Periodontopathogens Porphyromonas gingivalis and Fusobacterium nucleatum and Their Roles in the Progression of Respiratory Diseases
Abstract
1. Oral Microbiota and Systemic Diseases
2. Periodontal Diseases and Respiratory Diseases
3. Specific Respiratory Diseases Relating to P. gingivalis and F. nucleatum
3.1. Pneumonia
3.2. COPD
3.3. Lung Cancer
3.4. Asthma
3.5. Other Respiratory Diseases
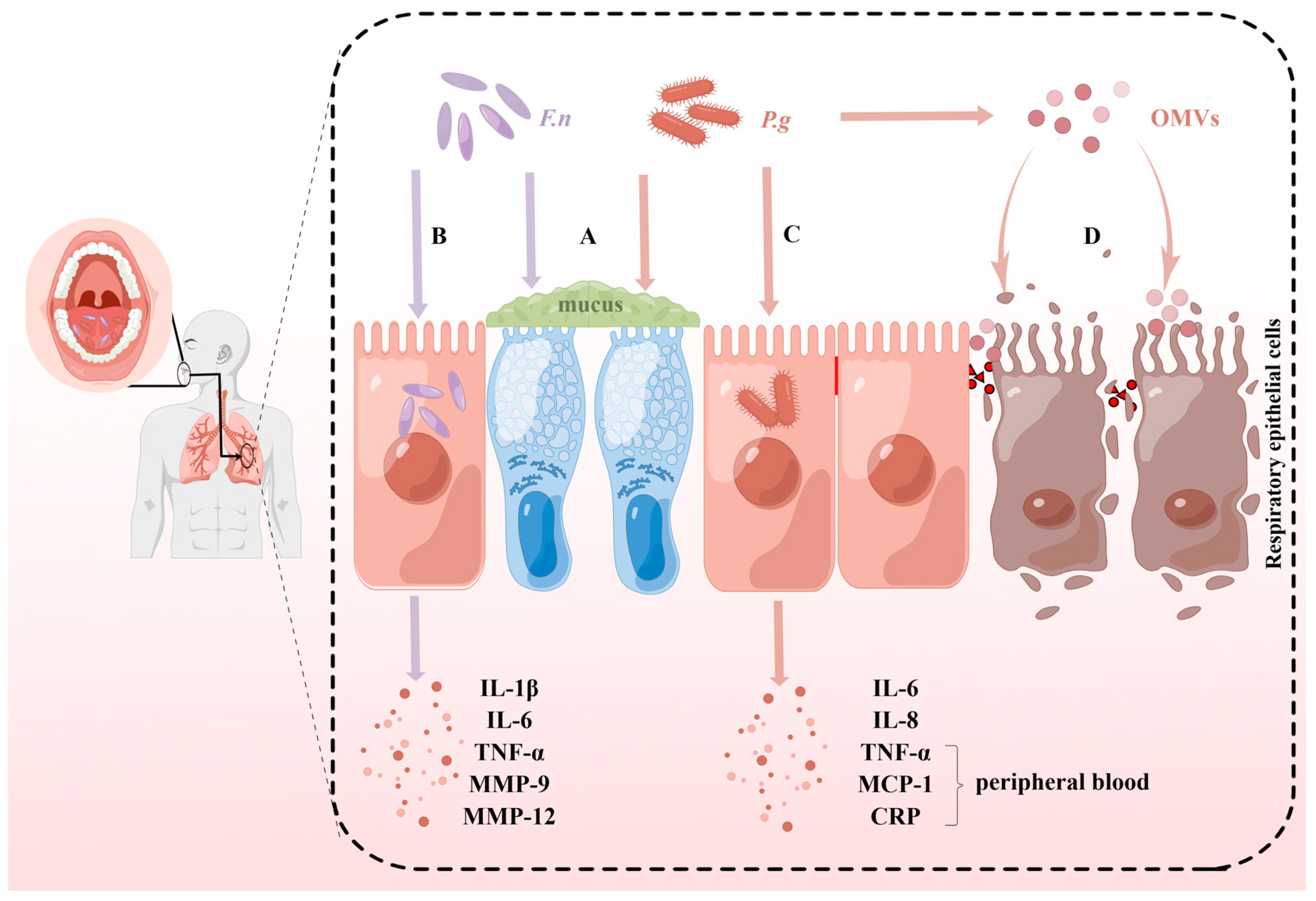
4. Potential Pathogenic Mechanisms of P. gingivalis and F. nucleatum in Respiratory Diseases
4.1. Induction of Mucus Hypersecretion and Airway Inflammation
4.2. Cytotoxic Effects
4.3. Synergistic Pathogenic Effects with Respiratory Pathogens
4.3.1. P. gingivalis and Streptococcus pneumoniae
4.3.2. P. gingivalis and Pseudomonas aeruginosa
4.3.3. F. nucleatum and P. aeruginosa
5. Discussion and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shang, Q.; Gao, Y.; Qin, T.; Wang, S.; Shi, Y.; Chen, T. Interaction of Oral and Toothbrush Microbiota Affects Oral Cavity Health. Front. Cell. Infect. Microbiol. 2020, 10, 17. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Drell, T.; Štšepetova, J.; Simm, J.; Rull, K.; Aleksejeva, A.; Antson, A.; Tillmann, V.; Metsis, M.; Sepp, E.; Salumets, A.; et al. The Influence of Different Maternal Microbial Communities on the Development of Infant Gut and Oral Microbiota. Sci. Rep. 2017, 7, 9940. [Google Scholar] [CrossRef]
- Bernardi, S.; Bianchi, S.; Tomei, A.R.; Continenza, M.A.; Macchiarelli, G. Microbiological and SEM-EDS Evaluation of Titanium Surfaces Exposed to Periodontal Gel: In Vitro Study. Materials 2019, 12, 1448. [Google Scholar] [CrossRef] [PubMed]
- Sara, B.; Giuseppe, M.; Adelaide, C.M. Dorsal Lingual Surface and Halitosis: A Morphological Point of View. Acta Stomatol. Croat. 2016, 50, 151–157. [Google Scholar] [CrossRef]
- Tuganbaev, T.; Yoshida, K.; Honda, K. The effects of oral microbiota on health. Science 2022, 376, 934–936. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.; Harjunmaa, U.; Doyle, R.; Mulewa, S.; Charlie, D.; Maleta, K.; Callard, R.; Walker, A.S.; Balloux, F.; Ashorn, P.; et al. Distinguishing the Signals of Gingivitis and Periodontitis in Supragingival Plaque: A Cross-Sectional Cohort Study in Malawi. Appl. Environ. Microbiol. 2016, 82, 6057–6067. [Google Scholar] [CrossRef]
- Teles, F.R.F.; Alawi, F.; Castilho, R.M.; Wang, Y. Association or Causation? Exploring the Oral Microbiome and Cancer Links. J. Dent. Res. 2020, 99, 1411–1424. [Google Scholar] [CrossRef]
- Watanabe, N.; Yokoe, S.; Ogata, Y.; Sato, S.; Imai, K. Exposure to Porphyromonas gingivalis Induces Production of Proinflammatory Cytokine via TLR2 from Human Respiratory Epithelial Cells. J. Clin. Med. 2020, 9, 3433. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.; Liu, W.; Huang, X.; Song, Y.; Wang, Z.; Jia, X. Periodontal Status and Microbiologic Pathogens in Patients with Chronic Obstructive Pulmonary Disease and Periodontitis: A Case-Control Study. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Li, W.; Wang, Q.; Chen, J.; Zu, Y.; Zhou, X.; Guo, Q. Relationships Between Oral Microecosystem and Respiratory Diseases. Front. Mol. Biosci. 2021, 8, 718222. [Google Scholar] [CrossRef] [PubMed]
- Stasiewicz, M.; Karpinski, T.M. The oral microbiota and its role in carcinogenesis. Semin. Cancer Biol. 2022, 86, 633–642. [Google Scholar] [CrossRef]
- Lee, J.B.; Kim, K.A.; Cho, H.Y.; Kim, D.; Kim, W.K.; Yong, D.; Lee, H.; Yoon, S.S.; Han, D.H.; Han, Y.D.; et al. Association between Fusobacterium nucleatum and patient prognosis in metastatic colon cancer. Sci. Rep. 2021, 11, 20263. [Google Scholar] [CrossRef] [PubMed]
- Read, E.; Curtis, M.A.; Neves, J.F. The role of oral bacteria in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 731–742. [Google Scholar] [CrossRef]
- Gao, S.G.; Yang, J.Q.; Ma, Z.K.; Yuan, X.; Zhao, C.; Wang, G.C.; Wei, H.; Feng, X.S.; Qi, Y.J. Preoperative serum immunoglobulin G and A antibodies to Porphyromonas gingivalis are potential serum biomarkers for the diagnosis and prognosis of esophageal squamous cell carcinoma. BMC Cancer 2018, 18, 17. [Google Scholar] [CrossRef]
