Resistance Profiles and Virulence Determinants in Biofilm-Forming Enterococcus faecium Isolated from Raw Seafood in Bangladesh
Abstract
:1. Introduction
2. Materials and Methods
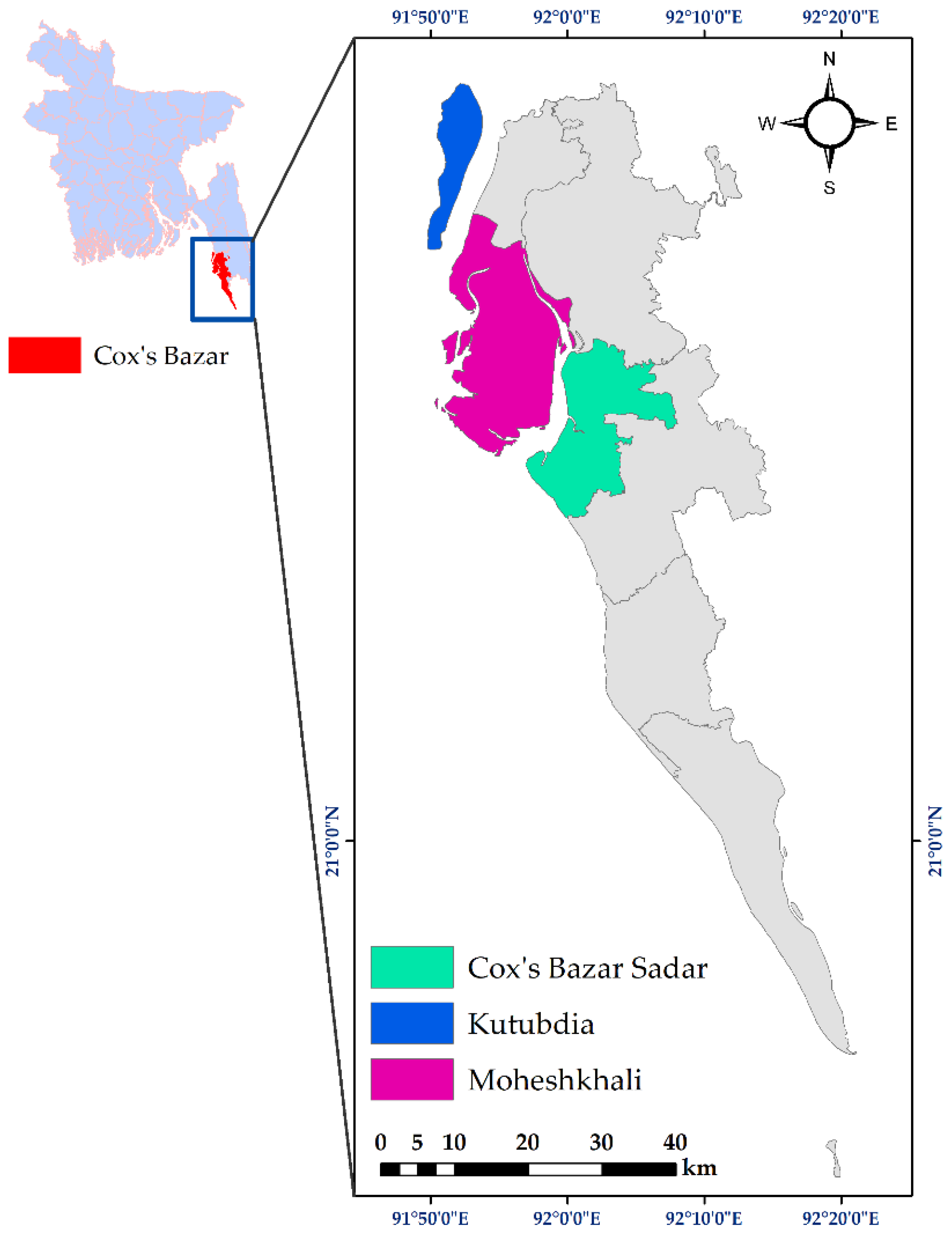
2.1. Sampling Sites
2.2. Sample Collection and Processing
2.3. Isolation and Molecular Detection of Enterococcus faecium
2.4. Determination of Biofilm-Forming Abilities of Enterococcus faecium
2.5. Molecular Detection of Virulence Genes in Enterococcus faecium
2.6. Antibiotic Susceptibility Test
2.7. Statistical Analysis
3. Results
3.1. Prevalence of Enterococcus faecium
