; Prabhakar, S.; Modi, M.; Khandelwal, N.; Garcia, H.H. A diagnostic and therapeutic scheme for a solitary cysticercus granuloma. Neurology 2010, 75, 2236–2245. [Google Scholar] [CrossRef]
- Carpio, A.; Romo, M.L. Multifactorial basis of epilepsy in patients with neurocysticercosis. Epilepsia 2015, 56, 973–974. [Google Scholar] [CrossRef] [PubMed]
- Gripper, L.B.; Welburn, S.C. The causal relationship between neurocysticercosis infection and the development of epilepsy—A systematic review. Infect. Dis. Poverty 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Ndimubanzi, P.C.; Carabin, H.; Budke, C.M.; Nguyen, H.; Qian, Y.-J.; Rainwater, E.; Dickey, M.; Reynolds, S.; Stoner, J.A. A Systematic Review of the Frequency of Neurocyticercosis with a Focus on People with Epilepsy. PLoS Negl. Trop. Dis. 2010, 4, e870. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Arroyo, G.; Del Brutto, V.J.; Zambrano, M.; Garcia, H.H. On the relationship between calcified neurocysticercosis and epilepsy in an endemic village: A largescale, computed tomography-based population in study in rural Ecuador. Epilepsia 2017, 58, 1955–2017. [Google Scholar]
- Del Brutto, O.H.; Recalde, B.Y.; Mera, R.M. Incidence of Adult-Onset Epilepsy and the Contributory Role of Neurocysticercosis in a Five-Year, Population-Based, Prospective Study in Rural Ecuador. Am. J. Trop. Med. Hyg. 2022, 106, 208–214. [Google Scholar] [CrossRef]
- Murthy, J.M.; Seshadri, V. Prevalence, clinical characteristics, and seizure outcomes of epilepsy due to calcific clinical stage of neurocysticercosis: Study in a rural community in south India. Epilepsy Behav. 2019, 98, 168–172. [Google Scholar] [CrossRef]
- Rajshekhar, V. Etiology and management of single small CT lesions in patients with seizures: Understanding a controversy. Acta Neurol. Scand. 1991, 84, 465–470. [Google Scholar] [CrossRef]
- Prasad, A.; Gupta, R.K.; Pradhan, S.; Tripathi, M.; Pandey, C.M.; Prasad, K.N. What triggers seizures in neurocysticercosis? A MRI-based study in pig farming community from a district of North India. Parasitol. Int. 2008, 57, 166–171. [Google Scholar] [CrossRef]
- Prabhakaran, V.; Rajshekhar, V.; Murrell, K.; Oommen, A. Taenia solium metacestode glycoproteins as diagnostic antigens for solitary cysticercus granuloma in Indian patients. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Rajshekhar, V.; Chandy, M.J. Incidence of solitary cysticercus granulomas. In Solitary Cysticercus Granuloma: The Disappearing Lesion; Rajshekhar, V., Chandy, M.J., Eds.; Orient Longman Limited: New Delhi, India, 2000; pp. 12–28. [Google Scholar]
- Garcia, H.H.; Gonzalez, A.E.; Rodriguez, S.; Tsang, V.C.W.; Pretell, E.J.; Gonzales, I.; Gilman, R.H. For The Cysticercosis Working Group in Peru Neurocysticercosis: Unraveling the nature of the single cysticercal granuloma. Neurology 2010, 75, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E.; Carpio, A.; Forsgren, L.; Hesdorffer, D.C.; Malmgren, K.; Sander, J.W.; Tomson, T.; Hauser, W.A. Recommendation for a definition of acute symptomatic seizure. Epilepsia 2010, 51, 671–675. [Google Scholar] [CrossRef]
- Escobar, A. The pathology of neurocysticercosis. In Cysticercosis of the Central Nervous System; Palacios, E., Rodriguez-Carbajal, I., Taveras, J., Eds.; Charles C. Thomas: Chicago, IL, USA, 1983; pp. 27–54. [Google Scholar]
