Taurine, a Component of the Tear Film, Exacerbates the Pathogenic Mechanisms of Acanthamoeba castellanii in the Ex Vivo Amoebic Keratitis Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Amoebae Culture
2.2. Reactivation of A. castellanii Virulence
2.3. Qualitative Evaluation of the Interaction of A. castellanii with Taurine in the Ex Vivo Model of Amoebic Keratitis
2.3.1. Surgical Extraction of Hamster Corneas
2.3.2. Ex Vivo Interaction of A. castellanii Trophozoites with Hamster Cornea
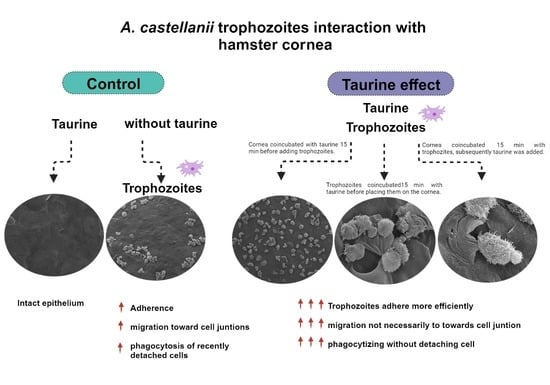
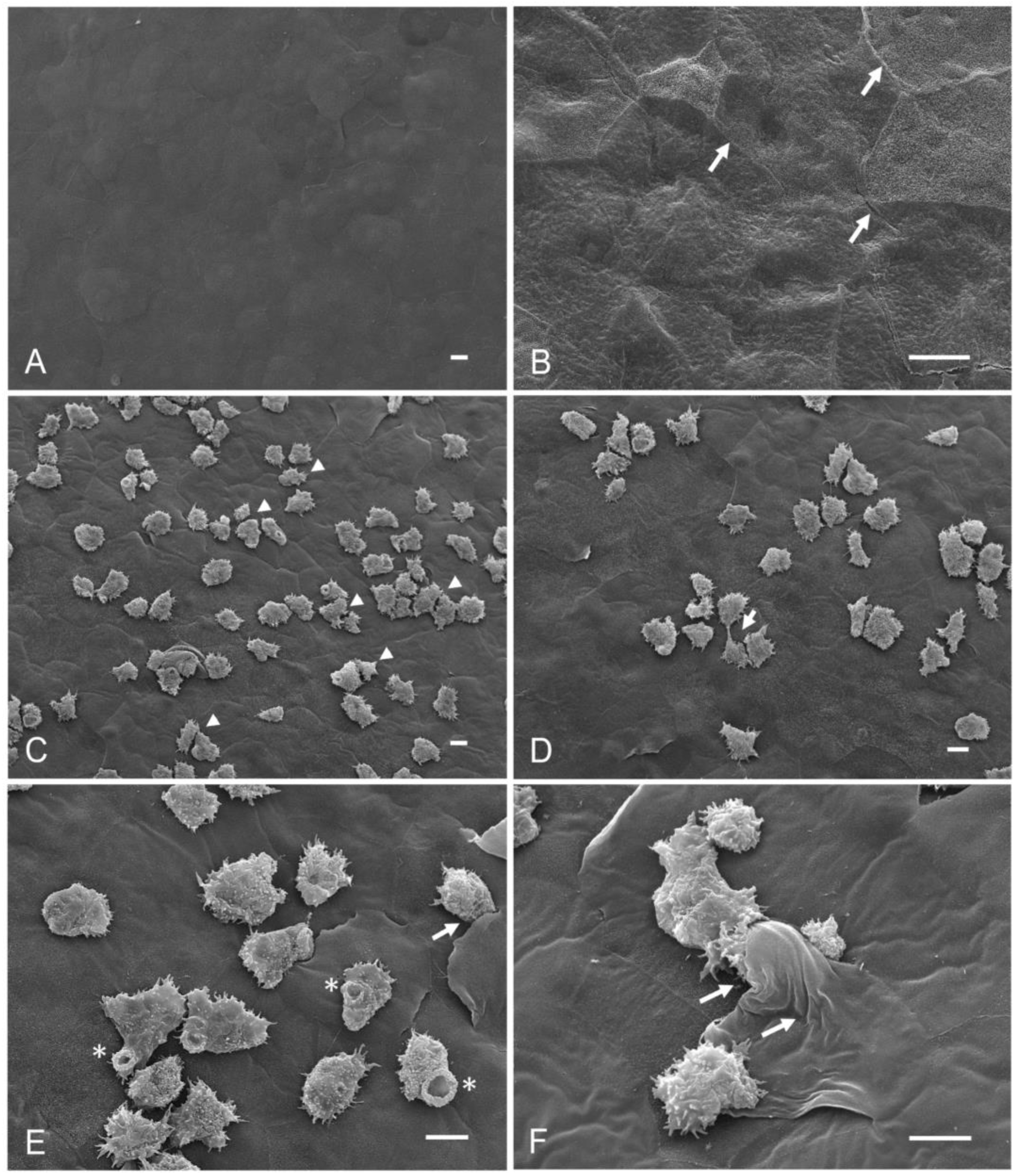
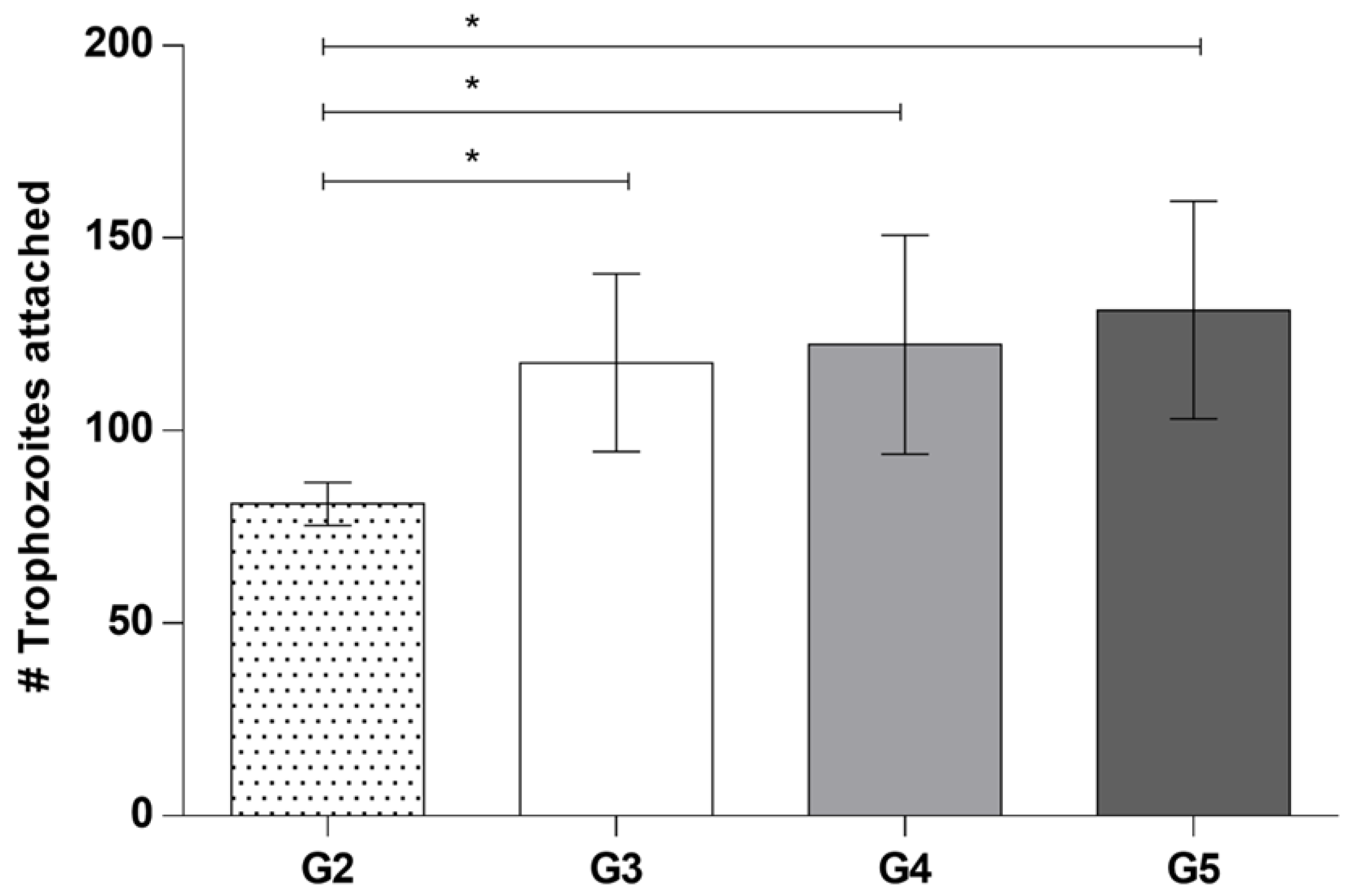
- Group 1: corneas coincubated with amoebic culture medium and taurine dissolved in the medium. Control (−).