3.2. Biofilm-Forming Ability of Isolated Enterococcus faecium
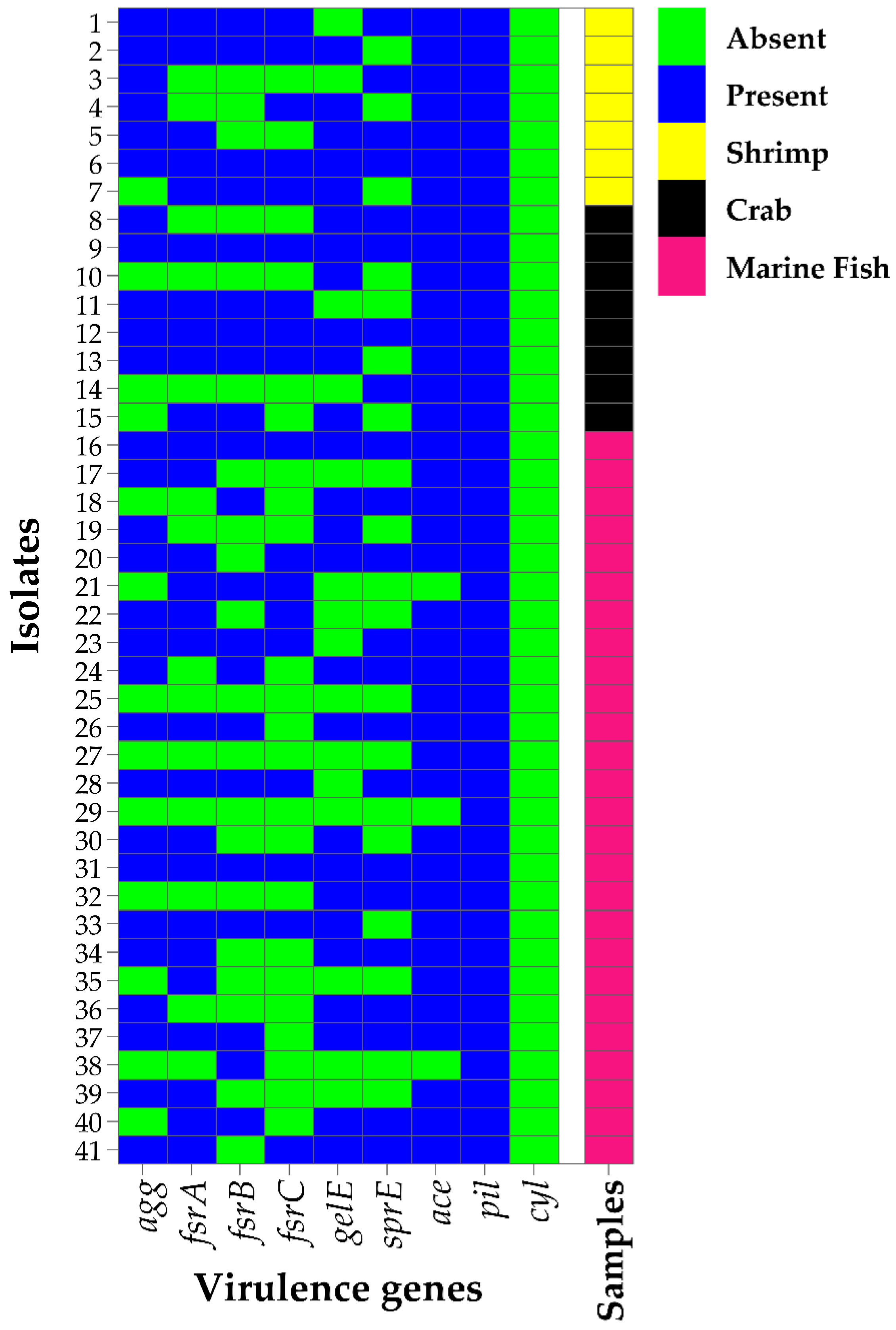
3.3. Prevalence of Virulence Factors in Biofilm-Forming Enterococcus faecium
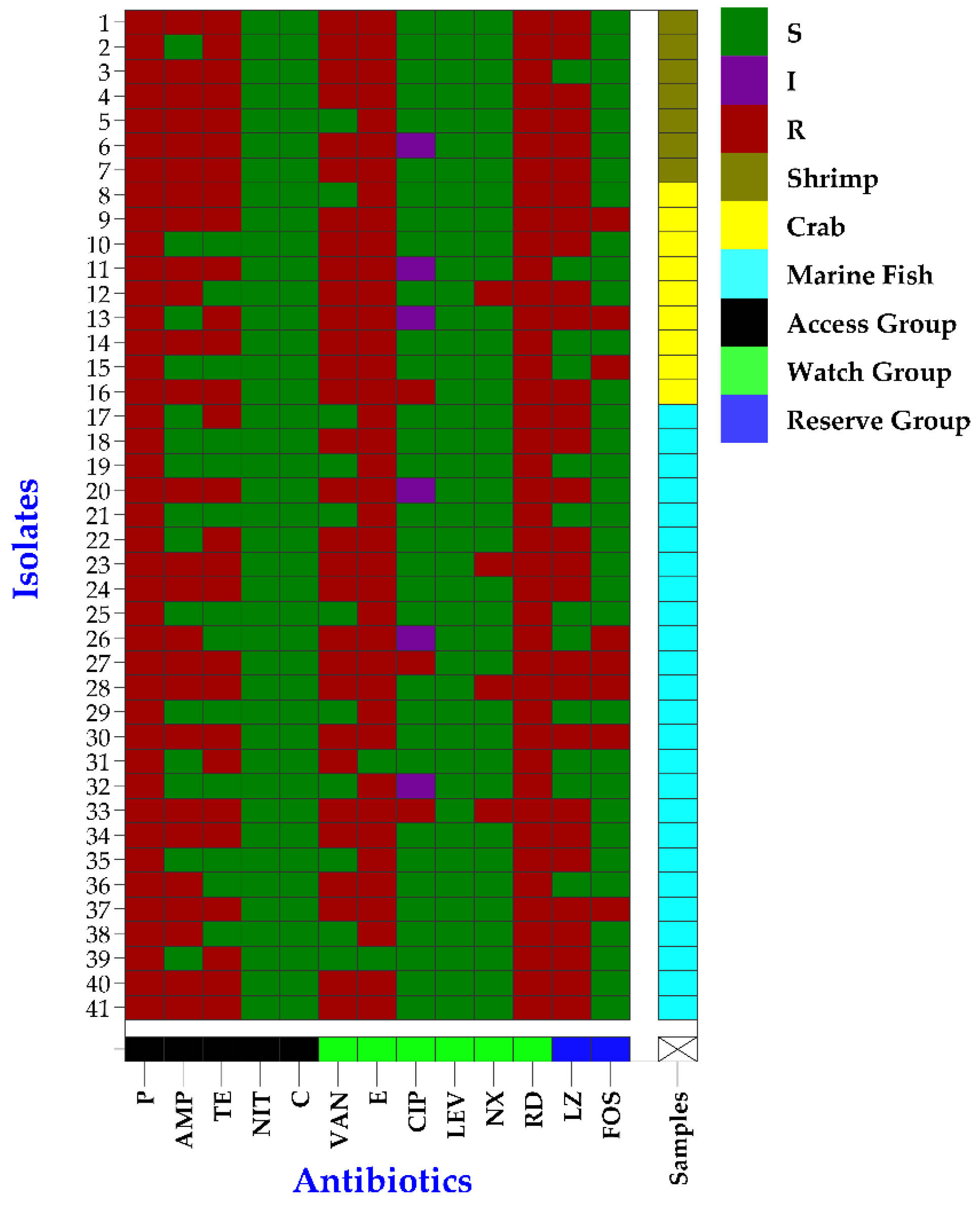
3.4. Antibiogram Profiles of Biofilm-Forming Enterococcus faecium
3.5. Phenotypic MDR and MAR Nature in Biofilm-Forming Enterococcus faecium
4. Discussion
4.1. Enterococcus faecium Isolated from Seafood
4.2. Biofilm Formation of Enterococcus faecium Isolated from Seafood
4.3. Virulence Profiles of Enterococcus faecium Isolated from Seafood
4.4. Antibiotic Resistance Profiles of Enterococcus faecium Isolated from Seafood
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, M.M.; Shamsuzzaman, M.M.; Mozumder, M.M.H.; Xiangmin, X.; Ming, Y.; Jewel, M.A.S. Exploitation and conservation of coastal and marine fisheries in Bangladesh: Do the fishery laws matter? Mar. Policy 2017, 76, 143–151. [Google Scholar] [CrossRef]
- Department of Fisheries (DoF). Yearbook of Fisheries Statistics of Bangladesh, 2021–2022, Fisheries Resources Survey System (FRSS), 2023, Volume 39, Ministry of Fisheries, Bangladesh. Available online: http://fisheries.portal.gov.bd/site/download/42836060-aa5e-491d-8309-cf750886813b (accessed on 2 August 2023).