- Chandy, M.J.; Rajshekhar, V.; Ghosh, S.; Prakash, S.; Joseph, T.; Abraham, J.; Chandi, S.M. Single small enhancing CT lesions in Indian patients with epilepsy: Clinical, radiological and pathological considerations. J. Neurol. Neurosurg. Psychiatry 1991, 54, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Murthy, J.M.K.; Vijaya, S.; Raju, R.C.; Thomas, J. Acute symptomatic seizures associated with neurocysticercosis: A community-based prevalence study and comprehensive rural epilepsy study in South India (CRESSI). Neurol. Asia 2004, 9 (Suppl. S1), 86. [Google Scholar]
- Jaiswal, S.K.; Murthy, J.M.K.; Reddy, M.P.; Srikrishna, S. Incidence of seizures due to degenerative phase of neurocysticercosis: A study in a cohort of primary school children in south India. Neurol. Asia 2019, 24, 121–125. [Google Scholar]
- Rajshekhar, V.; Jayaseelan, L. Seizure outcome in patients with a solitary cerebral cysticercus granuloma. Neurology 2004, 62, 2236–2240. [Google Scholar] [CrossRef]
- Rajshekhar, V. Rate of spontaneous resolution of a solitary cysticercus granuloma in patients with seizures. Neurology 2001, 57, 2315–2317. [Google Scholar] [CrossRef]
- Mahajan, L.; Malhotra, H.S.; Rizvi, I.; Kumar, N.; Garg, R.K.; Verma, R.; Sharma, P.K. Predictors of Lesion Calcification in Patients with Solitary Cysticercus Granuloma and New-Onset Seizures. Am. J. Trop. Med. Hyg. 2016, 95, 623–628. [Google Scholar] [CrossRef]
- Bustos, J.A.; Arooya, G.; Gilman, R.H.; Soto-Becerra, P.; Gonzales, I.; Saavera, H.; Pretell, E.J.; Nash, N.T.; O’Neal, S.E.; Del Brutto, O.H.; et al. Frequency and determinant factors for calcification neurocsyticercosis. Clin. Infect. Dis. 2021, 73, e2592–e2600. [Google Scholar] [CrossRef]
- Nash, T.E.; Del Brutto, O.H.; Butman, J.A.; Corona, T.; Delgado-Escueta, A.; Duron, R.M.; Evans, C.A.; Gilman, R.H.; Gonzalez, A.E.; Loeb, J.A.; et al. Calcific neurocysticercosis and epileptogenesis. Neurology 2004, 62, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Jama-António, J.M.C.; Yasuda, C.L.; Cendes, F. Intermittent perilesional edema and contrast enhancement in epilepsy with calcified neurocysticercosis may help to identify the seizure focus. Epilepsia Open 2019, 4, 351–354. [Google Scholar] [CrossRef]
- Nash, T.E.; Pretell, E.J.; Lescano, A.G.; Bustos, J.A.; Gilman, R.H.; Gonzalez, A.E.; Garcia, H.H. Cysticercosis Working Group in Peru, Perilesional brain edema and seizure activity in patients with calcified neurocysticercosis. Lancet Neurol. 2008, 7, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Nash, T. Edema surrounding calcified intracranial cysticercosis: Clinical manifestations, natural history and treatment. Pathog. Glob. Health 2012, 106, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Mahanty, S.; Zoghbi, S.S.; Araneta, M.D.F.; Hong, J.; Pike, V.W.; Innis, R.B.; Nash, T.E. PET Reveals Inflammation around Calcified Taenia solium Granulomas with Perilesional Edema. PLoS ONE 2013, 8, e74052. [Google Scholar] [CrossRef]
- de Souza, A.; Nalini, A.; Kovoor, J.M.E.; Yeshraj, G.; Siddalingaiah, H.S.; Thennarasu, K. Perilesional gliosis around solitary cerebral parenchymal cysticerci and long-term seizure outcome: A prospective study using serial magnetization transfer imaging. Epilepsia 2011, 52, 1918–1927. [Google Scholar] [CrossRef]
- Nash, T.E.; Bartelt, L.A.; Korpe, P.S.; Lopes, B.; Houpt, E.R. Calcified neurocysticercus, perilesionaledema, and histologic inflammation. Am. J. Trop. Med. Hyg. 2014, 90, 318–321. [Google Scholar] [CrossRef]