- Group 2: corneas coincubated with A. castellanii trophozoites. Control (+).
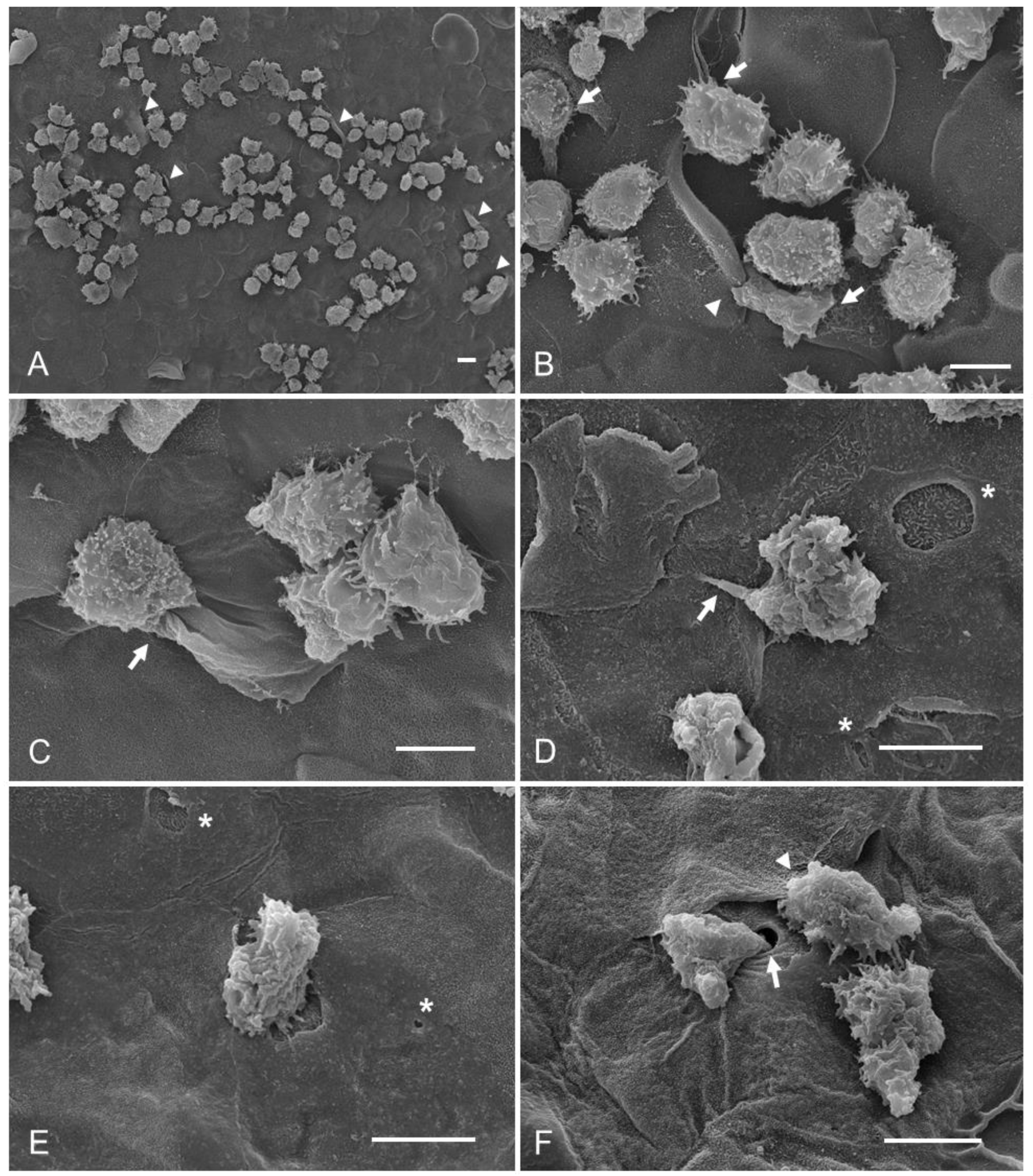
- Group 3: corneas coincubated with taurine interacting 15 min before adding trophozoites of A. castellanii.