- Novoslavskij, A.; Terentjeva, M.; Eizenberga, I.; Valciņa, O.; Bartkevičs, V.; Bērziņš, A. Major food borne pathogens in fish and fish products: A review. Ann. Microbiol. 2016, 66, 1–15. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Beshiru, A. Antimicrobial resistance, virulence determinants, and biofilm formation of Enterococcus species from ready-to-eat seafood. Front. Microbiol. 2019, 10, 728. [Google Scholar] [CrossRef]
- Ben Said, L.; Hamdaoui, M.; Klibi, A.; Ben Slama, K.; Torres, C.; Klibi, N. Diversity of species and antibiotic resistance in enterococci isolated from seafood in Tunisia. Ann. Microbiol. 2017, 67, 135–141. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; Dapkevicius, M.D.L.E.; Igrejas, G.; Poeta, P. Enterococci, from harmless bacteria to a pathogen. Microorganisms 2020, 8, 1118. [Google Scholar] [CrossRef]
- Torres, C.; Alonso, C.A.; Ruiz-Ripa, L.; León-Sampedro, R.; Del Campo, R.; Coque, T.M. Antimicrobial resistance in Enterococcus spp. of animal origin. Microbiol. Spectr. 2018, 6, 185–227. [Google Scholar] [CrossRef]
- ECDC. European Centre for Disease Prevention and Control Publishes Annual Epidemiological Report 2011. Euro. Surveill. 2011, 16, 20012. [Google Scholar]
- Costa, O.Y.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Souza, E.L.; Meira, Q.G.; Barbosa, I.D.; Athayde, A.J.; Conceição, M.L.; Siqueira Júnior, J.P. Biofilm formation by Staphylococcus aureus from food contact surfaces in a meat-based broth and sensitivity to sanitizers. Braz. J. Microbiol. 2014, 45, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Hashem, Y.A.; Amin, H.M.; Essam, T.M.; Yassin, A.S.; Aziz, R.K. Biofilm formation in enterococci: Genotype-phenotype correlations and inhibition by vancomycin. Sci. Rep. 2017, 7, 5733. [Google Scholar] [CrossRef]
- Hancock, L.E.; Gilmore, M.S. Pathogenicity of enterococci. In Gram-Positive Pathogens, 2nd ed.; Fischetti, V.A., Novick, R.P., Ferretti, J.J., Portnoy, D.A., Rood, J.I., Eds.; ASM Press: Washington, DC, USA, 2006; pp. 251–258. [Google Scholar]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The Antimicrobial Resistance Crisis: Causes, Consequences, and Management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef]
- Hammad, A.M.; Shimamoto, T.; Shimamoto, T. Genetic characterization of antibiotic resistance and virulence factors in Enterococcus spp. From Japanese retail ready-to-eat raw fish. Food Microbiol. 2014, 38, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, A.S.; Benomar, N.; Abriouel, H.; Cañamero, M.M.; Gálvez, A. Isolation and identification of Enterococcus faecium from seafoods: Antimicrobial resistance and production of bacteriocin-like substances. Food Microbiol. 2010, 27, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Samia, S.; Galib, H.T.; Tanvir, A.S.; Basudeb, C.S.; Walliullah, M.; Tasnia, A.; Sakil, M.M.; Afsana, F.N.; Sadia, K.P.; Kamal, K.D.; et al. Microbiological quality analysis of shrimps collected from local market around Dhaka city. Int. Food Res. J. 2014, 21, 33–38. [Google Scholar]
- Facklam, R.R.; da Carvalho, M.G.S.; Teixeira, L.M. History, Taxonomy, Biochemical Characteristics, and Antibiotic Susceptibility Testing of Enterococci. In The Enterococci; American Society of Microbiology: Washington, DC, USA, 2002; pp. 1–54. [Google Scholar]
- Dutka-Malen, S.; Evers, S.; Courvalin, P. Detection of glycopeptide resistance genotypes identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 1995, 33, 24–27. [Google Scholar] [CrossRef]
- Randall, L.P.; Cooles, S.W.; Osborn, M.K.; Piddock, L.J.V.; Woodward, M.J. Antibiotic resistance genes integrons multiple antibiotic resistance in thirty-five serotypes of Salmonella enterica isolated from humans animals in the UK. J. Antimicrob. Chemother. 2004, 53, 208–216. [Google Scholar] [CrossRef]
- Queipo-Ortuno, M.I.; De Dios Colmenero, J.; Macias, M.; Bravo, J.M.; Morata, P. Preparation of bacterial DNA template by boiling and effect of immunoglobulin G as an inhibitor in real-time PCR for serum samples from patients with brucellosis. Clin. Vaccine Immunol. 2008, 15, 293–296. [Google Scholar] [CrossRef]
- Zheng, J.X.; Bai, B.; Lin, Z.W.; Pu, Z.Y.; Yao, W.M.; Chen, Z.; Li, D.Y.; Deng, X.B.; Deng, Q.W.; Yu, Z.J. Characterization of biofilm formation by Enterococcus faecalis isolates derived from urinary tract infections in China. J. Med. Microbiol. 2018, 67, 60. [Google Scholar] [CrossRef]
- M100-S32; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022.
