Presence of Equine and Bovine Coronaviruses, Endoparasites, and Bacteria in Fecal Samples of Horses with Colic
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Handling and Sampling
2.2. Clinical Examination
2.3. Virological Examination—Molecular Investigation for Betacoronaviruses
2.4. Parasitological Examination—Fecal Parasitology and Molecular Investigation
2.5. Bacteriological Examination—Bacterial Culture and Classification Procedures
2.6. Statistical Analysis
3. Results
3.1. Clinical Findings
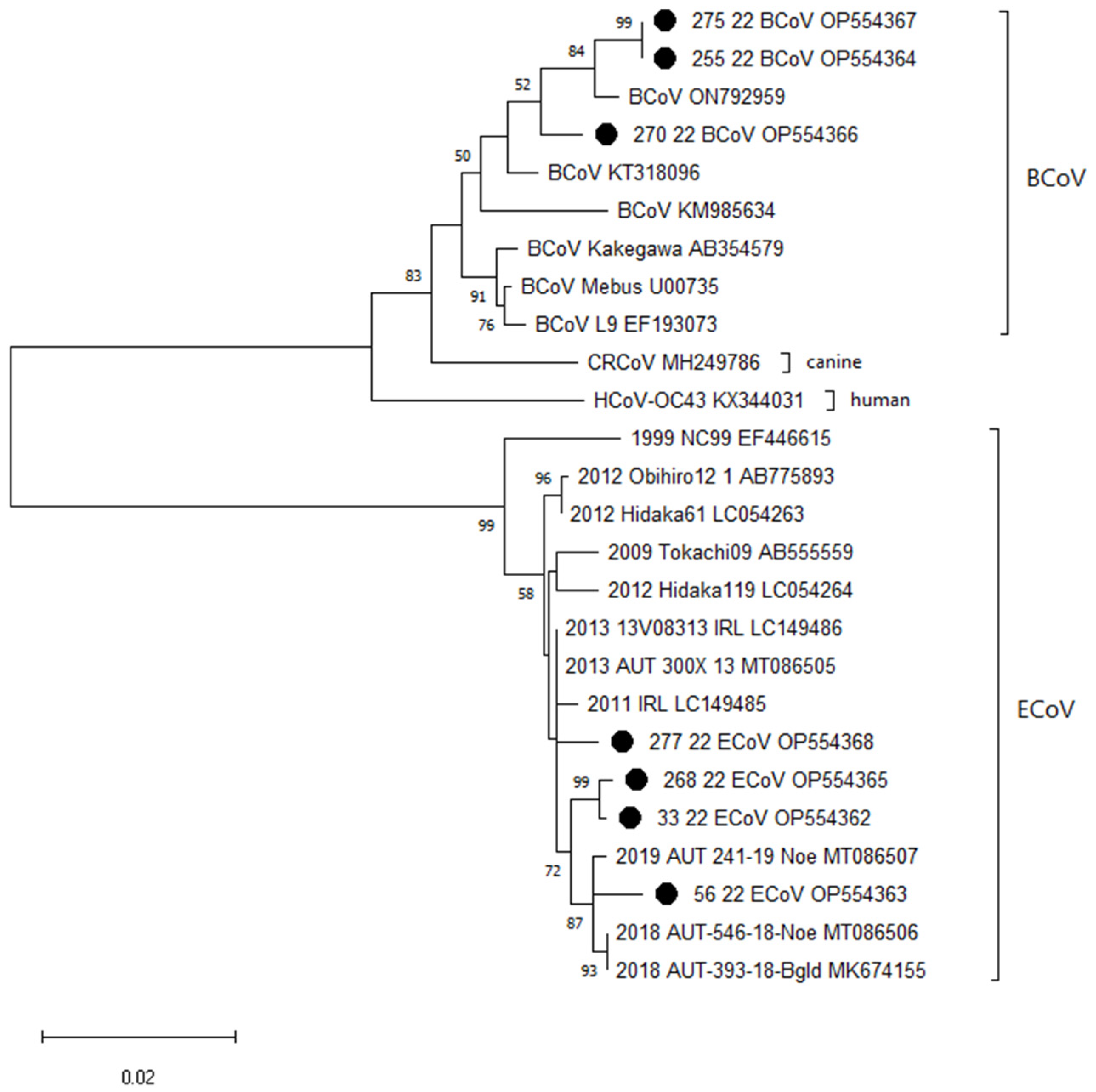
3.2. Virological Findings—Molecular Investigation for Betacoronaviruses
3.3. Parasitological Findings—Fecal Parasitology and Molecular Investigation
3.4. Bacteriological Findings—Bacterial Culture and Classification Procedures
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowden, A.; England, G.C.W.; Brennan, M.L.; Mair, T.S.; Furness, W.A.; Freeman, S.L.; Burford, J.H. Indicators of ‘Critical’ Outcomes in 941 Horses Seen ‘out-of-Hours’ for Colic. Vet. Rec. 2020, 187, 492. [Google Scholar] [CrossRef]
- Archer, D.C.; Proudman, C.J. Epidemiological Clues to Preventing Colic. Vet. J. 2006, 172, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Tinker, M.K.; White, N.A.; Lessard, P.; Thatcher, C.D.; Pelzer, K.D.; Davis, B.; Carmel, D.K. Prospective Study of Equine Colic Incidence and Mortality. Equine Vet. J. 1997, 29, 448–453. [Google Scholar] [CrossRef]
- Curtis, L.; Burford, J.H.; England, G.C.W.; Freeman, S.L. Risk Factors for Acute Abdominal Pain (Colic) in the Adult Horse: A Scoping Review of Risk Factors, and a Systematic Review of the Effect of Management-Related Changes. PLoS ONE 2019, 14, e0219307. [Google Scholar] [CrossRef] [PubMed]
- Oue, Y.; Ishihara, R.; Edamatsu, H.; Morita, Y.; Yoshida, M.; Yoshima, M.; Hatama, S.; Murakami, K.; Kanno, T. Isolation of an Equine Coronavirus from Adult Horses with Pyrogenic and Enteric Disease and Its Antigenic and Genomic Characterization in Comparison with the NC99 Strain. Vet. Microbiol. 2011, 150, 41–48. [Google Scholar] [CrossRef]
- Oue, Y.; Morita, Y.; Kondo, T.; Nemoto, M. Epidemic of Equine Coronavirus at Obihiro Racecourse, Hokkaido, Japan in 2012. J. Vet. Med. Sci. 2013, 75, 1261–1265. [Google Scholar] [CrossRef]
- Pusterla, N.; Mapes, S.; Wademan, C.; White, A.; Ball, R.; Sapp, K.; Burns, P.; Ormond, C.; Butterworth, K.; Bartol, J.; et al. Emerging Outbreaks Associated with Equine Coronavirus in Adult Horses. Vet. Microbiol. 2013, 162, 228–231. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; de Groot, R.J.; Haagmans, B.; Lau, S.K.P.; Neuman, B.W.; Perlman, S.; Sola, I.; van der Hoek, L.; Wong, A.C.P.; Yeh, S.-H. ICTV Virus Taxonomy Profile: Coronaviridae 2023. J. Gen. Virol. 2023, 104, 001843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guy, J.S.; Snijder, E.J.; Denniston, D.A.; Timoney, P.J.; Balasuriya, U.B.R. Genomic Characterization of Equine Coronavirus. Virology 2007, 369, 92–104. [Google Scholar] [CrossRef]