- Group 4: A. castellanii trophozoites interacting for 15 min with taurine before placing them on the cornea, in order to determine if taurine is capable of preventing the adhesion of trophozoites to the corneal epithelium.
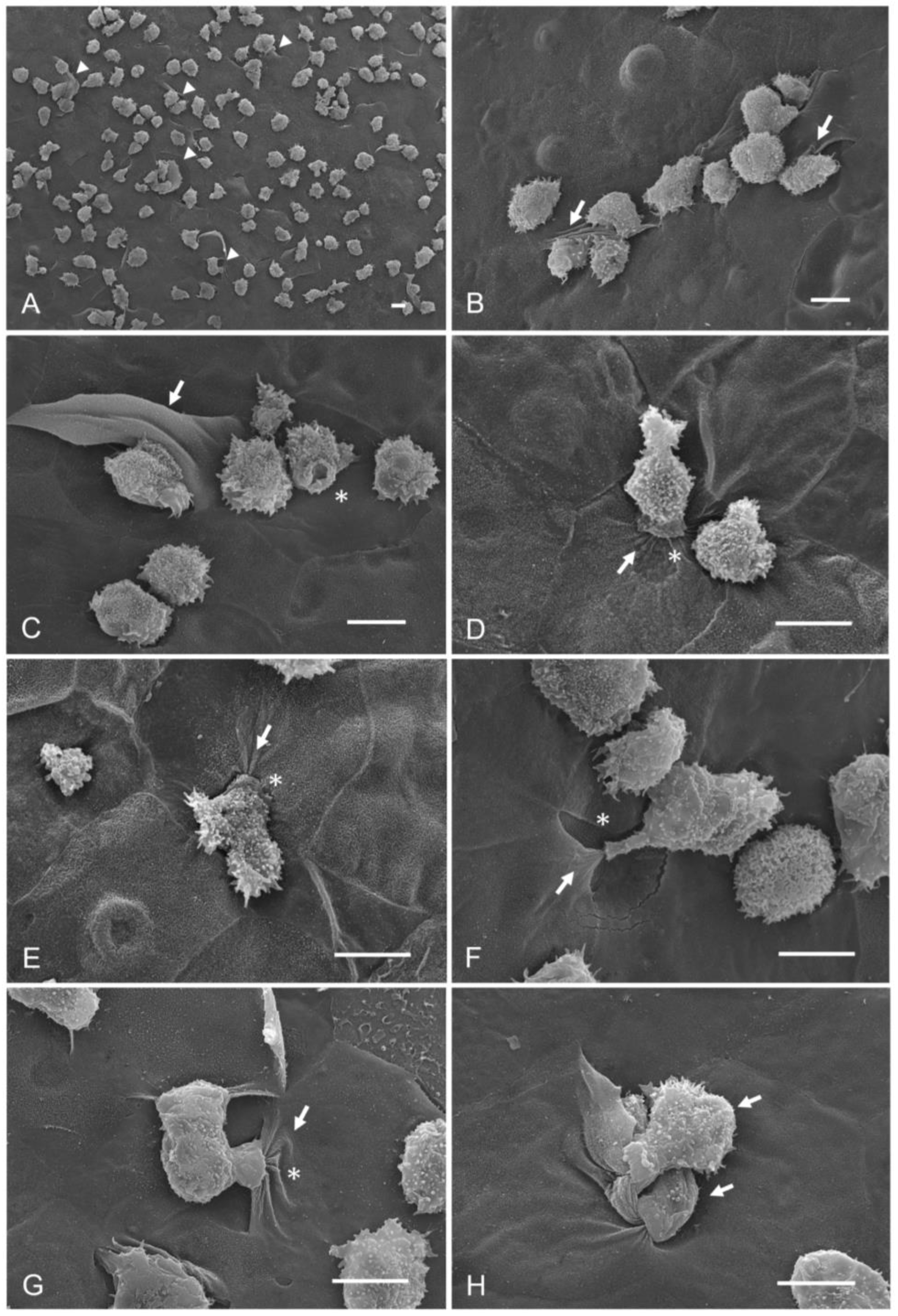
- Group 5: corneas interacting with A. castellanii for 15 min before subsequently adding taurine, to determine if taurine has any effect on trophozoites once they have adhered to the corneal epithelium.