- Bauer, A.T.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Krumperman, P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef]
- Brown, L.D.; Cai, T.T.; DasGupta, A. Interval estimation for a binomial proportion. Stat. Sci. 2001, 16, 101–133. [Google Scholar] [CrossRef]
- Boss, R.; Overesch, G.; Baumgartner, A. Antimicrobial Resistance of Escherichia coli, Enterococci, Pseudomonas aeruginosa, and Staphylococcus aureus from Raw Fish and Seafood Imported into Switzerland. J. Food Protect. 2016, 79, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Çardak, M.; Özmen Toğay, S.; Ay, M.; Karaalioğlu, O.; Erol, Ö.; Bağcı, U. Antibiotic resistance and virulence genes in Enterococcus species isolated from raw and processed seafood. J. Food Sci. Technol. 2022, 59, 2884–2893. [Google Scholar] [CrossRef] [PubMed]
- Chotinantakul, K.; Chansiw, N.; Okada, S. Antimicrobial resistance of Enterococcus spp. isolated from Thai fermented pork in Chiang Rai Province, Thailand. J. Glob. Antimicrob. Resist. 2018, 12, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Willems, R.J.; Friedrich, A.W.; Rossen, J.W.; Bathoorn, E. Enterococcus faecium: From microbiological insights to practical recommendations for infection control and diagnostics. Antimicrob. Resist. Infect. Control 2020, 9, 130. [Google Scholar] [CrossRef]
- Sieńko, A.; Wieczorek, P.; Majewski, P.; Ojdana, D.; Wieczorek, A.; Olszańska, D.; Tryniszewska, E. Comparison of antibiotic resistance and virulence between biofilm-producing and non-producing clinical isolates of Enterococcus faecium. Acta Biochim. Pol. 2015, 62, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Hentzer, M.; Riedel, K.; Rasmussen, T.B.; Heydorn, A.; Andersen, J.B.; Parsek, M.R.; Rice, S.A.; Eberl, L.; Molin, S.; Høiby, N.; et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 2002, 148, 87–102. [Google Scholar] [CrossRef]
- Gajewska, J.; Chajęcka-Wierzchowska, W.; Byczkowska-Rostkowska, Z.; Saki, M. Biofilm Formation Capacity and Presence of Virulence Determinants among Enterococcus Species from Milk and Raw Milk Cheeses. Life 2023, 13, 495. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, H.; Zhao, X.; Ge, C. Biofilm Formation and Control of Foodborne Pathogenic Bacteria. Molecules 2023, 28, 2432. [Google Scholar] [CrossRef]
- Tibúrcio, A.A.C.M.; Paiva, A.D.; Pedrosa, A.L.; Rodrigues, W.F.; da Silva, R.B.; Oliveira, A.G. Effect of sub-inhibitory concentrations of antibiotics on biofilm formation and expression of virulence genes in penicillin-resistant, ampicillin-susceptible Enterococcus faecalis. Heliyon 2022, 8, e11154. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, A.P.; Willems, R.J.; Bonten, M.J.; van Schaik, W. LPxTG surface proteins of enterococci. Trends Microbiol. 2009, 17, 423–430. [Google Scholar] [CrossRef]
- Roy, K.; Islam, M.S.; Paul, A.; Ievy, S.; Talukder, M.; Sobur, M.A.; Ballah, F.M.; Khan, M.S.R.; Rahman, M.T. Molecular detection and antibiotyping of multi-drug resistant Enterococcus faecium from healthy broiler chickens in Bangladesh. Vet. Med. Sci. 2022, 8, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; Łaniewska-Trokenheim, Ł. Virulence factors, antimicrobial resistance and biofilm formation in Enterococcus spp. isolated from retail shrimps. LWT-Food Sci. Technol. 2016, 69, 117–122. [Google Scholar] [CrossRef]