- Guy, J.S.; Breslin, J.J.; Breuhaus, B.; Vivrette, S.; Smith, L.G. Characterization of a Coronavirus Isolated from a Diarrheic Foal. J. Clin. Microbiol. 2000, 38, 4523–4526. [Google Scholar] [CrossRef]
- Nemoto, M.; Schofield, W.; Cullinane, A. The First Detection of Equine Coronavirus in Adult Horses and Foals in Ireland. Viruses 2019, 11, 946. [Google Scholar] [CrossRef]
- Narita, M.; Nobumoto, K.; Takeda, H.; Moryama, T.; Morita, Y.; Nakaoka, Y. Prevalence of Disease with Inference of Equine Coronavirus Infection Among Horses Stabled in a Draft-Horse Racecourse. J. Jpn. Vet. Med. Assoc. 2011, 64, 535–539. [Google Scholar] [CrossRef][Green Version]
- Fielding, C.L.; Higgins, J.K.; Higgins, J.C.; McIntosh, S.; Scott, E.; Giannitti, F.; Mete, A.; Pusterla, N. Disease Associated with Equine Coronavirus Infection and High Case Fatality Rate. J. Vet. Intern. Med. 2015, 29, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Auer, A.; Junge, H.K.; Glitz, F.; Dimmel, K.; Leidinger, J.; Cavalleri, J.; Rümenapf, T. Equine Coronavirus Is an Underestimated Pathogen in Austria. Vet. Med. Austria 2020, 107, 236–242. [Google Scholar]
- Nemoto, M.; Oue, Y.; Morita, Y.; Kanno, T.; Kinoshita, Y.; Niwa, H.; Ueno, T.; Katayama, Y.; Bannai, H.; Tsujimura, K.; et al. Experimental Inoculation of Equine Coronavirus into Japanese Draft Horses. Arch. Virol. 2014, 159, 3329–3334. [Google Scholar] [CrossRef]
- Giannitti, F.; Diab, S.; Mete, A.; Stanton, J.B.; Fielding, L.; Crossley, B.; Sverlow, K.; Fish, S.; Mapes, S.; Scott, L.; et al. Necrotizing Enteritis and Hyperammonemic Encephalopathy Associated with Equine Coronavirus Infection in Equids. Vet. Pathol. 2015, 52, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Vin, R.; Leutenegger, C.M.; Mittel, L.D.; Divers, T.J. Enteric Coronavirus Infection in Adult Horses. Vet. J. 2018, 231, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.; Harms, C.; Viner, M.; Barnum, S.; Pusterla, N. Investigation of an Experimental Infection Model of Equine Coronavirus in Adult Horses. J. Vet. Intern. Med. 2018, 32, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Berryhill, E.H.; Magdesian, K.G.; Aleman, M.; Pusterla, N. Clinical Presentation, Diagnostic Findings, and Outcome of Adult Horses with Equine Coronavirus Infection at a Veterinary Teaching Hospital: 33 Cases (2012–2018). Vet. J. 2019, 248, 95–100. [Google Scholar] [CrossRef]
- Goodrich, E.L.; Mittel, L.D.; Glaser, A.; Ness, S.L.; Radcliffe, R.M.; Divers, T.J. Novel Findings from a Beta Coronavirus Outbreak on an American Miniature Horse Breeding Farm in Upstate New York. Equine Vet. Educ. 2020, 32, 150–154. [Google Scholar] [CrossRef]
- Mattei, D.N.; Kopper, J.J.; Sanz, M.G. Equine Coronavirus-Associated Colitis in Horses: A Retrospective Study. J. Equine Vet. Sci. 2020, 87, 102906. [Google Scholar] [CrossRef]