- Hernández-Ruiz, P.; González-Pacheco, H.; Amezcua-Guerra, L.M.; Aguirre-García, M.M. Relación entre la disbiosis de la microbiota oral y la enfermedad cardiovascular aterosclerótica. Arch. Cardiol. Mex. 2022, 92, 371–376. [Google Scholar] [CrossRef]
- Loyola-Rodriguez, J.P.; Martinez-Martinez, R.E.; Abud-Mendoza, C.; Patiño-Marin, N.; Seymour, G.J. Rheumatoid arthritis and the role of oral bacteria. J. Oral Microbiol. 2010, 2, 5784. [Google Scholar] [CrossRef]
- Guo, H.; Li, B.; Yao, H.; Liu, D.; Chen, R.; Zhou, S.; Ji, Y.; Zeng, L.; Du, M. Profiling the oral microbiomes in patients with Alzheimer’s disease. Oral Dis. 2023, 29, 1341–1355. [Google Scholar] [CrossRef] [PubMed]
- Fardini, Y.; Chung, P.; Dumm, R.; Joshi, N.; Han, Y.W. Transmission of diverse oral bacteria to murine placenta: Evidence for the oral microbiome as a potential source of intrauterine infection. Infect. Immun. 2010, 78, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Matsha, T.E.; Prince, Y.; Davids, S.; Chikte, U.; Erasmus, R.T.; Kengne, A.P.; Davison, G.M. Oral Microbiome Signatures in Diabetes Mellitus and Periodontal Disease. J. Dent. Res. 2020, 99, 658–665. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Balejo, R.D.P.; Cortelli, J.R.; Costa, F.O.; Cyrino, R.M.; Aquino, D.R.; Cogo-Müller, K.; Miranda, T.B.; Moura, S.P.; Cortelli, S.C. Effects of chlorhexidine preprocedural rinse on bacteremia in periodontal patients: A randomized clinical trial. J. Appl. Oral Sci. 2017, 25, 586–595. [Google Scholar] [CrossRef]
- Marik, P.E. Aspiration pneumonitis and aspiration pneumonia. N. Engl. J. Med. 2001, 344, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Suda, W.; Luo, C.; Kawaguchi, T.; Motoo, I.; Narushima, S.; Kiguchi, Y.; Yasuma, K.; Watanabe, E.; Tanoue, T.; et al. Ectopic colonization of oral bacteria in the intestine drives T(H)1 cell induction and inflammation. Science 2017, 358, 359–365. [Google Scholar] [CrossRef]
- Madianos, P.N.; Bobetsis, Y.A.; Offenbacher, S. Adverse pregnancy outcomes (APOs) and periodontal disease: Pathogenic mechanisms. J. Clin. Periodontol. 2013, 40 (Suppl. S14), S170–S180. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouel, F. Periodontal Pathogens as Risk Factors of Cardiovascular Diseases, Diabetes, Rheumatoid Arthritis, Cancer, and Chronic Obstructive Pulmonary Disease-Is There Cause for Consideration? Microorganisms 2019, 7, 424. [Google Scholar] [CrossRef] [PubMed]
- Konkel, J.E.; O’Boyle, C.; Krishnan, S. Distal Consequences of Oral Inflammation. Front. Immunol. 2019, 10, 1403. [Google Scholar] [CrossRef]
- Inoue, K.; Takano, H.; Shimada, A.; Yanagisawa, R.; Sakurai, M.; Yoshino, S.; Sato, H.; Yoshikawa, T. Urinary trypsin inhibitor protects against systemic inflammation induced by lipopolysaccharide. Mol. Pharmacol. 2005, 67, 673–680. [Google Scholar] [CrossRef]
- Bonnington, K.E.; Kuehn, M.J. Protein selection and export via outer membrane vesicles. Biochim. Biophys. Acta 2014, 1843, 1612–1619. [Google Scholar] [CrossRef]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, B.; Brandt, B.W.; Cheng, L.; Zhou, X.; Exterkate, R.A.M.; Crielaard, W.; Deng, D.M. Comparison of Red-Complex Bacteria Between Saliva and Subgingival Plaque of Periodontitis Patients: A Systematic Review and Meta-Analysis. Front. Cell. Infect. Microbiol. 2021, 11, 727732. [Google Scholar] [CrossRef]
- Bostanci, N.; Belibasakis, G.N. Porphyromonas gingivalis: An invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 2012, 333, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Jenkinson, H.F. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol. Immunol. 2000, 15, 341–349. [Google Scholar] [CrossRef]
- Gonzales, J.R.; Groeger, S.; Johansson, A.; Meyle, J. T helper cells from aggressive periodontitis patients produce higher levels of interleukin-1 beta and interleukin-6 in interaction with Porphyromonas gingivalis. Clin. Oral Investig. 2014, 18, 1835–1843. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, J.T.; Gerow, E.A.; Schaff, R.; Taut, A.D.; Shin, S.Y.; Sugai, J.; Brand, D.; Burberry, A.; Jorns, J.; Lundy, S.K.; et al. Porphyromonas gingivalis oral infection exacerbates the development and severity of collagen-induced arthritis. Arthritis Res. Ther. 2013, 15, R186. [Google Scholar] [CrossRef] [PubMed]