2.3.3. Scanning Electron Microscopy (SEM)
2.4. Quantitative Evaluation of the Interaction of A. castellanii with Taurine in the Ex Vivo Model of Amoebic Keratitis
3. Results
3.1. Reactivation of A. castellanii Virulence
3.2. Qualitative Evaluation of the Interaction of A. castellanii with Taurine in the Ex Vivo Model of Amoebic Keratitis
3.3. Quantitative Evaluation of the Interaction of A. castellanii with Taurine in the Ex Vivo Model of Amoebic Keratitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Kot, K.; Łanocha-Arendarczyk, N.A.; Kosik-Bogacka, D.I. Amoebas from the genus Acanthamoeba and their pathogenic properties. Ann. Parasitol. 2018, 64, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Seal, D. Treatment of Acanthamoeba keratitis. Expert Rev. Anti-Infect. Ther. 2003, 1, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.W.; Niederkorn, J.Y. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol. 2006, 22, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Omaña-Molina, M.; González-Robles, A.; Salazar-Villatoro, L.I.; Lorenzo-Morales, J.; Cristóbal-Ramos, A.R.; Hernández-Ramírez, V.I.; Talamás-Rohana, P.; Cruz, A.R.M.; Martínez-Palomo, A. Reevaluating the role of Acanthamoeba proteases in tissue invasion: Observation of cytopathogenic mechanisms on MDCK cell monolayers and hamster corneal cells. Biomed Res. Int. 2013, 2013, 461329. [Google Scholar] [CrossRef] [PubMed]
- Omaña-Molina, M.; González-Robles, A.; Salazar-Villatoro, L.I.; Cristóbal-Ramos, A.R.; González-Lázaro, M.; Salinas-Moreno, E.; Méndez-Cruz, R.; Sánchez-Cornejo, M.; De la Torre-González, E.; Martínez-Palomo, A. Acanthamoeba castellanii: Morphological analysis of the interaction with human cornea. Exp. Parasitol. 2010, 126, 73–78. [Google Scholar] [CrossRef]
- González-Robles, A.; Castañón, G.; Cristóbal-Ramos, A.R.; Lázaro-Haller, A.; Omaña-Molina, M.; Bonilla, P.; Martínez-Palomo, A. Acanthamoeba castellanii: Structural basis of the cytopathic mechanisms. Exp. Parasitol. 2006, 114, 133–140. [Google Scholar] [CrossRef]
- Omaña-Molina, M.; Navarro-García, F.; González-Robles, A.; Serrano-Luna, J.; Campos-Rodríguez, R.; Martínez-Palomo, A.; Tsutsumi, V.; Shibayama, M. Induction of morphological and electrophysiological changes in hamster cornea after in vitro interaction with trophozoites of Acanthamoeba spp. Infect. Immun. 2004, 72, 3245–3251. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Willcox, M.D. Role of lactoferrin in the tear film. Biochimie 2009, 91, 35–43. [Google Scholar] [CrossRef]
- Knop, E.; Knop, N. The role of eye-associated lymphoid tissue in corneal immune protection. J. Anat. 2005, 206, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Saravanan, C.; Goldstein, M.H.; Wu, H.K.; Pasricha, G.; Sharma, S.; Panjwani, N. Effect of human tears on Acanthamoeba-induced cytopathic effect. Arch. Ophthalmol. 2008, 126, 348–352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marciano-Cabral, F.; Cabral, G. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 2003, 16, 273–307. [Google Scholar] [CrossRef] [PubMed]