- Banik, A.; Mohammad, N.; Akter, T.; Fatema, K.; Abony, M. Prevalence, identification and antibiotic susceptibility of Enterococcus Species isolated from chicken and pigeon meat in Gazipur area of Bangladesh. Open J. Med. Microbiol. 2018, 8, 74–83. [Google Scholar] [CrossRef]
- Roy, S.; Aung, M.S.; Paul, S.K.; Ahmed, S.; Haque, N.; Khan, E.R.; Barman, T.K.; Islam, A.; Abedin, S.; Sultana, C.; et al. Drug resistance determinants in clinical isolates of Enterococcus faecalis in Bangladesh: Identification of oxazolidinone resistance gene optrA in ST59 and ST902 lineages. Microorganisms 2020, 8, 1240. [Google Scholar] [CrossRef] [PubMed]
- Akter, T.; Foysal, M.J.; Alam, M.; Ehsan, R.; Paul, S.I.; Momtaz, F.; Siddik, M.A.; Tay, A.C.Y.; Fotedar, R.; Gupta, S.K.; et al. Involvement of Enterococcus species in streptococcosis of Nile tilapia in Bangladesh. Aquaculture 2021, 531, 735790. [Google Scholar] [CrossRef]
- Islam, M.S.; Paul, A.; Talukder, M.; Roy, K.; Sobur, M.A.; Ievy, S.; Nayeem, M.M.H.; Rahman, S.; Nazir, K.N.H.; Hossain, M.T.; et al. Migratory birds travelling to Bangladesh are potential carriers of multi-drug resistant Enterococcus spp., Salmonella spp., and Vibrio spp. Saudi J. Biol. Sci. 2021, 28, 5963–5970. [Google Scholar] [CrossRef]
- Sagor, M.S.; Hossain, M.S.; Islam, T.; Mahmud, M.A.; Miah, M.S.; Karim, M.R.; Giasuddin, M.; Samad, M.A. Phenotypic and genotypic antibiotic resistance and virulence profiling of Enterococcus faecalis isolated from poultry at two major districts in Bangladesh. Pak. Vet. J. 2022, 42, 153–160. [Google Scholar]
- Bag, M.A.S.; Arif, M.; Riaz, S.; Khan, M.S.R.; Islam, M.S.; Punom, S.A.; Ali, M.W.; Begum, F.; Islam, M.S.; Rahman, M.T.; et al. Antimicrobial resistance, virulence profiles, and public health significance of Enterococcus faecalis isolated from clinical mastitis of cattle in Bangladesh. BioMed Res. Int. 2022, 2022, 8101866. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.A.; Sagor, M.S.; Hossain, M.S.; Karim, M.R.; Mahmud, M.A.; Sarker, M.S.; Shownaw, F.A.; Mia, Z.; Card, R.M.; Agunos, A.; et al. High prevalence of vancomycin non-susceptible and multi-drug resistant enterococci in farmed animals and fresh retail meats in Bangladesh. Vet. Res. Commun. 2022, 46, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Akter, T.; Haque, M.N.; Ehsan, R.; Paul, S.I.; Foysal, M.J.; Tay, A.C.Y.; Islam, M.T.; Rahman, M.M. Virulence and antibiotic-resistance genes in Enterococcus faecalis associated with streptococcosis disease in fish. Sci. Rep. 2023, 13, 1551. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, F.B.; Islam, M.S.; Ullah, M.A.; Rana, M.L.; Punom, S.A.; Neloy, F.H.; Chowdhury, M.N.U.; Hassan, J.; Siddique, M.P.; Saha, S.; et al. Antimicrobial Resistance Profiles, Virulence Determinants, and Biofilm Formation in Enterococci Isolated from Rhesus Macaques (Macaca mulatta): A Potential Threat for Wildlife in Bangladesh? Animals 2023, 13, 2268. [Google Scholar] [CrossRef] [PubMed]
- Fioriti, S.; Coccitto, S.N.; Cedraro, N.; Simoni, S.; Morroni, G.; Brenciani, A.; Mangiaterra, G.; Vignaroli, C.; Vezzulli, L.; Biavasco, F.; et al. Linezolid resistance genes in enterococci isolated from sediment and zooplankton in two Italian coastal areas. Appl. Environ. Microbiol. 2021, 87, e02958-20. [Google Scholar] [CrossRef] [PubMed]