- Borgwardt, D.S.; Martin, A.D.; Van Hemert, J.R.; Yang, J.; Fischer, C.L.; Recker, E.N.; Nair, P.R.; Vidva, R.; Chandrashekaraiah, S.; Progulske-Fox, A.; et al. Histatin 5 binds to Porphyromonas gingivalis hemagglutinin B (HagB) and alters HagB-induced chemokine responses. Sci. Rep. 2014, 4, 3904. [Google Scholar] [CrossRef]
- Kadowaki, T.; Nakayama, K.; Okamoto, K.; Abe, N.; Baba, A.; Shi, Y.; Ratnayake, D.B.; Yamamoto, K. Porphyromonas gingivalis proteinases as virulence determinants in progression of periodontal diseases. J. Biochem. 2000, 128, 153–159. [Google Scholar] [CrossRef]
- Fleetwood, A.J.; Lee, M.K.S.; Singleton, W.; Achuthan, A.; Lee, M.C.; O’Brien-Simpson, N.M.; Cook, A.D.; Murphy, A.J.; Dashper, S.G.; Reynolds, E.C.; et al. Metabolic Remodeling, Inflammasome Activation, and Pyroptosis in Macrophages Stimulated by Porphyromonas gingivalis and Its Outer Membrane Vesicles. Front. Cell. Infect. Microbiol. 2017, 7, 351. [Google Scholar] [CrossRef]
- Thurnheer, T.; Karygianni, L.; Flury, M.; Belibasakis, G.N. Fusobacterium Species and Subspecies Differentially Affect the Composition and Architecture of Supra- and Subgingival Biofilms Models. Front. Microbiol. 2019, 10, 1716. [Google Scholar] [CrossRef]
- Sharma, A.; Inagaki, S.; Sigurdson, W.; Kuramitsu, H.K. Synergy between Tannerella forsythia and Fusobacterium nucleatum in biofilm formation. Oral Microbiol. Immunol. 2005, 20, 39–42. [Google Scholar] [CrossRef]
- Kaplan, C.W.; Lux, R.; Haake, S.K.; Shi, W. The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm. Mol. Microbiol. 2009, 71, 35–47. [Google Scholar] [CrossRef]
- Guo, L.; Shokeen, B.; He, X.; Shi, W.; Lux, R. Streptococcus mutans SpaP binds to RadD of Fusobacterium nucleatum ssp. polymorphum. Mol. Oral Microbiol. 2017, 32, 355–364. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W.; Ikegami, A.; Rajanna, C.; Kawsar, H.I.; Zhou, Y.; Li, M.; Sojar, H.T.; Genco, R.J.; Kuramitsu, H.K.; Deng, C.X. Identification and characterization of a novel adhesin unique to oral fusobacteria. J. Bacteriol. 2005, 187, 5330–5340. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Feng, Z.; Fujioka, H.; Lux, R.; McCormick, T.S.; Weinberg, A. Conceptual Perspectives: Bacterial Antimicrobial Peptide Induction as a Novel Strategy for Symbiosis with the Human Host. Front. Microbiol. 2018, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Ghosh, S.K.; Shokeen, B.; Eapan, B.; Lux, R.; Kiselar, J.; Nithianantham, S.; Young, A.; Pandiyan, P.; McCormick, T.S.; et al. FAD-I, a Fusobacterium nucleatum Cell Wall-Associated Diacylated Lipoprotein That Mediates Human Beta Defensin 2 Induction through Toll-Like Receptor-1/2 (TLR-1/2) and TLR-2/6. Infect. Immun. 2016, 84, 1446–1456. [Google Scholar] [CrossRef]
- Park, S.R.; Kim, D.J.; Han, S.H.; Kang, M.J.; Lee, J.Y.; Jeong, Y.J.; Lee, S.J.; Kim, T.H.; Ahn, S.G.; Yoon, J.H.; et al. Diverse Toll-like receptors mediate cytokine production by Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans in macrophages. Infect. Immun. 2014, 82, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Zar, H.J.; Ferkol, T.W. The global burden of respiratory disease-impact on child health. Pediatr. Pulmonol. 2014, 49, 430–434. [Google Scholar] [CrossRef]
- Hanada, S.; Pirzadeh, M.; Carver, K.Y.; Deng, J.C. Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia. Front. Immunol. 2018, 9, 2640. [Google Scholar] [CrossRef]
- Age-sex differences in the global burden of lower respiratory infections and risk factors, 1990-2019: Results from the Global Burden of Disease Study 2019. Lancet Infect. Dis. 2022, 22, 1626–1647. [CrossRef]
- Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [CrossRef]
- Society, A.T. Urgent Progress Needed to End the Preventable Burden of Pneumonia and Deaths: The Forum of International Respiratory Societies. Available online: https://www.thoracic.org/about/newsroom/press-releases/journal/2020/urgent-progress-needed-to-end-the-preventable-burden-of-pneumonia-and-deaths-firs.php (accessed on 18 August 2023).