- Campos-Rodríguez, R.; Oliver-Aguillón, G.; Vega-Pérez, L.M.; Jarillo-Luna, A.; Hernández-Martínez, D.; Rojas-Hernández, S.; Rodríguez-Monroy, M.A.; Rivera-Aguilar, V.; González-Robles, A. Human IgA inhibits adherence of Acanthamoeba polyphaga to epithelial cells and contact lenses. Can. J. Microbiol. 2004, 50, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Suzuki, C.; Wada, H.; Nomachi, M.; Imayasu, M.; Araki-Sasaki, K. Effects of lactoferrin on the viability and the encystment of Acanthamoeba trophozoites. Biochem. Cell. Biol. 2017, 95, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Alsam, S.; Jeong, S.R.; Dudley, R.; Khan, N.A. Role of human tear fluid in Acanthamoeba interactions with the human corneal epithelial cells. Int. J. Med. Microbiol. 2008, 298, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Nakatsukasa, M.; Sotozono, C.; Shimbo, K.; Ono, N.; Miyano, H.; Okano, A.; Hamuro, J.; Kinoshita, S. Amino Acid profiles in human tear fluids analyzed by high-performance liquid chromatography and electrospray ionization tandem mass spectrometry. Am. J. Ophthalmol. 2011, 151, 799–808.e1. [Google Scholar] [CrossRef]
- Skrovanek, S.; Valenzano, M.C.; Mullin, J.M. Restriction of sulfur-containing amino acids alters claudin composition and improves tight junction barrier function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1046–R1055. [Google Scholar] [CrossRef]
- Ripps, H.; Shen, W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar]
- Bucolo, C.; Fidilio, A.; Platania, C.; Geraci, F.; Lazzara, F.; Drago, F. Antioxidant and Osmoprotecting Activity of Taurine in Dry Eye Models. J. Ocul. Pharmacol. Ther. 2018, 34, 188–194. [Google Scholar] [CrossRef]
- Rusciano, D.; Roszkowska, A.M.; Gagliano, C.; Pezzino, S. Free amino acids: An innovative treatment for ocular surface disease. Eur. J. Pharmacol. 2016, 787, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Talamás-Lara, D.; Lagunes-Guillén, A.; Chávez-Munguía, B.; Salazar-Villatoro, L.; Acosta-Virgen, K.; Omaña-Molina, M.; Espinosa-Cantellano, M.; Martínez-Palomo, A. Acanthamoeba castellanii: Effect of neuroactive substances on trophozoite migration. Exp. Parasitol. 2022, 236–237, 108245. [Google Scholar] [CrossRef] [PubMed]
- Page, F.C. A new key to freshwater and soil Gymnamoebae: With instructions for culture. In Culture Collection of Algae and Protozoa; Freshwater Biological Association: Cumbria, UK, 1988; p. 122. [Google Scholar]
- Omaña-Molina, M.; Vanzzini-Zago, V.; Hernandez-Martinez, D.; Gonzalez-Robles, A.; Salazar-Villatoro, L.; Ramirez-Flores, E.; Oregon-Miranda, E.; Lorenzo-Morales, J.; Martinez-Palomo, A. Acanthamoeba genotypes T3 and T4 as causative agents of amoebic keratitis in Mexico. Parasitol. Res. 2016, 115, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Culbertson, C.G.; Smith, J.W.; Cohen, H.K.; Minner, J.R. Experimental infection of mice and monkeys by Acanthamoeba. Am. J. Pathol. 1959, 35, 185–197. [Google Scholar] [PubMed]
- Mazur, T.; Hadaś, E. The effect of the passages of Acanthamoeba strains through mice tissues on their virulence and its biochemical markers. Parasitol. Res. 1994, 80, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Im, K.I.; Park, K.M.; Yong, T.S.; Hong, Y.P.; Kim, T.E. Upregulated expression of the cDNA fragment possibly related to the virulence of Acanthamoeba culbertsoni. Korean J. Parasitol. 1999, 37, 257–263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, F.; Zhao, W.; Cao, S.; Fung, Y.S. Determination of taurine in human tear fluid by capillary electrophoresis with indirect amperometric detection based on electrogenerated bromine. J. Sep. Sci. 2015, 38, 3271–3278. [Google Scholar] [CrossRef]