- Ooi, W.W.; Wijemanne, S.; Thomas, C.B.; Quezado, M.; Brown, C.R.; Nash, T.E. Short report: A calcified Taenia solium granuloma associated with recurrent perilesional edema causing refractory seizures: Histopathological features. Am. J. Trop. Med. Hyg. 2011, 85, 460–463. [Google Scholar] [CrossRef]
- Rathore, C.; Thomas, B.; Kesavadas, C.; Abraham, M.; Radhakrishnan, K. Calcified neurocysticercosis lesions and antiepileptic drug resistant epilepsy: A surgically remediable syndrome? Epilepsia 2013, 54, 1815–1822. [Google Scholar] [CrossRef]
- Marchi, N.; Angelov, L.; Masaryk, T.; Fazio, V.; Granata, T.; Hernandez, N.; Hallene, K.; Diglaw, T.; Franic, L.; Najm, I.; et al. Seizure-Promoting Effect of Blood?Brain Barrier Disruption. Epilepsia 2007, 48, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Murthy, J.M.; Reddy, V.S. Clinical characteristics, seizure spread patterns and prognosis of seizures associated with a single small cerebral calcific CT lesion. Seizure 1998, 7, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Sachdev, M.S.; Tirath, A.; Gupta, A.K.; Avasthi, G. Focal Cortical-Subcortical Calcifications (FCSCs) and Epilepsy in the Indian Subcontinent. Epilepsia 2000, 41, 718–726. [Google Scholar] [CrossRef]
- Rajshekhar, V.; Raghava, M.V.; Prabhakaran, V.; Oommen, A.; Muliyil, J. Active epilepsy as an index of burden of neurocysticercosis in Vellore district, India. Neurology 2006, 67, 2135–2139. [Google Scholar] [CrossRef] [PubMed]
- Goel, D.; Dhanai, J.; Agarwal, A.; Mehlotra, V.; Saxena, V. Neurocysticercosis and its impact on crude prevalence rate of epilepsy in an Indian community. Neurol. India 2011, 59, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Murthy, J.M.K.; Jaiswal, S.K.; Reddy, M.P.; Srikrishna, S. Incidence Study of Epilepsy Using the ILAE 2017 Classification of Epilepsies in a Cohort of School Children Accessing Education in Government Primary Schools in South India. Neurol. India 2020, 68, 1389–1393. [Google Scholar]
- Murthy, J.M.; Deshmukh, D.S. Convulsive status epilepticus due to different evolutionary stages of neurocysticercosis—Solitary cyticercus granuloma, low cyst load, and single calcific lesion in an endemic country: Clinical profile. Seizure 2019, 71, 229–232. [Google Scholar] [CrossRef]
- Escalaya, A.L.; Burneo, J.G. Epilepsy surgery and neurocysticercosis: Assessing the role of the cysticercotic lesion in medically-refractory epilepsy. Epilepsy Behav. 2017, 76, 178–181. [Google Scholar] [CrossRef]
- Nash, T.E.; Mahanty, S.; Loeb, J.A.; Theodore, W.H.; Friedman, A.; Sander, J.W.; Garcia, H.H.; Singh, G.; Del Brutto, O.H.; Takayanagui, O.M.; et al. Neurocysticercosis: A natural human model of epileptogenesis. Epilepsia 2015, 56, 177–183. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kumar, R.; Chawla, S.; Pradhan, S. Demonstration of Scolex within Calcified Cysticercus Cyst: Its Possible Role in the Pathogenesis of Perilesional Edema. Epilepsia 2002, 43, 1502–1508. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murthy, J.M.K. Epilepsy Due to Solitary Calcified Cysticercus Granuloma. Pathogens 2023, 12, 1037. https://doi.org/10.3390/pathogens12081037
Murthy JMK. Epilepsy Due to Solitary Calcified Cysticercus Granuloma. Pathogens. 2023; 12(8):1037. https://doi.org/10.3390/pathogens12081037
Chicago/Turabian StyleMurthy, Jagarlapudi M. K. 2023. "Epilepsy Due to Solitary Calcified Cysticercus Granuloma" Pathogens 12, no. 8: 1037. https://doi.org/10.3390/pathogens12081037
APA StyleMurthy, J. M. K. (2023). Epilepsy Due to Solitary Calcified Cysticercus Granuloma. Pathogens, 12(8), 1037. https://doi.org/10.3390/pathogens12081037