- Huang, J.; Deng, Y.; Tin, M.S.; Lok, V.; Ngai, C.H.; Zhang, L.; Lucero-Prisno, D.E., 3rd; Xu, W.; Zheng, Z.J.; Elcarte, E.; et al. Distribution, Risk Factors, and Temporal Trends for Lung Cancer Incidence and Mortality: A Global Analysis. Chest 2022, 161, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Bassis, C.M.; Erb-Downward, J.R.; Dickson, R.P.; Freeman, C.M.; Schmidt, T.M.; Young, V.B.; Beck, J.M.; Curtis, J.L.; Huffnagle, G.B. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 2015, 6, e00037. [Google Scholar] [CrossRef]
- Invernizzi, R.; Lloyd, C.M.; Molyneaux, P.L. Respiratory microbiome and epithelial interactions shape immunity in the lungs. Immunology 2020, 160, 171–182. [Google Scholar] [CrossRef]
- Patel, V.S.; Sitapara, R.A.; Gore, A.; Phan, B.; Sharma, L.; Sampat, V.; Li, J.H.; Yang, H.; Chavan, S.S.; Wang, H.; et al. High Mobility Group Box-1 mediates hyperoxia-induced impairment of Pseudomonas aeruginosa clearance and inflammatory lung injury in mice. Am. J. Respir Cell. Mol. Biol. 2013, 48, 280–287. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kawanami, T.; Yatera, K.; Fukuda, K.; Noguchi, S.; Nagata, S.; Nishida, C.; Kido, T.; Ishimoto, H.; Taniguchi, H.; et al. Significance of anaerobes and oral bacteria in community-acquired pneumonia. PLoS ONE 2013, 8, e63103. [Google Scholar] [CrossRef]
- Sapey, E.; Yonel, Z.; Edgar, R.; Parmar, S.; Hobbins, S.; Newby, P.; Crossley, D.; Usher, A.; Johnson, S.; Walton, G.M.; et al. The clinical and inflammatory relationships between periodontitis and chronic obstructive pulmonary disease. J. Clin. Periodontol. 2020, 47, 1040–1052. [Google Scholar] [CrossRef]
- Gomes-Filho, I.S.; de Oliveira, T.F.; da Cruz, S.S.; Passos-Soares Jde, S.; Trindade, S.C.; Oliveira, M.T.; Souza-Machado, A.; Cruz, Á.A.; Barreto, M.L.; Seymour, G.J. Influence of periodontitis in the development of nosocomial pneumonia: A case control study. J. Periodontol. 2014, 85, e82–e90. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.T.; Xia, L.Y.; Zhang, Y.G.; Li, S.; Leng, W.D.; Kwong, J.S. Periodontal Disease and Incident Lung Cancer Risk: A Meta-Analysis of Cohort Studies. J. Periodontol. 2016, 87, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G.; Gorbach, S.L.; Finegold, S.M. The bacteriology of aspiration pneumonia. Am. J. Med. 1974, 56, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Kimizuka, R.; Abe, S.; Kato, T.; Ishihara, K. Involvement of periodontopathic anaerobes in aspiration pneumonia. J. Periodontol. 2005, 76, 2154–2160. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.M.R.; Saraiva, L.; Romito, G.A.; Pannuti, C.M.; Oliveira, H.P.; Peres, M.; Carmona, M.J.C.; Villar, C.C. Periodontopathogenic bacteria in subglottic samples from patients undergoing elective intubation for general anesthesia: A pilot study. J. Periodontol. 2021, 92, e94–e102. [Google Scholar] [CrossRef]
- Takahashi, T.; Muro, S.; Tanabe, N.; Terada, K.; Kiyokawa, H.; Sato, S.; Hoshino, Y.; Ogawa, E.; Uno, K.; Naruishi, K.; et al. Relationship between periodontitis-related antibody and frequent exacerbations in chronic obstructive pulmonary disease. PLoS ONE 2012, 7, e40570. [Google Scholar] [CrossRef][Green Version]
- Tan, L.; Wang, H.; Li, C.; Pan, Y. 16S rDNA-based metagenomic analysis of dental plaque and lung bacteria in patients with severe acute exacerbations of chronic obstructive pulmonary disease. J. Periodontal. Res. 2014, 49, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, J.; Xu, M.; Zhu, D.; Wang, X.; Chen, Y.; Wu, J.; Cui, C.; Zhang, W.; Yu, L. 16S rDNA analysis of periodontal plaque in chronic obstructive pulmonary disease and periodontitis patients. J. Oral Microbiol. 2017, 9, 1324725. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Tang, X.; Pan, C.; Wang, H.; Pan, Y. Relationship among clinical periodontal, microbiologic parameters and lung function in participants with chronic obstructive pulmonary disease. J. Periodontol. 2019, 90, 134–140. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, X.; Chen, K.; Zhou, F.; Yang, H.; Yang, H.; Qi, Y.; Kong, J.; Sun, W.; Gao, S. Clinical significance and prognostic value of Porphyromonas gingivalis infection in lung cancer. Transl. Oncol. 2021, 14, 100972. [Google Scholar] [CrossRef]
- Ampomah, N.K.; Teles, F.; Martin, L.M.; Lu, J.; Koestler, D.C.; Kelsey, K.T.; Beck, J.D.; Platz, E.A.; Michaud, D.S. Circulating IgG antibodies to periodontal bacteria and lung cancer risk in the CLUE cohorts. JNCI Cancer Spectr. 2023, 7, pkad029. [Google Scholar] [CrossRef]