- González-Robles, A.; Omaña-Molina, M.; Salazar-Villatoro, L.; Flores-Maldonado, C.; Lorenzo-Morales, J.; Reyes-Batlle, M.; Arnalich-Montiel, F.; Martínez-Palomo, A. Acanthamoeba culbertsoni isolated from a clinical case with intraocular dissemination: Structure and in vitro analysis of the interaction with hamster cornea and MDCK epithelial cell monolayers. Exp. Parasitol. 2017, 183, 245–253. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N.A. Biology and pathogenesis of Acanthamoeba. Parasit. Vectors 2012, 5, 6. [Google Scholar] [CrossRef]
- Chalmers, R.M. Chapter Fourteen-Acanthamoeba. In Microbiology of Waterborne Diseases; Percival, S.L., Yates, M.V., Chalmers, R.M., Gray, N.F., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 263–276. [Google Scholar]
- Kennett, M.J.; Hook, R.R., Jr.; Franklin, C.L.; Riley, L.K. Acanthamoeba castellanii: Characterization of an adhesin molecule. Exp. Parasitol. 1999, 92, 161–169. [Google Scholar] [CrossRef]
- Garate, M.; Cubillos, I.; Marchant, J.; Panjwani, N. Biochemical characterization and functional studies of Acanthamoeba mannose-binding protein. Infect. Immun. 2005, 73, 5775–5781. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.L.; Nordin, A.; Abd Ghafar, N.; Suboh, Y.; Ab Rahim, N.; Chua, K.H. Acanthamoeba-mediated cytopathic effect correlates with MBP and AhLBP mRNA expression. Parasit. Vectors 2017, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Omaña-Molina, M.; Hernández, D.; González-Robles, A.; Salazar-Villatoro, L.; Castañón, G.; Campos-Rodríguez, R.; Bonilla, P. Relationship between adhesion and cytopathic effect of four strains of Acanthamoeba spp. J. Eukaryot. Microbiol. 2006, 53, S18–S19. [Google Scholar] [CrossRef] [PubMed]
- González-Robles, A.; Salazar-Villatoro, L.; Omaña-Molina, M.; Reyes-Batlle, M.; Martín-Navarro, C.M.; Lorenzo-Morales, J. Morphological Features and In Vitro Cytopathic Effect of Acanthamoeba griffini Trophozoites Isolated from a Clinical Case. J. Parasitol. Res. 2014, 2014, 256310. [Google Scholar] [CrossRef]
- Allen, P.G.; Dawidowicz, E.A. Phagocytosis in Acanthamoeba: I. A mannose receptor is responsible for the binding and phagocytosis of yeast. J. Cell. Physiol. 1990, 145, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Schuster, F.L.; Levandowsky, M. Chemosensory responses of Acanthamoeba castellanii: Visual analysis of random movement and responses to chemical signals. J. Eukaryot. Microbiol. 1996, 43, 150–158. [Google Scholar] [CrossRef]
- Pettit, D.A.; Williamson, J.; Cabral, G.A.; Marciano-Cabral, F. In vitro destruction of nerve cell cultures by Acanthamoeba spp.: A transmission and scanning electron microscopy study. J. Parasitol. 1996, 82, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Palomo, A.; González-Robles, A.; Chávez, B.; Orozco, E.; Fernández-Castelo, S.; Cervantes, A. Structural bases of the cytolytic mechanisms of Entamoeba histolytica. J. Protozool. 1985, 32, 166–175. [Google Scholar] [CrossRef]
- Marciano-Cabral, F.M.; Fulford, D.E. Cytopathology of pathogenic and nonpathogenic Naegleria species for cultured rat neuroblastoma cells. Appl. Environ. Microbiol. 1986, 51, 1133–1137. [Google Scholar] [CrossRef]
- Willcox, M.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef]
- Fürnkranz, U.; Nagl, M.; Gottardi, W.; Köhsler, M.; Aspöck, H.; Walochnik, J. Cytotoxic activity of N-chlorotaurine on Acanthamoeba spp. Antimicrob. Agents Chemother. 2008, 52, 470–476. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teuchner, B.; Wibmer, I.D.; Schaumann, P.; Seifarth, C.; Walochnik, J.; Nagl, M. N-chlorotaurine Inactivates Acanthamoeba and Candida albicans in the Porcine Ex Vivo Corneal Infection Model. Cornea 2019, 38, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.; Osuna, A.; Rosales, M.J.; Cifuentes, J.; Mascaró, C. Sucker-like structures in two strains of Acanthamoeba: Scanning electron microscopy study. Int. J. Parasitol. 1991, 21, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol. Rev. 2006, 30, 564–595. [Google Scholar] [CrossRef] [PubMed]