- Love, S.; Murphy, D.; Mellor, D. Pathogenicity of Cyathostome Infection. Vet. Parasitol. 1999, 85, 113–122. [Google Scholar] [CrossRef]
- Murphy, D.; Love, S. The Pathogenic Effects of Experimental Cyathostome Infections in Ponies. Vet. Parasitol. 1997, 70, 99–110. [Google Scholar] [CrossRef]
- Gehlen, H.; Wulke, N.; Ertelt, A.; Nielsen, M.K.; Morelli, S.; Traversa, D.; Merle, R.; Wilson, D.; von Samson-Himmelstjerna, G. Comparative Analysis of Intestinal Helminth Infections in Colic and Non-Colic Control Equine Patients. Animals 2020, 10, 1916. [Google Scholar] [CrossRef] [PubMed]
- Reinemeyer, C.R.; Nielsen, M.K. Parasitism and Colic. Vet. Clin. N. Am.-Equine Pract. 2009, 25, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.; Slocombe, D. Pathogenesis of Helminths in Equines. Vet. Parasitol. 1985, 18, 139–153. [Google Scholar] [CrossRef]
- Stancampiano, L.; Usai, F.; Marigo, A.; Rinnovati, R. Are Small Strongyles (Cyathostominae) Involved in Horse Colic Occurrence? Vet. Parasitol. 2017, 247, 33–36. [Google Scholar] [CrossRef]
- Fabiani, J.V.; Lyons, E.T.; Nielsen, M.K. Dynamics of Parascaris and Strongylus spp. Parasites in Untreated Juvenile Horses. Vet. Parasitol. 2016, 230, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Proudman, C.J.; French, N.P.; Trees, A.J. Tapeworm Infection Is a Significant Risk Factor for Spasmodic Colic and Ileal Impaction Colic in the Horse. Equine Vet. J. 1998, 30, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Hedberg-Alm, Y.; Penell, J.; Riihimäki, M.; Osterman-Lind, E.; Nielsen, M.K.; Tydén, E. Parasite Occurrence and Parasite Management in Swedish Horses Presenting with Gastrointestinal Disease-a Case-Control Study. Animals 2020, 10, 638. [Google Scholar] [CrossRef]
- Jürgenschellert, L.; Krücken, J.; Austin, C.J.; Lightbody, K.L.; Bousquet, E.; Von Samson-Himmelstjerna, G. Investigations on the Occurrence of Tapeworm Infections in German Horse Populations with Comparison of Different Antibody Detection Methods Based on Saliva and Serum Samples. Parasit. Vectors 2020, 13, 462. [Google Scholar] [CrossRef]
- Costa, M.C.; Arroyo, L.G.; Allen-Vercoe, E.; Stämpfli, H.R.; Kim, P.T.; Sturgeon, A.; Weese, J.S. Comparison of the Fecal Microbiota of Healthy Horses and Horses with Colitis by High Throughput Sequencing of the V3–V5 Region of the 16s RRNA Gene. PLoS ONE 2012, 7, e41484. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.; Hastie, P.; Murray, J.A. Factors Influencing Equine Gut Microbiota: Current Knowledge. J. Equine Vet. Sci. 2020, 88, 102943. [Google Scholar] [CrossRef] [PubMed]
