Exploring Factors Influencing Changes in Incidence and Severity of Multisystem Inflammatory Syndrome in Children
Abstract
:1. Introduction
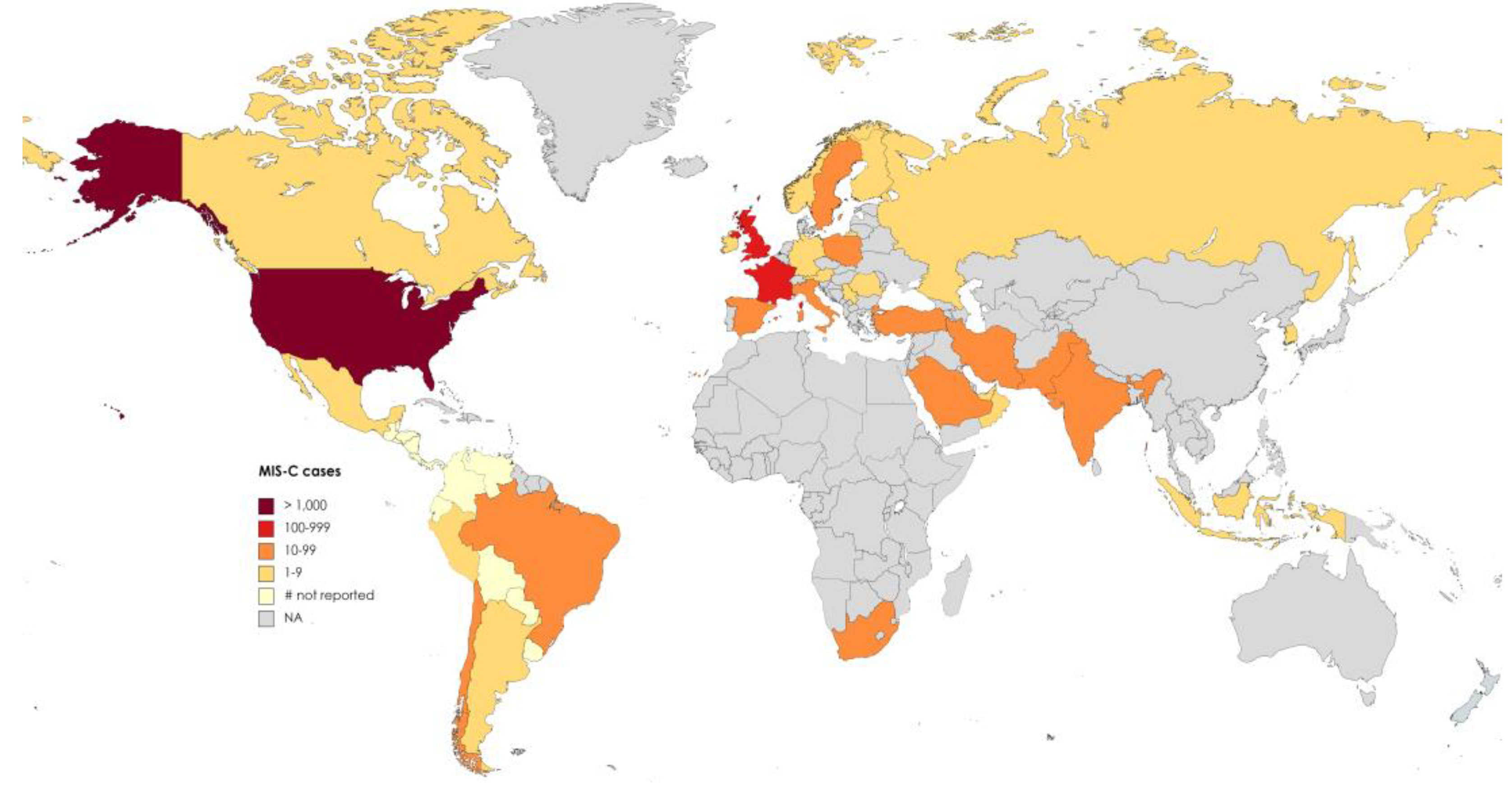
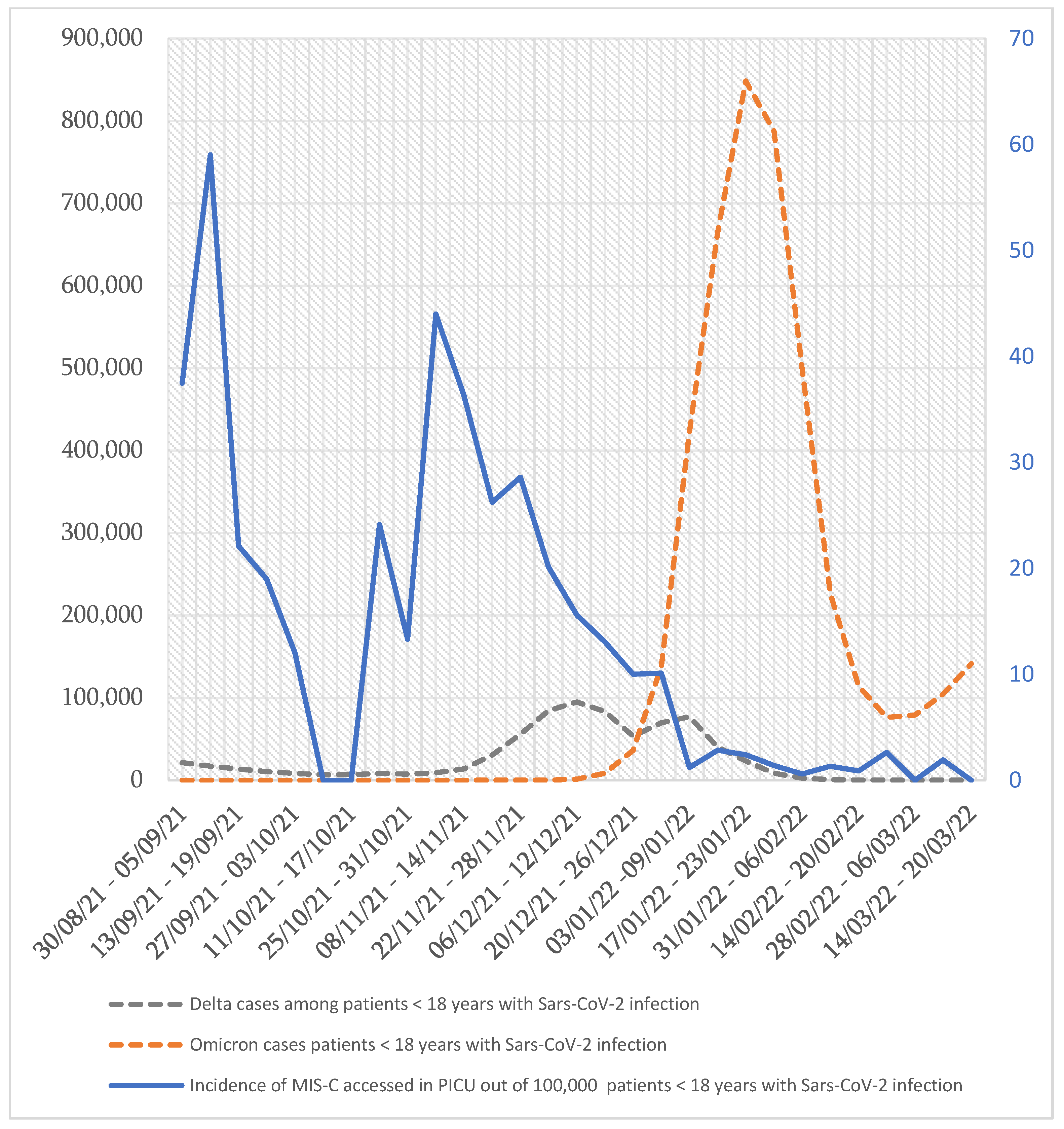
2. Are We Facing a Global Reduction in the Incidence of MIS-C?
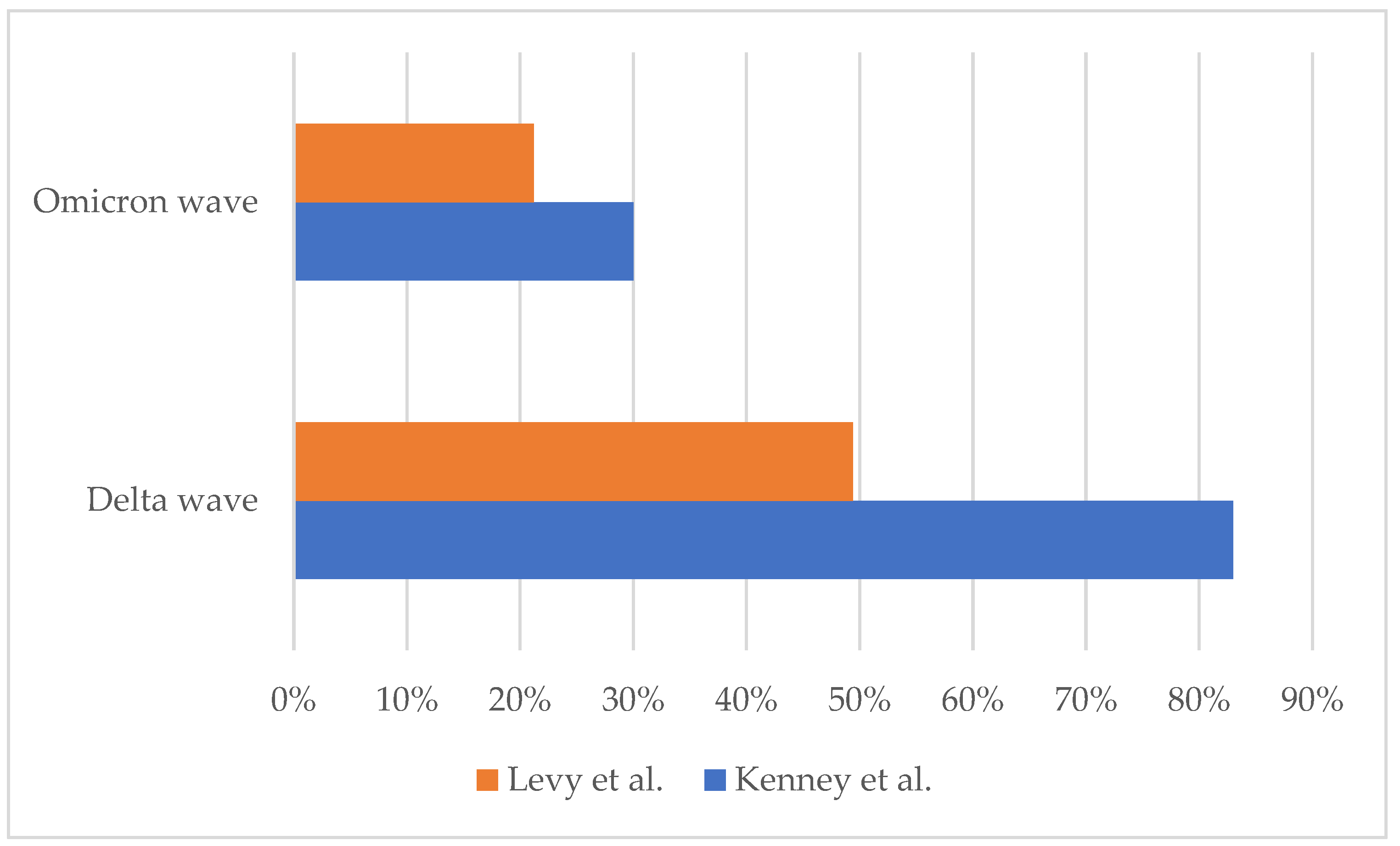
3. Are We Assisting in the Reduction in the Severity of MIS-C?
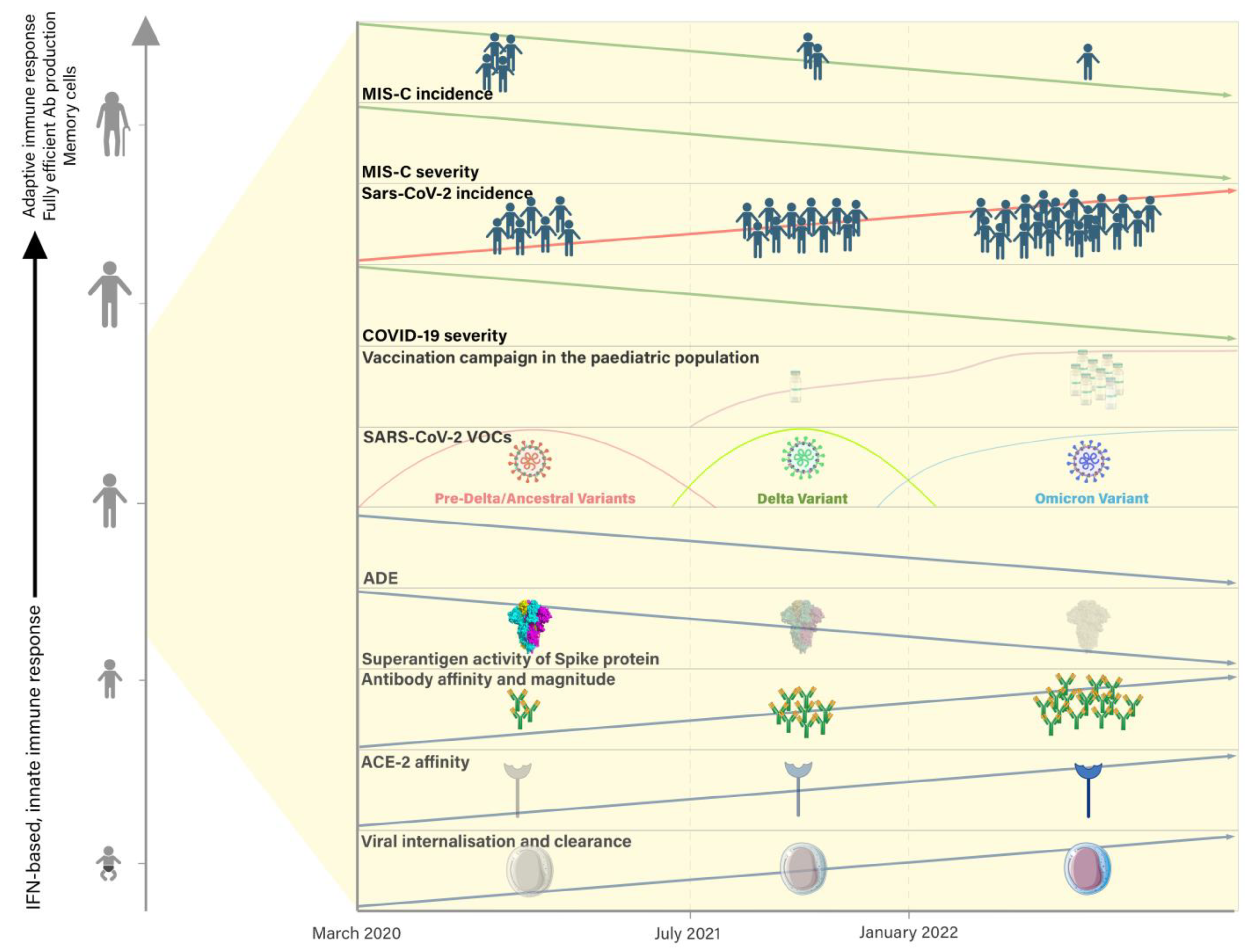
4. What Is Contributing to the Change in MIS-C Features over Time?
4.1. Elements of Pathogenesis of MIS-C
4.1.1. From SARS-CoV-2 Infection to Persistent Antigenemia
4.1.2. From Antigenemia to Altered Antibody Production and Inflammation
4.2. The Role of the Immune System in the Change in MIS-C Epidemiology
4.3. The Role of Variants of SARS-CoV-2 (VOCs) in the Change in MIS-C Epidemiology
4.4. The Role of Vaccination in the Change in MIS-C Epidemiology
4.5. The Role of Therapeutic Novelties in the Change in MIS-C Severity
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- CDC/CSTE Standardized Case Definition for Surveillance of Multisystem Inflammatory Syndrome in Children Associated with SARS-CoV-2 Infection. Available online: https://cdn.ymaws.com/www.cste.org/resource/resmgr/ps/ps2022/22-ID-02_MISC.pdf (accessed on 27 January 2023).
- Multisystem Inflammatory Syndrome in Children and Adolescents with COVID-19. Available online: https://www.who.int/publications-detail-redirect/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed on 29 March 2023).