- Abbott, I.J.; van Gorp, E.; van der Meijden, A.; Wijma, R.A.; Meletiadis, J.; Roberts, J.A.; Mouton, J.W.; Peleg, A.Y. Oral fosfomycin treatment for enterococcal urinary tract infections in a dynamic in vitro model. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as promoters of bacterial antibiotic resistance and tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]

| Target Organism and Determinants | Target Genes | Primer Sequences (5′-3′) | Annealing Tm (°C) | Size (bp) | References |
|---|---|---|---|---|---|
| Enterococcus faecium | ddlE. faecium | F: GCAAGGCTTCTTAGAGA R: CATCGTGTAAGCTAACTTC | 50 | 550 | [19] |
| Virulence | Agg | F: TCTTGGACACGACCCATGAT R: AGAAAGAACATCACCACGAGC | 58 | 413 | [11] |
| fsrA | F: CGTTCCGTCTCTCATAGTTA R: GCAGGATTTGAGGTTGCTAA | 53 | 474 | [11] | |
| fsrB | F: TAATCTAGGCTTAGTTCCCAC R: CTAAATGGCTCTGTCGTCTAG | 55 | 428 | [11] | |
| fsrC | F: GTGTTTTTGATTTCGCCAGAGA R: TATAACAATCCCCAACCGTG | 54 | 716 | [11] | |
| gelE | F: GGTGAAGAAGTTACTCTGAC R: GGTATTGAGTTATGAGGGGC | 52 | 704 | [11] | |
| sprE | F: CTGAGGACAGAAGACAAGAAG R: GGTTTTTCTCACCTGGATAG | 53 | 432 | [11] | |
| Ace | F: GAATGACCGAGAACGATGGC R: CTTGATGTTGGCCTGCTTCC | 58 | 615 | [11] | |
| Pil | F: GAAGAAACCAAAGCACCTAC R: CTACCTAAGAAAAGAAACGCG | 53 | 620 | [11] | |
| Cyl | F: TGGCGGTATTTTTACTGGAG R: TGAATCGCTCCATTTCTTC | 52 | 186 | [11] | |
| Antibiotic resistance | blaTEM | F: CATTTCCGTGTCGCCCTTAT R: TCCATAGTTGCCTGACTCCC | 56 | 793 | [20] |
| Type of Samples (N*) | n (%) P [95% CI] Q | p-Value | n* (%) A [95% CI] B |
|---|---|---|---|
| Shrimp (50) | 7 (14 a) [7.0–26.1] | 0.034 | 41 (27.3%) [20.8–34.9] |
| Crabs (25) | 8 (32 a,b) [17.2–51.6] | ||
| Marine fish (75) | 26 (34.7 b) [24.9–45.9] |
| Virulence Genes | Virulence in Different Degrees of Biofilm Formation | Total No. of Positive Isolates (%) [95% CI] | p-Value | ||
|---|---|---|---|---|---|
| No. (%) of Strong Biofilm Former (n = 18) | No. (%) of Intermediate Biofilm Former (n = 14) | No. (%) of Non-Biofilm Former (n = 9) | |||
| agg | 16 (88.9 a) | 8 (57.1 a,b) | 4 (44.4 b) | 28 (68.3) [53.0–80.4] | 0.035 |
| fsrA | 16 (88.9 a) | 7 (50 b) | 4 (44.4 b) | 27 (65.9) [50.6–78.4] | 0.022 |
| fsrB | 14 (77.8 a) | 6 (42.9 a,b) | 1 (11.1 b) | 21 (51.2) [36.5–65.8] | 0.004 |
| fsrC | 13 (72.2 a) | 4 (28.6 b) | 1 (11.1 b) | 18 (43.9) [29.9–59.0] | 0.004 |
| pil | 18 (100 a) | 14 (100 a) | 9 (100 a) | 41 (100) [91.4–100] | NA |
| gelE | 15 (83.3 a) | 8 (57.1 a,b) | 3 (33.3 b) | 26 (63.4) [48.1–76.4] | 0.033 |
| sprE | 13 (72.2 a) | 7 (50 a,b) | 2 (22.2 b) | 22 (53.7) [38.8–67.9] | 0.046 |
| ace | 18 (100 a) | 13 (92.9 a) | 7 (77.8 a) | 38 (92.7) [80.6–97.5] | 0.112 |
| cyl | 0 (0 a) | 0 (0 a) | 0 (0 a) | 0 (0) [0.0–8.6] | NA |
| Categories | Antibiotics | Antibiotic Resistance in Different Degrees of Biofilm Formation | Total No. of Positive Isolates (%) [95% CI] | p-Value | ||
|---|---|---|---|---|---|---|
| No. (%) of Strong Biofilm Former (n = 18) | No. (%) of Intermediate Biofilm Former (n = 14) | No. (%) of Non-Biofilm Former (n = 9) | ||||
| Phenotypic | CIP | 2 (11.1 a) | 1 (7.1 a) | 0 (0 a) | 3 (7.3) [2.5–19.4] | 0.579 |
| TE | 17 (94.4 a) | 8 (57.1 b) | 3 (33.3 b) | 28 (68.3) [53.0–80.4] | 0.003 | |
| LEV | 0 (0 a) | 0 (0 a) | 0 (0 a) | 0 (0) [0.0–8.6] | NA | |
| FOS | 6 (33.3 a) | 2 (14.3 a) | 0 (0 a) | 8 (19.5) [10.2–34.0] | 0.100 | |
| RD | 18 (100 a) | 14 (100 a) | 9 (100 a) | 41 (100) [91.4–100] | NA | |