- Stewart, H.L.; Pitta, D.; Indugu, N.; Vecchiarelli, B.; Hennessy, M.L.; Engiles, J.B.; Southwood, L.L. Changes in the Faecal Bacterial Microbiota during Hospitalisation of Horses with Colic and the Effect of Different Causes of Colic. Equine Vet. J. 2021, 53, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Uzal, F.A.; Navarro, M.A.; Asin, J.; Henderson, E.E. Clostridial Diseases of Horses: A Review. Vaccines 2022, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Staempfli, H.R.; Prescott, J.F. A Prospective Study of the Roles of Clostridium difficile and Enterotoxigenic Clostridium perfringens in Equine Diarrhoea. Equine Vet. J. 2001, 33, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Schoster, A.; Kunz, T.; Lauper, M.; Graubner, C.; Schmitt, S.; Weese, J.S. Prevalence of Clostridium difficile and Clostridium perfringens in Swiss Horses with and without Gastrointestinal Disease and Microbiota Composition in Relation to Clostridium difficile Shedding. Vet. Microbiol. 2019, 239, 108433. [Google Scholar] [CrossRef] [PubMed]
- Stewart, H.L.; Southwood, L.L.; Indugu, N.; Vecchiarelli, B.; Engiles, J.B.; Pitta, D. Differences in the Equine Faecal Microbiota between Horses Presenting to a Tertiary Referral Hospital for Colic Compared with an Elective Surgical Procedure. Equine Vet. J. 2019, 51, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Manship, A.J.; Blikslager, A.T.; Elfenbein, J.R. Disease Features of Equine Coronavirus and Enteric Salmonellosis Are Similar in Horses. J. Vet. Intern. Med. 2019, 33, 912–917. [Google Scholar] [CrossRef]
- Dallap Schaer, B.L.; Aceto, H.; Caruso, M.A.; Brace, M.A. Identification of Predictors of Salmonella Shedding in Adult Horses Presented for Acute Colic. J. Vet. Intern. Med. 2012, 26, 1177–1185. [Google Scholar] [CrossRef]
- Spiss, S.; Benetka, V.; Künzel, F.; Sommerfeld-Stur, I.; Walk, K.; Latif, M.; Möstl, K. Enteric and Respiratory Coronavirus Infections in Austrian Dogs: Serological and Virological Investigations of Prevalence and Clinical Importance in Respiratory and Enteric Disease. Vet. Med. Austria 2012, 99, 67–81. [Google Scholar]
- Mapes, S.; Rhodes, D.M.; Wilson, W.D.; Leutenegger, C.M.; Pusterla, N. Comparison of Five Real-Time PCR Assays for Detecting Virulence Genes in Isolates of Escherichia Coli from Septicaemic Neonatal Foals. Vet. Rec. 2007, 161, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.K.; Peterson, D.S.; Monrad, J.; Thamsborg, S.M.; Olsen, S.N.; Kaplan, R.M. Detection and Semi-Quantification of Strongylus vulgaris DNA in Equine Faeces by Real-Time Quantitative PCR. Int. J. Parasitol. 2008, 38, 443–453. [Google Scholar] [CrossRef]
- Jürgenschellert, L.; Krücken, J.; Bousquet, E.; Bartz, J.; Heyer, N.; Nielsen, M.K.; von Samson-Himmelstjerna, G. Occurrence of Strongylid Nematode Parasites on Horse Farms in Berlin and Brandenburg, Germany, with High Seroprevalence of Strongylus vulgaris Infection. Front. Vet. Sci. 2022, 9, 892920. [Google Scholar] [CrossRef]
- Spergser, J.; Loncaric, I.; Tichy, A.; Fritz, J.; Scope, A. The Cultivable Autochthonous Microbiota of the Critically Endangered Northern Bald Ibis (Geronticus eremita). PLoS ONE 2018, 13, e0195255. [Google Scholar] [CrossRef]