- Son, M.B.F.; Burns, J.C.; Newburger, J.W. A New Definition for Multisystem Inflammatory Syndrome in Children. Pediatrics 2023, 151, e2022060302. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.T.; Trachtenberg, F.L.; Pearson, G.D.; Dionne, A.; Elias, M.D.; Friedman, K.; Hayes, K.H.; Mahony, L.; McCrindle, B.W.; Oster, M.E.; et al. The NHLBI Study on Long-TerM OUtcomes after the Multisystem Inflammatory Syndrome in Children (MUSIC): Design and Objectives. Am. Heart J. 2022, 243, 43–53. [Google Scholar] [CrossRef]
- Matsubara, D.; Chang, J.; Kauffman, H.L.; Wang, Y.; Nadaraj, S.; Patel, C.; Paridon, S.M.; Fogel, M.A.; Quartermain, M.D.; Banerjee, A. Longitudinal Assessment of Cardiac Outcomes of Multisystem Inflammatory Syndrome in Children Associated With COVID-19 Infections. J. Am. Heart Assoc. 2022, 11, e023251. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Rose, E.B.; Dapul, H.; Soma, V.L.; Maddux, A.B.; Mourani, P.M.; Bowens, C.; et al. Characteristics and Outcomes of US Children and Adolescents with Multisystem Inflammatory Syndrome in Children (MIS-C) Compared with Severe Acute COVID-19. JAMA 2021, 325, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Nakra, N.A.; Blumberg, D.A.; Herrera-Guerra, A.; Lakshminrusimha, S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children 2020, 7, 69. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Porritt, R.A.; Cheng, M.H.; Bahar, I.; Arditi, M. Multisystem Inflammatory Syndrome in Children and Long COVID: The SARS-CoV-2 Viral Superantigen Hypothesis. Front. Immunol. 2022, 13, 941009. [Google Scholar] [CrossRef]
- Lee, D.; Le Pen, J.; Yatim, A.; Dong, B.; Aquino, Y.; Ogishi, M.; Pescarmona, R.; Talouarn, E.; Rinchai, D.; Zhang, P.; et al. Inborn Errors of OAS-RNase L in SARS-CoV-2-Related Multisystem Inflammatory Syndrome in Children. Science 2022, 379, eabo3627. [Google Scholar] [CrossRef]
- Chou, J.; Platt, C.D.; Habiballah, S.; Nguyen, A.A.; Elkins, M.; Weeks, S.; Peters, Z.; Day-Lewis, M.; Novak, T.; Armant, M.; et al. Mechanisms Underlying Genetic Susceptibility to Multisystem Inflammatory Syndrome in Children (MIS-C). J. Allergy Clin. Immunol. 2021, 148, 732–738.e1. [Google Scholar] [CrossRef]
- Neri, I.; Conti, F.; Virdi, A.; Guglielmo, A.; Leonardi, L.; Corsini, I.; Ghizzi, C.; Gabrielli, L.; Lazzarotto, T.; Lanari, M.; et al. Chilblains in a Child with Confirmed SARS-CoV-2 Infection: A Red Flag for Late-onset Skin Manifestation in Previously Infected Individuals. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e357–e359. [Google Scholar] [CrossRef]
- Sancho-Shimizu, V.; Brodin, P.; Cobat, A.; Biggs, C.M.; Toubiana, J.; Lucas, C.L.; Henrickson, S.E.; Belot, A.; MIS-C@CHGE; Tangye, S.G.; et al. SARS-CoV-2-Related MIS-C: A Key to the Viral and Genetic Causes of Kawasaki Disease? J. Exp. Med. 2021, 218, e20210446. [Google Scholar] [CrossRef] [PubMed]
- Bellini, T.; Bustaffa, M.; Caorsi, R.; Tibaldi, J.; Gattorno, M.; Piccotti, E. The Incidence of Multisystem Inflammatory Syndrome in a Paediatric Emergency Department. Acta Paediatr. 2022, 111, 1819–1820. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance (accessed on 5 May 2023).
- Lopez, L.; Burgner, D.; Glover, C.; Carr, J.; Clark, J.; Boast, A.; Vasilunas, N.; McMullan, B.; Francis, J.R.; Bowen, A.C.; et al. Lower Risk of Multi-System Inflammatory Syndrome in Children (MIS-C) with the Omicron Variant. Lancet Reg. Health-West. Pac. 2022, 27, 100604. [Google Scholar] [CrossRef]
- Holm, M.; Espenhain, L.; Glenthøj, J.; Schmidt, L.S.; Nordly, S.B.; Hartling, U.B.; Nygaard, U. Risk and Phenotype of Multisystem Inflammatory Syndrome in Vaccinated and Unvaccinated Danish Children Before and During the Omicron Wave. JAMA Pediatr. 2022, 176, 821–823. [Google Scholar] [CrossRef]
- Sorg, A.L.; Schönfeld, V.; Siedler, A.; Hufnagel, M.; Doenhardt, M.; Diffloth, N.; Berner, R.; V Kries, R.; Armann, J. SARS-CoV-2 Variants and the Risk of Pediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2 among Children in Germany. Infection 2022, 51, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Ptak, K.; Szymońska, I.; Olchawa-Czech, A.; Kukla, K.; Cisowska, M.; Kwinta, P. Comparison of the Course of Multisystem Inflammatory Syndrome in Children during Different Pandemic Waves. Eur. J. Pediatr. 2023, 182, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Levy, N.; Koppel, J.H.; Kaplan, O.; Yechiam, H.; Shahar-Nissan, K.; Cohen, N.K.; Shavit, I. Severity and Incidence of Multisystem Inflammatory Syndrome in Children During 3 SARS-CoV-2 Pandemic Waves in Israel. JAMA 2022, 327, 2452–2454. [Google Scholar] [CrossRef]
- Cohen, J.M.; Carter, M.J.; Cheung, C.R.; Ladhani, S. Evelina Paediatric Inflammatory Multisystem Syndrome Temporally related to SARS-CoV-2 (PIMS-TS) Study Group Lower Risk of Multisystem Inflammatory Syndrome in Children with the Delta and Omicron Variants of Severe Acute Respiratory Syndrome Coronavirus 2. Clin. Infect. Dis. 2023, 76, e518–e521. [Google Scholar] [CrossRef]