- González-Robles, A.; Salazar-Villatoro, L.; Omaña-Molina, M.; Lorenzo-Morales, J.; Martínez-Palomo, A. Acanthamoeba royreba: Morphological features and in vitro cytopathic effect. Exp. Parasitol. 2013, 133, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Castelan-Ramírez, I.; Salazar-Villatoro, L.; Chávez-Munguía, B.; Salinas-Lara, C.; Sánchez-Garibay, C.; Flores-Maldonado, C.; Hernández-Martínez, D.; Anaya-Martínez, V.; Ávila-Costa, M.R.; Méndez-Cruz, A.R.; et al. Schwann Cell Autophagy and Necrosis as Mechanisms of Cell Death by Acanthamoeba. Pathogens 2020, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Behar, T.N.; Smith, S.V.; Kennedy, R.T.; McKenzie, J.M.; Maric, I.; Barker, J.L. GABA(B) receptors mediate motility signals for migrating embryonic cortical cells. Cereb. Cortex 2001, 11, 744–753. [Google Scholar] [CrossRef]
- Nishiyama, S.; Takahashi, Y.; Yamamoto, K.; Suzuki, D.; Itoh, Y.; Sumita, K.; Uchida, Y.; Homma, M.; Imada, K.; Kawagishi, I. Identification of a Vibrio cholerae chemoreceptor that senses taurine and amino acids as attractants. Sci. Rep. 2016, 6, 20866. [Google Scholar] [CrossRef]
- Worku, M.L.; Karim, Q.N.; Spencer, J.; Sidebotham, R.L. Chemotactic response of Helicobacter pylori to human plasma and bile. J. Med. Microbiol. 2004, 53, 807–811. [Google Scholar] [CrossRef]
- Baig, A.M.; Ahmad, H.R. Correction to: Evidence of a M1-muscarinic GPCR homolog in unicellular eukaryotes: Featuring Acanthamoeba spp. bioinformatics 3D-modelling and experimentations. J. Recept. Signal. Transduct. Res. 2017, 37, 267–275. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar-Villatoro, L.; Chávez-Munguía, B.; Guevara-Estrada, C.E.; Lagunes-Guillén, A.; Hernández-Martínez, D.; Castelan-Ramírez, I.; Omaña-Molina, M. Taurine, a Component of the Tear Film, Exacerbates the Pathogenic Mechanisms of Acanthamoeba castellanii in the Ex Vivo Amoebic Keratitis Model. Pathogens 2023, 12, 1049. https://doi.org/10.3390/pathogens12081049
Salazar-Villatoro L, Chávez-Munguía B, Guevara-Estrada CE, Lagunes-Guillén A, Hernández-Martínez D, Castelan-Ramírez I, Omaña-Molina M. Taurine, a Component of the Tear Film, Exacerbates the Pathogenic Mechanisms of Acanthamoeba castellanii in the Ex Vivo Amoebic Keratitis Model. Pathogens. 2023; 12(8):1049. https://doi.org/10.3390/pathogens12081049
Chicago/Turabian StyleSalazar-Villatoro, Lizbeth, Bibiana Chávez-Munguía, Celia Esther Guevara-Estrada, Anel Lagunes-Guillén, Dolores Hernández-Martínez, Ismael Castelan-Ramírez, and Maritza Omaña-Molina. 2023. "Taurine, a Component of the Tear Film, Exacerbates the Pathogenic Mechanisms of Acanthamoeba castellanii in the Ex Vivo Amoebic Keratitis Model" Pathogens 12, no. 8: 1049. https://doi.org/10.3390/pathogens12081049
APA StyleSalazar-Villatoro, L., Chávez-Munguía, B., Guevara-Estrada, C. E., Lagunes-Guillén, A., Hernández-Martínez, D., Castelan-Ramírez, I., & Omaña-Molina, M. (2023). Taurine, a Component of the Tear Film, Exacerbates the Pathogenic Mechanisms of Acanthamoeba castellanii in the Ex Vivo Amoebic Keratitis Model. Pathogens, 12(8), 1049. https://doi.org/10.3390/pathogens12081049