- Jones, G.F.; Ward, G.E.; Murtaugh, M.P.; Lin, G.; Gebhart, C.J. Enhanced Detection of Intracellular Organism of Swine Proliferative Enteritis, Ileal Symbiont Intracellularis, in Feces by Polymerase Chain Reaction. J. Clin. Microbiol. 1993, 31, 2611–2615. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 July 2023).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Hepworth-Warren, K.L.; Erwin, S.J.; Moore, C.B.; Talbot, J.R.; Young, K.A.S.; Neault, M.J.; Haugland, J.C.; Robertson, J.B.; Blikslager, A.T. Risk Factors Associated with an Outbreak of Equine Coronavirus at a Large Farm in North Carolina. Front. Vet. Sci. 2023, 10, 1060759. [Google Scholar] [CrossRef]
- Hierweger, M.M.; Remy-Wohlfender, F.; Franzen, J.; Koch, M.C.; Blau, D.; Schoster, A.; Nicholson, P.; Gerber, V.; Gurtner, C.; Fouché, N.; et al. Outbreak of Equine Coronavirus Disease in Adult Horses, Switzerland 2021. Transbound. Emerg. Dis. 2022, 69, 1691–1694. [Google Scholar] [CrossRef]
- Pusterla, N. Equine Coronaviruses. Vet. Clin. N. Am.-Equine Pract. 2023, 39, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.D.; Gibbs, P.G.; Woods, A.M. Dietary and Other Management Factors Associated with Colic in Horses. J. Am. Vet. Med. Assoc. 1999, 215, 53–60. [Google Scholar]
- Daniels, S.P.; Leng, J.; Swann, J.R.; Proudman, C.J. Bugs and Drugs: A Systems Biology Approach to Characterising the Effect of Moxidectin on the Horse’s Faecal Microbiome. Anim. Microbiome 2020, 2, 38. [Google Scholar] [CrossRef]
- Herholz, C.; Miserez, R.; Nicolet, J.; Frey, J.; Popoff, M.; Gibert, M.; Gerber, H.; Straub, R. Prevalence of Beta2-Toxigenic Clostridium perfringens in Horses with Intestinal Disorders. J. Clin. Microbiol. 1999, 37, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Burgess, B.A. Salmonella in Horses. Vet. Clin. N. Am.-Equine Pract. 2023, 39, 25–35. [Google Scholar] [CrossRef]
- Sanz, M.G.; Kwon, S.Y.; Pusterla, N.; Gold, J.R.; Bain, F.; Evermann, J. Evaluation of Equine Coronavirus Fecal Shedding among Hospitalized Horses. J. Vet. Intern. Med. 2019, 33, 918–922. [Google Scholar] [CrossRef]
- Barros, I.N.; Silva, S.O.S.; Nogueira Neto, F.S.; Asano, K.M.; Souza, S.P.; Richtzenhain, L.J.; Brandao, P.E. A Multigene Approach for Comparing Genealogy of Betacoronavirus from Cattle and Horses. Sci. World J. 2013, 2013, 349702. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, B.; Sun, D. Advances in Bovine Coronavirus Epidemiology. Viruses 2022, 14, 1109. [Google Scholar] [CrossRef]
- Shally-Jensen, M. Encyclopedia of Contemporary American Social Issues; Abc-Clio: Santa Barbara, CA, USA, 2011. [Google Scholar]
- Vijgen, L.; Keyaerts, E.; Moës, E.; Thoelen, I.; Wollants, E.; Lemey, P.; Vandamme, A.-M.; Van Ranst, M. Complete Genomic Sequence of Human Coronavirus OC43: Molecular Clock Analysis Suggests a Relatively Recent Zoonotic Coronavirus Transmission Event. J. Virol. 2005, 79, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Berche, P. The Enigma of the 1889 Russian Flu Pandemic: A Coronavirus? La Presse Médicale 2022, 51, 104111. [Google Scholar] [CrossRef]