- Buonsenso, D.; Perramon, A.; Català, M.; Torres, J.P.; Camacho-Moreno, G.; Rojas-Solano, M.; Ulloa-Gutierrez, R.; Camacho-Badilla, K.; Pérez-Corrales, C.; Cotugno, N.; et al. Multisystem Inflammatory Syndrome in Children in Western Countries? Decreasing Incidence as the Pandemic Progresses?: An Observational Multicenter International Cross-Sectional Study. Pediatr. Infect. Dis. J. 2022, 41, 989–993. [Google Scholar] [CrossRef]
- Chen, J.; Wang, R.; Gilby, N.B.; Wei, G.-W. Omicron Variant (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. J. Chem. Inf. Model. 2022, 62, 412–422. [Google Scholar] [CrossRef]
- Kenney, P.O.; Chang, A.J.; Krabill, L.; Hicar, M.D. Decreased Clinical Severity of Pediatric Acute COVID-19 and MIS-C and Increase of Incidental Cases during the Omicron Wave in Comparison to the Delta Wave. Viruses 2023, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Recher, M.; Leteurtre, S.; Javouhey, E.; Morin, L.; Baudin, F.; Rambaud, J.; Mortamet, G.; Hubert, H.; Angoulvant, F.; Levy, M.; et al. Risk of Admission to the Pediatric Intensive Care Unit for SARS-CoV-2 Delta and Omicron Infections. J. Pediatr. Infect. Dis. Soc. 2023, 12, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.R.; Butters, C.; Abdulbari Yunis, N.; Lishman, J.; Scott, C.; van der Zalm, M.M.; Zühlke, L.; Rabie, H.; Webb, K. The Impact of SARS-CoV-2 Variants on the Clinical Phenotype and Severity of Multisystem Inflammatory Syndrome in Children in South Africa. Pediatr. Infect. Dis. J. 2022, 41, e510–e512. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Newhams, M.M.; Olson, S.M.; Halasa, N.B.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Kamidani, S.; Tarquinio, K.M.; Maddux, A.B.; et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) MRNA Vaccination Against Multisystem Inflammatory Syndrome in Children Among Persons Aged 12–18 Years—United States, July–December 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 52–58. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn Errors of Type I IFN Immunity in Patients with Life-Threatening COVID-19. Science 2020, 370, eabd4570. [Google Scholar] [CrossRef]
- Sacco, K.; Castagnoli, R.; Vakkilainen, S.; Liu, C.; Delmonte, O.M.; Oguz, C.; Kaplan, I.M.; Alehashemi, S.; Burbelo, P.D.; Bhuyan, F.; et al. Immunopathological Signatures in Multisystem Inflammatory Syndrome in Children and Pediatric COVID-19. Nat. Med. 2022, 28, 1050–1062. [Google Scholar] [CrossRef]
- Vella, L.A.; Rowley, A.H. Current Insights into the Pathophysiology of Multisystem Inflammatory Syndrome in Children. Curr. Pediatr. Rep. 2021, 9, 83–92. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the Immune System in Humans from Infancy to Old Age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef]
- de Bree, L.C.J.; Koeken, V.A.C.M.; Joosten, L.A.B.; Aaby, P.; Benn, C.S.; van Crevel, R.; Netea, M.G. Non-Specific Effects of Vaccines: Current Evidence and Potential Implications. Semin. Immunol. 2018, 39, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.; Greve-Isdahl, M.; Bøås, H.; Suren, P.; Buanes, E.A.; Veneti, L. COVID-19 Hospitalization Among Children <18 Years by Variant Wave in Norway. Pediatrics 2022, 150, e2022057564. [Google Scholar] [CrossRef]
- Meyts, I.; Bucciol, G.; Quinti, I.; Neven, B.; Fischer, A.; Seoane, E.; Lopez-Granados, E.; Gianelli, C.; Robles-Marhuenda, A.; Jeandel, P.-Y.; et al. Coronavirus Disease 2019 in Patients with Inborn Errors of Immunity: An International Study. J. Allergy Clin. Immunol. 2021, 147, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Giardino, G.; Milito, C.; Lougaris, V.; Punziano, A.; Carrabba, M.; Cinetto, F.; Scarpa, R.; Dellepiane, R.M.; Ricci, S.; Rivalta, B.; et al. The Impact of SARS-CoV-2 Infection in Patients with Inborn Errors of Immunity: The Experience of the Italian Primary Immunodeficiencies Network (IPINet). J. Clin. Immunol. 2022, 42, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Pacillo, L.; Amodio, D.; Rivalta, B.; Moratti, M.; Campoli, C.; Zama, D.; Corsini, I.; Giancotta, C.; Bernardi, S.; et al. SARS-CoV-2 Infection and Treatment in a Cohort of Patients with Inborn Errors of Immunity. Pediatr. Allergy Immunol. 2022, 33, e13833. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.N.; Patel, R.S.; Trachtman, R.; Lepow, L.; Amanat, F.; Krammer, F.; Wilson, K.M.; Onel, K.; Geanon, D.; Tuballes, K.; et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 2020, 183, 982–995.e14. [Google Scholar] [CrossRef]
- Gartlan, C.; Tipton, T.; Salguero, F.J.; Sattentau, Q.; Gorringe, A.; Carroll, M.W. Vaccine-Associated Enhanced Disease and Pathogenic Human Coronaviruses. Front. Immunol. 2022, 13, 882972. [Google Scholar] [CrossRef]