- Zhou, B.; Lu, J.; Beck, J.D.; Moss, K.L.; Prizment, A.E.; Demmer, R.T.; Porosnicu Rodriguez, K.A.; Joshu, C.E.; Michaud, D.S.; Platz, E.A. Periodontal and Other Oral Bacteria and Risk of Lung Cancer in the Atherosclerosis Risk in Communities (ARIC) Study. Cancer Epidemiol. Biomark. Prev. 2023, 32, 505–515. [Google Scholar] [CrossRef]
- Arbes, S.J., Jr.; Sever, M.L.; Vaughn, B.; Cohen, E.A.; Zeldin, D.C. Oral pathogens and allergic disease: Results from the Third National Health and Nutrition Examination Survey. J. Allergy Clin. Immunol. 2006, 118, 1169–1175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Card, J.W.; Carey, M.A.; Voltz, J.W.; Bradbury, J.A.; Ferguson, C.D.; Cohen, E.A.; Schwartz, S.; Flake, G.P.; Morgan, D.L.; Arbes, S.J., Jr.; et al. Modulation of allergic airway inflammation by the oral pathogen Porphyromonas gingivalis. Infect. Immun. 2010, 78, 2488–2496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Irani, S.; Schmidlin, P.R.; Bolivar, I.; Speich, R.; Boehler, A. Evidence for graft colonization with periodontal pathogens in lung transplant recipients. A pilot study. Schweiz Mon. Zahnmed 2011, 121, 1144–1149. [Google Scholar]
- Tanaka, A.; Kogami, M.; Nagatomo, Y.; Takeda, Y.; Kanzawa, H.; Kawaguchi, Y.; Ono, S.; Furukawa, K.; Nakamura, H.; Aoshiba, K. Subcutaneous abscess due to empyema necessitans caused by Porphyromonas gingivalis in a patient with periodontitis. IDCases 2022, 27, e01458. [Google Scholar] [CrossRef]
- Nagaoka, K.; Yanagihara, K.; Harada, Y.; Yamada, K.; Migiyama, Y.; Morinaga, Y.; Izumikawa, K.; Kohno, S. Quantitative detection of periodontopathic bacteria in lower respiratory tract specimens by real-time PCR. J. Infect. Chemother. 2017, 23, 69–73. [Google Scholar] [CrossRef]
- De Carvalho Baptista, I.M.; Martinho, F.C.; Nascimento, G.G.; da Rocha Santos, C.E.; Prado, R.F.D.; Valera, M.C. Colonization of oropharynx and lower respiratory tract in critical patients: Risk of ventilator-associated pneumonia. Arch. Oral Biol. 2018, 85, 64–69. [Google Scholar] [CrossRef]
- Hoffmeister, B.C.; Ducasse, C.K.; González, L.M.; Quilodrán, S.C.; Joyas, M.A. Pulmonary and thoracic infection by Fusobacterium nucleatum. Andes Pediatr. Rev. Chil. Pediatr. 2021, 92, 93–98. [Google Scholar] [CrossRef]
- Wolff, L.; Martiny, D.; Deyi, V.Y.M.; Maillart, E.; Clevenbergh, P.; Dauby, N. COVID-19-Associated Fusobacterium nucleatum Bacteremia, Belgium. Emerg. Infect. Dis. 2021, 27, 975–977. [Google Scholar] [CrossRef]
- Bao, L.; Zhang, C.; Lyu, J.; Yan, C.; Cao, R.; Pan, M.; Li, Y. Beware of pharyngeal Fusobacterium nucleatum in COVID-19. BMC Microbiol. 2021, 21, 277. [Google Scholar] [CrossRef]
- Li, Q.; Tan, L.; Wang, H.; Kou, Y.; Shi, X.; Zhang, S.; Pan, Y. Fusobacterium nucleatum Interaction with Pseudomonas aeruginosa Induces Biofilm-Associated Antibiotic Tolerance via Fusobacterium Adhesin A. ACS Infect. Dis. 2020, 6, 1686–1696. [Google Scholar] [CrossRef]
- Mai, X.; Genco, R.J.; LaMonte, M.J.; Hovey, K.M.; Freudenheim, J.L.; Andrews, C.A.; Wactawski-Wende, J. Periodontal Pathogens and Risk of Incident Cancer in Postmenopausal Females: The Buffalo OsteoPerio Study. J. Periodontol. 2016, 87, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Cheng, Z.; Yin, Z.; Xu, J.; Wu, F.; Jin, Y.; Yang, G. Airway Fusobacterium is Associated with Poor Response to Immunotherapy in Lung Cancer. OncoTargets Ther. 2022, 15, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Dong, H.; Zhang, N.; Zhao, P.; Qi, Y.; Yang, X.; Wang, L. Empyema caused by Fusobacterium nucleatum with squamous cell carcinoma of the lung: A case report and literature review. Front. Med. 2023, 10, 1099040. [Google Scholar] [CrossRef]
- Brook, I.; Frazier, E.H. Immune response to Fusobacterium nucleatum and Prevotella intermedia in the sputum of patients with acute exacerbation of chronic bronchitis. Chest 2003, 124, 832–833. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Sauer-Heilborn, A.; Welte, T.; Jauregui, R.; Brettar, I.; Guzman, C.A.; Höfle, M.G. High individuality of respiratory bacterial communities in a large cohort of adult cystic fibrosis patients under continuous antibiotic treatment. PLoS ONE 2015, 10, e0117436. [Google Scholar] [CrossRef]
- Bansal, M.; Khatri, M.; Taneja, V. Potential role of periodontal infection in respiratory diseases—A review. J. Med. Life 2013, 6, 244–248. [Google Scholar]
- Scannapieco, F.A. Role of oral bacteria in respiratory infection. J. Periodontol. 1999, 70, 793–802. [Google Scholar] [CrossRef]
- Liu, J.; Ren, X.; Zhang, M.; Lei, Y.; Chen, Y.; He, H. Roles of Wnt3a and Dkk1 in experimental periodontitis. J. Dent. Sci. 2017, 12, 220–225. [Google Scholar] [CrossRef]