- Kaneshima, T.; Hohdatsu, T.; Hagino, R.; Hosoya, S.; Nojiri, Y.; Murata, M.; Takano, T.; Tanabe, M.; Tsunemitsu, H.; Koyama, H. The Infectivity and Pathogenicity of a Group 2 Bovine Coronavirus in Pups. J. Vet. Med. Sci. 2007, 69, 301–303. [Google Scholar] [CrossRef][Green Version]
- Erles, K.; Shiu, K.B.; Brownlie, J. Isolation and Sequence Analysis of Canine Respiratory Coronavirus. Virus Res. 2007, 124, 78–87. [Google Scholar] [CrossRef]
- Amer, H.M. Bovine-like Coronaviruses in Domestic and Wild Ruminants. Anim. Health Res. Rev. 2018, 19, 113–124. [Google Scholar] [CrossRef]
- Savard, C.; Provost, C.; Ariel, O.; Morin, S.; Fredrickson, R.; Gagnon, C.A.; Broes, A.; Wang, L. First Report and Genomic Characterization of a Bovine-like Coronavirus Causing Enteric Infection in an Odd-Toed Non-Ruminant Species (Indonesian Tapir, Acrocodia Indica) during an Outbreak of Winter Dysentery in a Zoo. Transbound. Emerg. Dis. 2022, 69, 3056–3065. [Google Scholar] [CrossRef]
- Leopardi, S.; Desiato, R.; Mazzucato, M.; Orusa, R.; Obber, F.; Averaimo, D.; Berjaoui, S.; Canziani, S.; Capucchio, M.T.; Conti, R.; et al. One health surveillance strategy for coronaviruses in Italian wildlife. Epidemiol. Infect. 2023, 151, e96. [Google Scholar] [CrossRef]
| Primer/Probe Sequences | Sequence 5′-3′ | Source |
|---|---|---|
| qBetaCoV1-F | ACGTGGTGTTCCTGTTGTTATAGG | [41] |
| qBetaCoV1-R | AACATCTTTAATAAGGCGACGTAACAT | |
| qBetaCoV1-P | FAM-CCACTAAGTTTTATGGCGGCTGGGATG-BHQ1 | |
| qECoV-N-F | TGGGAACAGGCCCGC | [7] |
| qECoV-N-R | CCTAGTCGGAATAGCCTCATCAC | |
| qECoV-N-P | FAM-TGGGTCGCTAACAAG-BHQ1 | |
| ECoV-29316-F | CAGGCATGGACACCGCATTG | [14] |
| ECoV-30730-R | CCAGGTGCCGACATAAGGTTCAT | [5] |
| BetaCoV-30000-R 1 | CTTGATCCTGCACTAGAGGCTC | [14] |
| ECoV-30509-F 1 | GATGATGGGACGAATATGAGC | This study |
| BCoV-30578-F 1 | GTACACTTTCAGGTTTTGAGACC |
| Group | Horse ID | Virus and Viral Loads | Rectal Temp. [°C] | Fecal Consistency | Findings of Rectal Examination | Severity of Colic | Strongyles | Bacteria |
|---|---|---|---|---|---|---|---|---|
| P | 33/22 | ECoV 1.14 × 109 | 38.0 | Soft | No abnormal findings | ++ | - | +++ E. coli +++ S. dysgalactiae ssp. equisimilis ++ Enterococcus faecalis +++ Candida spp. |
| P | 255/22 | BCoV 3.47 × 107 | 37.2 | formed | Colonic impaction | + | - | ++ E. coli ++ Enterococcus faecalis +++ Cl. perfringens |
| P | 268/22 | ECoV 1.87 × 109 | 37.3 * | formed | Colonic impaction and left dorsal displacement of the ascending colon | + | - ** | ++ E. coli +++ S. equinus ++ Enterococcus faecalis |
| P | 270/22 | BCoV 1.77 × 106 | 37.5 | formed | No abnormal findings | + | + | ++ E. coli ++ Enterococcus faecalis ++ Cl. perfringens +/++ Cs. difficile |
| P | 275/22 | BCoV 3.19 × 106 | 37.5 * | formed | Colonic impaction | ++ | - ** | ++ E. coli +++ S. equinus ++ Enterococcus faecium |