- Junqueira, C.; Crespo, Â.; Ranjbar, S.; de Lacerda, L.B.; Lewandrowski, M.; Ingber, J.; Parry, B.; Ravid, S.; Clark, S.; Schrimpf, M.R.; et al. FcγR-Mediated SARS-CoV-2 Infection of Monocytes Activates Inflammation. Nature 2022, 606, 576–584. [Google Scholar] [CrossRef]
- Menni, C.; Valdes, A.M.; Polidori, L.; Antonelli, M.; Penamakuri, S.; Nogal, A.; Louca, P.; May, A.; Figueiredo, J.C.; Hu, C.; et al. Symptom Prevalence, Duration, and Risk of Hospital Admission in Individuals Infected with SARS-CoV-2 during Periods of Omicron and Delta Variant Dominance: A Prospective Observational Study from the ZOE COVID Study. Lancet 2022, 399, 1618–1624. [Google Scholar] [CrossRef]
- Post, N.; Eddy, D.; Huntley, C.; van Schalkwyk, M.C.I.; Shrotri, M.; Leeman, D.; Rigby, S.; Williams, S.V.; Bermingham, W.H.; Kellam, P.; et al. Antibody Response to SARS-CoV-2 Infection in Humans: A Systematic Review. PLoS ONE 2020, 15, e0244126. [Google Scholar] [CrossRef]
- Dunay, G.A.; Barroso, M.; Woidy, M.; Danecka, M.K.; Engels, G.; Hermann, K.; Neumann, F.S.; Paul, K.; Beime, J.; Escherich, G.; et al. Long-Term Antibody Response to SARS-CoV-2 in Children. J. Clin. Immunol. 2023, 43, 46–56. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, J.; Mangalam, A.K.; Channappanavar, R.; Fett, C.; Meyerholz, D.K.; Agnihothram, S.; Baric, R.S.; David, C.S.; Perlman, S. Airway Memory CD4(+) T Cells Mediate Protective Immunity against Emerging Respiratory Coronaviruses. Immunity 2016, 44, 1379–1391. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yin, L.; Patel, J.; Tang, L.; Huang, Y. The Inflammatory Markers of Multisystem Inflammatory Syndrome in Children (MIS-C) and Adolescents Associated with COVID-19: A Meta-Analysis. J. Med. Virol. 2021, 93, 4358–4369. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Okarska-Napierała, M.; Mańdziuk, J.; Feleszko, W.; Stelmaszczyk-Emmel, A.; Panczyk, M.; Demkow, U.; Kuchar, E. Recurrent Assessment of Lymphocyte Subsets in 32 Patients with Multisystem Inflammatory Syndrome in Children (MIS-C). Pediatr. Allergy Immunol. 2021, 32, 1857–1865. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Wang, X.-M.; Xing, X.; Xu, Z.; Zhang, C.; Song, J.-W.; Fan, X.; Xia, P.; Fu, J.-L.; Wang, S.-Y.; et al. Single-Cell Landscape of Immunological Responses in Patients with COVID-19. Nat. Immunol. 2020, 21, 1107–1118. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; COVID-19 Genomics UK (COG-UK) Consortium; et al. SARS-CoV-2 Variants, Spike Mutations and Immune Escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Edridge, A.W.D.; Kaczorowska, J.; Hoste, A.C.R.; Bakker, M.; Klein, M.; Loens, K.; Jebbink, M.F.; Matser, A.; Kinsella, C.M.; Rueda, P.; et al. Seasonal Coronavirus Protective Immunity Is Short-Lasting. Nat. Med. 2020, 26, 1691–1693. [Google Scholar] [CrossRef] [PubMed]
- Vanshylla, K.; Di Cristanziano, V.; Kleipass, F.; Dewald, F.; Schommers, P.; Gieselmann, L.; Gruell, H.; Schlotz, M.; Ercanoglu, M.S.; Stumpf, R.; et al. Kinetics and Correlates of the Neutralizing Antibody Response to SARS-CoV-2 Infection in Humans. Cell Host Microbe 2021, 29, 917–929.e4. [Google Scholar] [CrossRef]
- Dowell, A.C.; Butler, M.S.; Jinks, E.; Tut, G.; Lancaster, T.; Sylla, P.; Begum, J.; Bruton, R.; Pearce, H.; Verma, K.; et al. Children Develop Robust and Sustained Cross-Reactive Spike-Specific Immune Responses to SARS-CoV-2 Infection. Nat. Immunol. 2022, 23, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Adaptive Immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Cross-Reactive CD4+ T Cells Enhance SARS-CoV-2 Immune Responses upon Infection and Vaccination. Available online: https://pubmed.ncbi.nlm.nih.gov/34465633/ (accessed on 6 May 2023).
- Newell, K.L.; Clemmer, D.C.; Cox, J.B.; Kayode, Y.I.; Zoccoli-Rodriguez, V.; Taylor, H.E.; Endy, T.P.; Wilmore, J.R.; Winslow, G.M. Switched and Unswitched Memory B Cells Detected during SARS-CoV-2 Convalescence Correlate with Limited Symptom Duration. PLoS ONE 2021, 16, e0244855. [Google Scholar] [CrossRef]
- Soysal, A.; Topçu, B.; Atıcı, S.; Gönüllü, E.; Öner, C.N. MIS-C Patients Who Were Reinfected with SARS-CoV-2. Report of 2 Cases. Arch. Argent. Pediatr. 2023, e202202893. [Google Scholar] [CrossRef]
- Buddingh, E.P.; Vossen, A.C.T.M.; Lamb, H.J.; van der Palen, R.L.F.; Brinkman, D.M.C. Reinfection with Severe Acute Respiratory Syndrome Coronavirus 2 Without Recurrence of Multisystem Inflammatory Syndrome in Children. Pediatr. Infect. Dis. J. 2021, 40, e491–e492. [Google Scholar] [CrossRef] [PubMed]
- Porritt, R.A.; Paschold, L.; Rivas, M.N.; Cheng, M.H.; Yonker, L.M.; Chandnani, H.; Lopez, M.; Simnica, D.; Schultheiß, C.; Santiskulvong, C.; et al. HLA Class I-Associated Expansion of TRBV11-2 T Cells in Multisystem Inflammatory Syndrome in Children. J. Clin. Investig. 2021, 131, e146614. [Google Scholar] [CrossRef]