- Morris, J.F.; Sewell, D.L. Necrotizing pneumonia caused by mixed infection with Actinobacillus actinomycetemcomitans and Actinomyces israelii: Case report and review. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1994, 18, 450–452. [Google Scholar] [CrossRef]
- Finegold, S.M.; Strong, C.A.; McTeague, M.; Marina, M. The importance of black-pigmented gram-negative anaerobes in human infections. FEMS Immunol. Med. Microbiol. 1993, 6, 77–82. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Shay, K. Oral health disparities in older adults: Oral bacteria, inflammation, and aspiration pneumonia. Dent. Clin. North Am. 2014, 58, 771–782. [Google Scholar] [CrossRef]
- Yoneyama, T.; Yoshida, M.; Ohrui, T.; Mukaiyama, H.; Okamoto, H.; Hoshiba, K.; Ihara, S.; Yanagisawa, S.; Ariumi, S.; Morita, T.; et al. Oral care reduces pneumonia in older patients in nursing homes. J. Am. Geriatr. Soc. 2002, 50, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Kikutani, T.; Tamura, F.; Takahashi, N.; Tohara, T.; Nawachi, K.; Maekawa, K.; Kuboki, T. Relationship between oral environment and development of pneumonia and acute viral respiratory infection in dependent older individuals. Geriatr. Gerontol. Int. 2019, 19, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Naruishi, K.; Nishikawa, Y.; Kido, J.I.; Fukunaga, A.; Nagata, T. Relationship of aspiration pneumonia to cognitive impairment and oral condition: A cross-sectional study. Clin. Oral Investig. 2018, 22, 2575–2580. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [CrossRef] [PubMed]
- Scannapieco, F.A.; Cantos, A. Oral inflammation and infection, and chronic medical diseases: Implications for the elderly. Periodontology 2000 2016, 72, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Dessì, A.; Bosco, A.; Pintus, R.; Orrù, G.; Fanos, V. Fusobacterium nucleatum and alteration of the oral microbiome: From pregnancy to SARS-CoV-2 infection. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4579–4596. [Google Scholar] [CrossRef]
- Wu, X.; Wei, X.; Li, X.; Deng, J.; Zhang, J. Diversity of Fungi and Bacteria in Bronchoalveolar Lavage Fluid during Development of Chronic Obstructive Pulmonary Disease. Jpn. J. Infect. Dis. 2022, 75, 560–568. [Google Scholar] [CrossRef]
- Segal, L.N.; Alekseyenko, A.V.; Clemente, J.C.; Kulkarni, R.; Wu, B.; Gao, Z.; Chen, H.; Berger, K.I.; Goldring, R.M.; Rom, W.N.; et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome 2013, 1, 19. [Google Scholar] [CrossRef]
- Lafuente Ibáñez de Mendoza, I.; Maritxalar Mendia, X.; García de la Fuente, A.M.; Quindós Andrés, G.; Aguirre Urizar, J.M. Role of Porphyromonas gingivalis in oral squamous cell carcinoma development: A systematic review. J. Periodontal. Res. 2020, 55, 13–22. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, Y.; Li, G.; Lan, Z.; Ma, M.; Li, H.; Kong, J.; Sun, J.; Hou, G.; Hou, X.; et al. Blockade of Immune-Checkpoint B7-H4 and Lysine Demethylase 5B in Esophageal Squamous Cell Carcinoma Confers Protective Immunity against P. gingivalis Infection. Cancer Immunol. Res. 2019, 7, 1440–1456. [Google Scholar] [CrossRef] [PubMed]
- Perrone, F.; Belluomini, L.; Mazzotta, M.; Bianconi, M.; Di Noia, V.; Meacci, F.; Montrone, M.; Pignataro, D.; Prelaj, A.; Rinaldi, S.; et al. Exploring the role of respiratory microbiome in lung cancer: A systematic review. Crit. Rev. Oncol. Hematol. 2021, 164, 103404. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, N.; Völzke, H.; Schwahn, C.; Kramer, A.; Jünger, M.; Schäfer, T.; John, U.; Kocher, T. Inverse association between periodontitis and respiratory allergies. Clin. Exp. Allergy 2006, 36, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Zhang, Y.; Tong, H.; Zhang, Y.; Lu, T. Potential Mechanisms for Traditional Chinese Medicine in Treating Airway Mucus Hypersecretion Associated with Coronavirus Disease 2019. Front. Mol. Biosci. 2020, 7, 577285. [Google Scholar] [CrossRef] [PubMed]
- Caramori, G.; Casolari, P.; Di Gregorio, C.; Saetta, M.; Baraldo, S.; Boschetto, P.; Ito, K.; Fabbri, L.M.; Barnes, P.J.; Adcock, I.M.; et al. MUC5AC expression is increased in bronchial submucosal glands of stable COPD patients. Histopathology 2009, 55, 321–331. [Google Scholar] [CrossRef]
- Lai, H.Y.; Rogers, D.F. Mucus hypersecretion in asthma: Intracellular signalling pathways as targets for pharmacotherapy. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 67–76. [Google Scholar] [CrossRef]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef]
- Fahy, J.V. Goblet cell and mucin gene abnormalities in asthma. Chest 2002, 122, 320s–326s. [Google Scholar] [CrossRef]