| P | 277/22 | ECoV 2.57 × 108 | 37.9 | Soft, sticky | pelvic flexure impaction | + | - | +++ Bacillus spp. ++ S. equinus ++ Enterococcus faecium |
| C | 56/22 | ECoV 6.20 × 106 | Referred for an ophthalmologic consult. No clinical evidence for viral infection or gastrointestinal disease. | - | + E. coli +++ Enterococcus faecalis | |||
| Strongylid Egg Shedding | Patients | Controls | N Total |
|---|---|---|---|
| negative | 71 (67.6%) | 17 (47.2%) | 88 |
| low-grade | 18 (17.1%) | 5 (13.9%) | 23 |
| moderate | 2 (1.9%) | 6 (16.7%) | 8 |
| high-grade | 14 (13.3%) | 8 (22.2%) | 22 |
| Organism | Family | Order | Patients | Controls | FET Odds Ratio | FDR |
|---|---|---|---|---|---|---|
| E. coli | Enterobacteriaceae | Enterobacterales | 95 (90.5%) | 28 (80%) | 2.36 | 0.244 |
| S. equinus | Streptococcaceae | Lactobacillales | 66 (62.9%) | 14 (40%) | 2.52 | 0.107 |
| S. dysgalactiae | Streptococcaceae | Lactobacillales | 14 (13.3%) | 4 (11.4%) | 1.19 | 1 |
| Enterococcus spp. | Enterococcaceae | Lactobacillales | 86 (81.9%) | 28 (80%) | 1.13 | 0.984 |
| Bacillus spp. | Bacillaceae | Bacillales | 33 (31.4%) | 18 (51.4%) | 0.44 | 0.118 |
| Lactobacillus spp. | Lactobacillaceae | Lactobacillales | 30 (28.6%) | 16 (45.7%) | 0.48 | 0.21 |
| Cl. perfringens | Clostridiaceae | Clostridiales | 41 (39%) | 6 (17.1%) | 4.12 | 0.01 |
| Cs. difficile | Peptostreptococcaceae | Eubacteriales | 22 (21%) | 2 (5.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stummer, M.; Frisch, V.; Glitz, F.; Hinney, B.; Spergser, J.; Krücken, J.; Diekmann, I.; Dimmel, K.; Riedel, C.; Cavalleri, J.-M.V.; et al. Presence of Equine and Bovine Coronaviruses, Endoparasites, and Bacteria in Fecal Samples of Horses with Colic. Pathogens 2023, 12, 1043. https://doi.org/10.3390/pathogens12081043
Stummer M, Frisch V, Glitz F, Hinney B, Spergser J, Krücken J, Diekmann I, Dimmel K, Riedel C, Cavalleri J-MV, et al. Presence of Equine and Bovine Coronaviruses, Endoparasites, and Bacteria in Fecal Samples of Horses with Colic. Pathogens. 2023; 12(8):1043. https://doi.org/10.3390/pathogens12081043
Chicago/Turabian StyleStummer, Moritz, Vicky Frisch, Frauke Glitz, Barbara Hinney, Joachim Spergser, Jürgen Krücken, Irina Diekmann, Katharina Dimmel, Christiane Riedel, Jessika-Maximiliane V. Cavalleri, and et al. 2023. "Presence of Equine and Bovine Coronaviruses, Endoparasites, and Bacteria in Fecal Samples of Horses with Colic" Pathogens 12, no. 8: 1043. https://doi.org/10.3390/pathogens12081043
APA StyleStummer, M., Frisch, V., Glitz, F., Hinney, B., Spergser, J., Krücken, J., Diekmann, I., Dimmel, K., Riedel, C., Cavalleri, J.-M. V., Rümenapf, T., Joachim, A., Lyrakis, M., & Auer, A. (2023). Presence of Equine and Bovine Coronaviruses, Endoparasites, and Bacteria in Fecal Samples of Horses with Colic. Pathogens, 12(8), 1043. https://doi.org/10.3390/pathogens12081043