- Amormino, C.; Tedeschi, V.; Paldino, G.; Arcieri, S.; Fiorillo, M.T.; Paiardini, A.; Tuosto, L.; Kunkl, M. SARS-CoV-2 Spike Does Not Possess Intrinsic Superantigen-like Inflammatory Activity. Cells 2022, 11, 2526. [Google Scholar] [CrossRef]
- Burns, J.C. MIS-C: Myths Have Been Debunked, but Mysteries Remain. Nat. Rev. Rheumatol. 2023, 19, 70–71. [Google Scholar] [CrossRef]
- Gazali, F.M.; Wijayanti, N.; Hakim, M.S.; Supriyati, E.; Arguni, E.; Daniwijaya, M.E.W.; Nuryastuti, T.; Nuhamunada, M.; Nabilla, R.; Haryana, S.M.; et al. The High Mutation Rate at the D614G Hotspot-Furin Cleavage Site Region Increases the Priming Efficiency of the Spike Protein by Furin Protease: Analysis of Indonesian SARS-CoV-2 G614 Variants Obtained during the Early COVID-19 Pandemic. Virusdisease 2023, 34, 321–330. [Google Scholar] [CrossRef]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta Variant of SARS-CoV-2: A Comparative Computational Study of Spike Protein. J. Med. Virol. 2022, 94, 1641–1649. [Google Scholar] [CrossRef]
- Jain, M.; Patil, N.; Gor, D.; Sharma, M.K.; Goel, N.; Kaushik, P. Proteomic Approach for Comparative Analysis of the Spike Protein of SARS-CoV-2 Omicron (B.1.1.529) Variant and Other Pango Lineages. Proteomes 2022, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Yin, J.; Zhang, M.; Li, J.; Wu, Y.; Chen, J.; Miao, H. Study on the Clinical Significance of ACE2 and Its Age-Related Expression. J. Inflamm. Res. 2021, 14, 2873–2882. [Google Scholar] [CrossRef] [PubMed]
- Pawłowski, P.H. Additional Positive Electric Residues in the Crucial Spike Glycoprotein S Regions of the New SARS-CoV-2 Variants. Infect. Drug Resist. 2021, 14, 5099–5105. [Google Scholar] [CrossRef]
- Pawłowski, P.H.; Institute of Biochemistry and Biophysics; Polish Academy of Sciences, Warszawa, Poland. Charged Amino Acids May Promote Coronavirus SARS-CoV-2 Fusion with the Host Cell. AIMS Biophys. 2021, 8, 111–121. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef] [PubMed]
- Suryadevara, N.; Shiakolas, A.R.; VanBlargan, L.A.; Binshtein, E.; Chen, R.E.; Case, J.B.; Kramer, K.J.; Armstrong, E.C.; Myers, L.; Trivette, A.; et al. An Antibody Targeting the N-Terminal Domain of SARS-CoV-2 Disrupts the Spike Trimer. J. Clin. Investig. 2022, 132, e159062. [Google Scholar] [CrossRef] [PubMed]
- McCallum, M.; De Marco, A.; Lempp, F.A.; Tortorici, M.A.; Pinto, D.; Walls, A.C.; Beltramello, M.; Chen, A.; Liu, Z.; Zatta, F.; et al. N-Terminal Domain Antigenic Mapping Reveals a Site of Vulnerability for SARS-CoV-2. Cell 2021, 184, 2332–2347.e16. [Google Scholar] [CrossRef]
- Overview of the Implementation of COVID-19 Vaccination Strategies and Deployment Plans in the EU/EEA. Available online: https://www.ecdc.europa.eu/en/publications-data/overview-implementation-covid-19-vaccination-strategies-anddeployment-plans (accessed on 9 July 2023).
- Yousaf, A.R.; Cortese, M.M.; Taylor, A.W.; Broder, K.R.; Oster, M.E.; Wong, J.M.; Guh, A.Y.; McCormick, D.W.; Kamidani, S.; Schlaudecker, E.P.; et al. Reported Cases of Multisystem Inflammatory Syndrome in Children Aged 12–20 Years in the USA Who Received a COVID-19 Vaccine, December, 2020, through August, 2021: A Surveillance Investigation. Lancet Child Adolesc. Health 2022, 6, 303–312. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of Antibody Immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Varnaitė, R.; García, M.; Glans, H.; Maleki, K.T.; Sandberg, J.T.; Tynell, J.; Christ, W.; Lagerqvist, N.; Asgeirsson, H.; Ljunggren, H.-G.; et al. Expansion of SARS-CoV-2-Specific Antibody-Secreting Cells and Generation of Neutralizing Antibodies in Hospitalized COVID-19 Patients. J. Immunol. 2020, 205, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Su, B.; Guo, X.; Sun, W.; Deng, Y.; Bao, L.; Zhu, Q.; Zhang, X.; Zheng, Y.; Geng, C.; et al. Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients’ B Cells. Cell 2020, 182, 73–84.e16. [Google Scholar] [CrossRef]
- Jiang, Q.; Cao, Y.; Ruan, J.W.; Hu, P. A Comparative Immune Response to COVID-19 Vaccination between Children and Adults. Influ. Other Respir. Viruses 2023, 17, e13070. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Harahsheh, A.S.; Handoko, R.; Raghuveer, G.; Portman, M.A.; Khoury, M.; Newburger, J.W.; Lee, S.; Jain, S.S.; Khare, M.; et al. SARS-CoV-2 Variants and Multisystem Inflammatory Syndrome in Children. N. Engl. J. Med. 2023, 388, 1624–1626. [Google Scholar] [CrossRef]
- Chaudhary, J.K.; Yadav, R.; Chaudhary, P.K.; Maurya, A.; Kant, N.; Rugaie, O.A.; Haokip, H.R.; Yadav, D.; Roshan, R.; Prasad, R.; et al. Insights into COVID-19 Vaccine Development Based on Immunogenic Structural Proteins of SARS-CoV-2, Host Immune Responses, and Herd Immunity. Cells 2021, 10, 2949. [Google Scholar] [CrossRef]