- King, P.T. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin. Transl. Med. 2015, 4, 68. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Okuda, K.; Chen, G.; Subramani, D.B.; Wolf, M.; Gilmore, R.C.; Kato, T.; Radicioni, G.; Kesimer, M.; Chua, M.; Dang, H.; et al. Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways. Am. J. Respir. Crit. Care Med. 2019, 199, 715–727. [Google Scholar] [CrossRef]
- Miya, C.; Cueno, M.E.; Suzuki, R.; Maruoka, S.; Gon, Y.; Kaneko, T.; Yonehara, Y.; Imai, K. Porphyromonas gingivalis gingipains potentially affect MUC5AC gene expression and protein levels in respiratory epithelial cells. FEBS Open Bio. 2021, 11, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, K.; Yanagihara, K.; Harada, Y.; Yamada, K.; Migiyama, Y.; Morinaga, Y.; Hasegawa, H.; Izumikawa, K.; Kakeya, H.; Nishimura, M.; et al. Macrolides inhibit Fusobacterium nucleatum-induced MUC5AC production in human airway epithelial cells. Antimicrob. Agents Chemother. 2013, 57, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Radicioni, G.; Ceppe, A.; Ford, A.A.; Alexis, N.E.; Barr, R.G.; Bleecker, E.R.; Christenson, S.A.; Cooper, C.B.; Han, M.K.; Hansel, N.N.; et al. Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: An analysis of the SPIROMICS cohort. Lancet Respir. Med. 2021, 9, 1241–1254. [Google Scholar] [CrossRef]
- Benedyk, M.; Mydel, P.M.; Delaleu, N.; Płaza, K.; Gawron, K.; Milewska, A.; Maresz, K.; Koziel, J.; Pyrc, K.; Potempa, J. Gingipains: Critical Factors in the Development of Aspiration Pneumonia Caused by Porphyromonas gingivalis. J. Innate Immun. 2016, 8, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, U.C.; Chwieralski, C.E.; Reinhold, D.; Welte, T.; Buhling, F. Radiation-induced matrix production of lung fibroblasts is regulated by interleukin-8. Int. J. Radiat. Biol. 2009, 85, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Grubek-Jaworska, H.; Paplińska, M.; Hermanowicz-Salamon, J.; Białek-Gosk, K.; Dąbrowska, M.; Grabczak, E.; Domagała-Kulawik, J.; Stępień, J.; Chazan, R. IL-6 and IL-13 in induced sputum of COPD and asthma patients: Correlation with respiratory tests. Respiration 2012, 84, 101–107. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, C. The Significance of Serum Interleukin-8 in Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Tanaffos 2018, 17, 13–21. [Google Scholar]
- Benoot, T.; Piccioni, E.; De Ridder, K.; Goyvaerts, C. TNFα and Immune Checkpoint Inhibition: Friend or Foe for Lung Cancer? Int. J. Mol. Sci. 2021, 22, 8691. [Google Scholar] [CrossRef]
- Yu, S.; Xue, M.; Yan, Z.; Song, B.; Hong, H.; Gao, X. Correlation between TNF-α −308 and +489 Gene Polymorphism and Acute Exacerbation of Chronic Obstructive Pulmonary Diseases. Biomed. Res. Int. 2021, 2021, 6661281. [Google Scholar] [CrossRef]
- Traves, S.L.; Culpitt, S.V.; Russell, R.E.; Barnes, P.J.; Donnelly, L.E. Increased levels of the chemokines GROalpha and MCP-1 in sputum samples from patients with COPD. Thorax 2002, 57, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Pink, I.; Raupach, D.; Fuge, J.; Vonberg, R.P.; Hoeper, M.M.; Welte, T.; Rademacher, J. C-reactive protein and procalcitonin for antimicrobial stewardship in COVID-19. Infection 2021, 49, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.C.; Gillespie, D.; White, P.; Bates, J.; Lowe, R.; Thomas-Jones, E.; Wootton, M.; Hood, K.; Phillips, R.; Melbye, H.; et al. C-Reactive Protein Testing to Guide Antibiotic Prescribing for COPD Exacerbations. N. Engl. J. Med. 2019, 381, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.R.; Donaldson, G.C.; Perera, W.R.; Wilkinson, T.M.; Bilello, J.A.; Hagan, G.W.; Vessey, R.S.; Wedzicha, J.A. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006, 174, 867–874. [Google Scholar] [CrossRef]
- Hayata, M.; Watanabe, N.; Tamura, M.; Kamio, N.; Tanaka, H.; Nodomi, K.; Miya, C.; Nakayama, E.; Ueda, K.; Ogata, Y.; et al. The Periodontopathic Bacterium Fusobacterium nucleatum Induced Proinflammatory Cytokine Production by Human Respiratory Epithelial Cell Lines and in the Lower Respiratory Organs in Mice. Cell. Physiol. Biochem. 2019, 53, 49–61. [Google Scholar] [CrossRef]
- Koike, R.; Cueno, M.E.; Nodomi, K.; Tamura, M.; Kamio, N.; Tanaka, H.; Kotani, A.; Imai, K. Heat-Killed Fusobacterium nucleatum Triggers Varying Heme-Related Inflammatory and Stress Responses Depending on Primary Human Respiratory Epithelial Cell Type. Molecules 2020, 25, 3839. [Google Scholar] [CrossRef]