| P | 18 (100 a) | 14 (100 a) | 9 (100 a) | 41 (100) [91.4–100] | NA | |
| LZD | 16 (88.9 a) | 9 (64.3 a,b) | 4 (44.4 b) | 29 (70.7) [55.5–82.4] | 0.046 | |
| NOR | 3 (16.7 a) | 1 (7.7 a) | 0 (0 a) | 4 (9.8) [3.9–22.6] | 0.374 | |
| NIT | 0 (0 a) | 0 (0 a) | 0 (0 a) | 0 (0) [0.0–8.6] | NA | |
| AMP | 17 (94.4 a) | 8 (57.1 b) | 1 (11.1 b) | 26 (63.4) [48.1–76.4] | <0.001 | |
| C | 0 (0 a) | 0 (0 a) | 0 (0 a) | 0 (0) [0.0–8.6] | NA | |
| VA | 18 (100 a) | 12 (85.7 a) | 0 (0 a) | 30 (73.2) [58.1–84.3] | <0.001 | |
| E | 18 (100 a) | 13 (92.9 a) | 8 (88.9 a) | 39 (95.1) [83.9–99.1] | 0.400 | |
| Genotypic | blaTEM | 12 (66.7 a) | 9 (64.3 a) | 4 (44.4 a) | 25 (61.0) [45.7–74.3] | 0.511 |
| No. of Patterns | Antibiotic Resistance Patterns | No. of Antibiotics (Classes) | No. of Isolates | Overall MDR Isolates (%) | MAR Index |
|---|---|---|---|---|---|
| 1 | P, AMP, VAN, E, TE, CIP, NX, LZ, FOS, RD | 10 (8) | 1 | 41 (100) | 0.8 |
| 2 | P, AMP, VAN, E, TE, NX, LZ, FOS, RD | 9 (8) | 1 | 0.7 | |
| 3 | P, AMP, VAN, E, TE, CIP, LZ, FOS, RD | 9 (8) | 1 | ||
| 4 | P, AMP, VAN, E, TE, CIP, NX, LZ, RD | 9 (7) | 1 | ||
| 5 | P, AMP, VAN, E, TE, NX, LZ, RD | 8 (7) | 1 | 0.6 | |
| 6 | P, AMP, VAN, E, TE, CIP, LZ, RD | 8 (7) | 1 | ||
| 7 | P, AMP, VAN, E, TE, LZ, FOS, RD | 8 (7) | 2 | ||
| 8 | P, VAN, E, TE, LZ, FOS, RD | 7 (7) | 1 | 0.5 | |
| 9 | P, AMP, VAN, E, TE, LZ, RD | 7 (6) | 9 | ||
| 10 | P, AMP, VAN, E, NX, LZ, RD | 7 (6) | 1 | ||
| 11 | P, VAN, E, TE, LZ, RD | 6 (6) | 3 | 0.5 | |
| 12 | P, VAN, E, TE, FOS, RD | 6 (6) | 1 | ||
| 13 | P, AMP, VAN, E, TE, RD | 6 (5) | 3 | ||
| 14 | P, AMP, E, TE, LZ, RD | 6 (5) | 2 | ||
| 15 | P, AMP, VAN, E, LZ, RD | 6 (5) | 1 | ||
| 16 | P, VAN, E, LZ, RD | 5 (5) | 1 | 0.4 | |
| 17 | P, E, TE, LZ, RD | 5 (5) | 1 | ||
| 18 | P, AMP, VAN, TE, RD | 5 (4) | 1 | ||
| 19 | P, AMP, VAN, E, RD | 5 (4) | 1 | ||
| 20 | P, AMP, E, LZ, RD | 5 (4) | 1 | ||
| 21 | P, E, TE, RD | 4 (4) | 1 | 0.3 | |
| 22 | P, E, LZ, RD | 4 (4) | 1 | ||
| 23 | P, TE, LZ, RD | 4 (4) | 1 | ||
| 24 | P, E, RD | 3 (3) | 4 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, M.A.; Islam, M.S.; Rana, M.L.; Ferdous, F.B.; Neloy, F.H.; Firdous, Z.; Hassan, J.; Rahman, M.T. Resistance Profiles and Virulence Determinants in Biofilm-Forming Enterococcus faecium Isolated from Raw Seafood in Bangladesh. Pathogens 2023, 12, 1101. https://doi.org/10.3390/pathogens12091101
Ullah MA, Islam MS, Rana ML, Ferdous FB, Neloy FH, Firdous Z, Hassan J, Rahman MT. Resistance Profiles and Virulence Determinants in Biofilm-Forming Enterococcus faecium Isolated from Raw Seafood in Bangladesh. Pathogens. 2023; 12(9):1101. https://doi.org/10.3390/pathogens12091101
Chicago/Turabian StyleUllah, Md. Ashek, Md. Saiful Islam, Md. Liton Rana, Farhana Binte Ferdous, Fahim Haque Neloy, Zannatul Firdous, Jayedul Hassan, and Md. Tanvir Rahman. 2023. "Resistance Profiles and Virulence Determinants in Biofilm-Forming Enterococcus faecium Isolated from Raw Seafood in Bangladesh" Pathogens 12, no. 9: 1101. https://doi.org/10.3390/pathogens12091101
APA StyleUllah, M. A., Islam, M. S., Rana, M. L., Ferdous, F. B., Neloy, F. H., Firdous, Z., Hassan, J., & Rahman, M. T. (2023). Resistance Profiles and Virulence Determinants in Biofilm-Forming Enterococcus faecium Isolated from Raw Seafood in Bangladesh. Pathogens, 12(9), 1101. https://doi.org/10.3390/pathogens12091101