- Castro Dopico, X.; Ols, S.; Loré, K.; Karlsson Hedestam, G.B. Immunity to SARS-CoV-2 Induced by Infection or Vaccination. J. Intern. Med. 2022, 291, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of Myocarditis, Pericarditis, and Cardiac Arrhythmias Associated with COVID-19 Vaccination or SARS-CoV-2 Infection. Nat. Med. 2022, 28, 410–422. [Google Scholar] [CrossRef]
- Yasuhara, J.; Masuda, K.; Aikawa, T.; Shirasu, T.; Takagi, H.; Lee, S.; Kuno, T. Myopericarditis After COVID-19 MRNA Vaccination Among Adolescents and Young Adults: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2023, 177, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, L.D.; Newhams, M.M.; Olson, S.M.; Halasa, N.B.; Price, A.M.; Orzel, A.O.; Young, C.C.; Boom, J.A.; Sahni, L.C.; Maddux, A.B.; et al. BNT162b2 MRNA Vaccination Against Coronavirus Disease 2019 Is Associated with a Decreased Likelihood of Multisystem Inflammatory Syndrome in Children Aged 5-18 Years-United States, July 2021–April 2022. Clin. Infect. Dis. 2023, 76, e90–e100. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.R.; Majumdar, S.N. Condensation Transition in Large Deviations of Self-Similar Gaussian Processes with Stochastic Resetting. J. Stat. Mech. 2022, 2022, 053212. [Google Scholar] [CrossRef]
- Stojkoski, V.; Sandev, T.; Kocarev, L.; Pal, A. Geometric Brownian Motion under Stochastic Resetting: A Stationary yet Nonergodic Process. Phys. Rev. E 2021, 104, 014121. [Google Scholar] [CrossRef] [PubMed]
- Vinod, D.; Cherstvy, A.G.; Wang, W.; Metzler, R.; Sokolov, I.M. Nonergodicity of Reset Geometric Brownian Motion. Phys. Rev. E 2022, 105, L012106. [Google Scholar] [CrossRef]
- Abrams, J.Y.; Belay, E.D.; Godfred-Cato, S.; Campbell, A.P.; Zambrano, L.D.; Kunkel, A.; Miller, A.D.; Wu, M.J.; Meng, L.; Shah, A.B.; et al. Trends in Treatments for Multisystem Inflammatory Syndrome in Children (MIS-C), United States, February 2020–July 2021. Clin. Infect. Dis. 2022, 75, 1201–1209. [Google Scholar] [CrossRef]
- Mahmoud, S.; El-Kalliny, M.; Kotby, A.; El-Ganzoury, M.; Fouda, E.; Ibrahim, H. Treatment of MIS-C in Children and Adolescents. Curr. Pediatr. Rep. 2022, 10, 1–10. [Google Scholar] [CrossRef]
- Whitworth, H.; Sartain, S.E.; Kumar, R.; Armstrong, K.; Ballester, L.; Betensky, M.; Cohen, C.T.; Diaz, R.; Diorio, C.; Goldenberg, N.A.; et al. Rate of Thrombosis in Children and Adolescents Hospitalized with COVID-19 or MIS-C. Blood 2021, 138, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.H.; Wood, K.E.; Burghardt, E.L.; Koestner, B.P.; Wendt, L.H.; Badheka, A.V.; Sharathkumar, A.A. Thromboprophylaxis for Children Hospitalized with COVID-19 and MIS-C. Res. Pract. Thromb. Haemost. 2022, 6, e12780. [Google Scholar] [CrossRef] [PubMed]
- Guner Ozenen, G.; Akaslan Kara, A.; Boncuoglu, E.; Kiymet, E.; Cem, E.; Sahinkaya, S.; Yilmaz Celebi, M.; Gulderen, M.; Kacar, P.; Uras, M.; et al. Evaluation of Antithrombotic Prophylaxis and Thrombotic Events in Children with COVID-19 or MIS-C: A Tertiary Pediatric Center Experience. Arch. Pediatr. 2023, 30, 172–178. [Google Scholar] [CrossRef] [PubMed]
| SARS-COV-2 Wave in Relation to the Variant | |||
|---|---|---|---|
| Pre-Delta Wave | Delta Wave | Omicron Wave | |
| USA [15] | 16/100.000 | 7/100.000 | 3/100.000 |
| Australia [16] | 1.3/100.000 | 0.5/100.000 | 0.08/100.000 |
| Denmark [17] | 25/100.000 | 28/100.000 | 2/100.000 |
| Germany [18] | 62/100.000 | 17/100.000 | 3/100.000 |
| Israel [20] | 54.5/100.000 | 49.2/100.000 | 3.8/100.000 |
| England [21] | 231/100.000 | 79–102/100.000 | 12/100.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castaldo, P.; d’Alanno, G.; Biserni, G.B.; Moratti, M.; Conti, F.; Fabi, M.; Lanari, M. Exploring Factors Influencing Changes in Incidence and Severity of Multisystem Inflammatory Syndrome in Children. Pathogens 2023, 12, 997. https://doi.org/10.3390/pathogens12080997
Castaldo P, d’Alanno G, Biserni GB, Moratti M, Conti F, Fabi M, Lanari M. Exploring Factors Influencing Changes in Incidence and Severity of Multisystem Inflammatory Syndrome in Children. Pathogens. 2023; 12(8):997. https://doi.org/10.3390/pathogens12080997
Chicago/Turabian StyleCastaldo, Pasquale, Gabriele d’Alanno, Giovanni Battista Biserni, Mattia Moratti, Francesca Conti, Marianna Fabi, and Marcello Lanari. 2023. "Exploring Factors Influencing Changes in Incidence and Severity of Multisystem Inflammatory Syndrome in Children" Pathogens 12, no. 8: 997. https://doi.org/10.3390/pathogens12080997
APA StyleCastaldo, P., d’Alanno, G., Biserni, G. B., Moratti, M., Conti, F., Fabi, M., & Lanari, M. (2023). Exploring Factors Influencing Changes in Incidence and Severity of Multisystem Inflammatory Syndrome in Children. Pathogens, 12(8), 997. https://doi.org/10.3390/pathogens12080997