- Takahashi, Y.; Watanabe, N.; Kamio, N.; Yokoe, S.; Suzuki, R.; Sato, S.; Iinuma, T.; Imai, K. Expression of the SARS-CoV-2 Receptor ACE2 and Proinflammatory Cytokines Induced by the Periodontopathic Bacterium Fusobacterium nucleatum in Human Respiratory Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 1352. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Tan, L.; Zhang, S.; Lin, L.; Tang, X.; Pan, Y. Oral Pathogen Fusobacterium nucleatum Coaggregates with Pseudomonas aeruginosa to Modulate the Inflammatory Cytotoxicity of Pulmonary Epithelial Cells. Front. Cell. Infect. Microbiol. 2021, 11, 643913. [Google Scholar] [CrossRef]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef]
- Kim, R.Y.; Pinkerton, J.W.; Essilfie, A.T.; Robertson, A.A.B.; Baines, K.J.; Brown, A.C.; Mayall, J.R.; Ali, M.K.; Starkey, M.R.; Hansbro, N.G.; et al. Role for NLRP3 Inflammasome-mediated, IL-1β-Dependent Responses in Severe, Steroid-Resistant Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 283–297. [Google Scholar] [CrossRef]
- Chiang, T.Y.; Tsao, S.M.; Yeh, C.B.; Yang, S.F. Matrix metalloproteinases in pneumonia. Clin. Chim. Acta 2014, 433, 272–277. [Google Scholar] [CrossRef]
- Opdenakker, G.; Van den Steen, P.E.; Van Damme, J. Gelatinase B: A tuner and amplifier of immune functions. Trends Immunol. 2001, 22, 571–579. [Google Scholar] [CrossRef]
- Grzela, K.; Litwiniuk, M.; Zagorska, W.; Grzela, T. Airway Remodeling in Chronic Obstructive Pulmonary Disease and Asthma: The Role of Matrix Metalloproteinase-9. Arch. Immunol. Ther. Exp. 2016, 64, 47–55. [Google Scholar] [CrossRef]
- Suzuki, R.; Kamio, N.; Sugimoto, K.; Maruoka, S.; Gon, Y.; Kaneko, T.; Yonehara, Y.; Imai, K. Periodontopathic Bacterium Fusobacterium nucleatum Affects Matrix Metalloproteinase-9 Expression in Human Alveolar Epithelial Cells and Mouse Lung. In Vivo 2022, 36, 649–656. [Google Scholar] [CrossRef]
- Suzuki, R.; Kamio, N.; Kaneko, T.; Yonehara, Y.; Imai, K. Fusobacterium nucleatum exacerbates chronic obstructive pulmonary disease in elastase-induced emphysematous mice. FEBS Open Bio 2022, 12, 638–648. [Google Scholar] [CrossRef]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell. Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef]
- He, Y.; Shiotsu, N.; Uchida-Fukuhara, Y.; Guo, J.; Weng, Y.; Ikegame, M.; Wang, Z.; Ono, K.; Kamioka, H.; Torii, Y.; et al. Outer membrane vesicles derived from Porphyromonas gingivalis induced cell death with disruption of tight junctions in human lung epithelial cells. Arch. Oral Biol. 2020, 118, 104841. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, R.; Yi, Z.; Li, Y.; Fu, Y.; Zhang, Y.; Li, P.; Li, X.; Pan, Y. Porphyromonas gingivalis induced inflammatory responses and promoted apoptosis in lung epithelial cells infected with H1N1 via the Bcl-2/Bax/Caspase-3 signaling pathway. Mol. Med. Rep. 2018, 18, 97–104. [Google Scholar] [CrossRef]
- Hashimoto, R.; Takahashi, J.; Shirakura, K.; Funatsu, R.; Kosugi, K.; Deguchi, S.; Yamamoto, M.; Tsunoda, Y.; Morita, M.; Muraoka, K.; et al. SARS-CoV-2 disrupts respiratory vascular barriers by suppressing Claudin-5 expression. Sci. Adv. 2022, 8, eabo6783. [Google Scholar] [CrossRef]
- Lappi-Blanco, E.; Lehtonen, S.T.; Sormunen, R.; Merikallio, H.M.; Soini, Y.; Kaarteenaho, R.L. Divergence of tight and adherens junction factors in alveolar epithelium in pulmonary fibrosis. Hum. Pathol. 2013, 44, 895–907. [Google Scholar] [CrossRef]
- Park, S.; Lee, P.H.; Baek, A.R.; Park, J.S.; Lee, J.; Park, S.W.; Kim, D.J.; Jang, A.S. Association of the Tight Junction Protein Claudin-4 with Lung Function and Exacerbations in Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 2735–2740. [Google Scholar] [CrossRef]
- Haurat, M.F.; Aduse-Opoku, J.; Rangarajan, M.; Dorobantu, L.; Gray, M.R.; Curtis, M.A.; Feldman, M.F. Selective sorting of cargo proteins into bacterial membrane vesicles. J. Biol. Chem. 2011, 286, 1269–1276. [Google Scholar] [CrossRef]
- Mantri, C.K.; Chen, C.H.; Dong, X.; Goodwin, J.S.; Pratap, S.; Paromov, V.; Xie, H. Fimbriae-mediated outer membrane vesicle production and invasion of Porphyromonas gingivalis. Microbiologyopen 2015, 4, 53–65. [Google Scholar] [CrossRef]
- Veith, P.D.; Chen, Y.Y.; Gorasia, D.G.; Chen, D.; Glew, M.D.; O’Brien-Simpson, N.M.; Cecil, J.D.; Holden, J.A.; Reynolds, E.C. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J. Proteome Res. 2014, 13, 2420–2432. [Google Scholar] [CrossRef]
- Pallazola, A.M.; Rao, J.X.; Mengistu, D.T.; Morcos, M.S.; Toma, M.S.; Stolberg, V.R.; Tretyakova, A.; McCloskey, L.; Curtis, J.L.; Freeman, C.M. Human lung cDC1 drive increased perforin-mediated NK cytotoxicity in chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 321, L1183–L1193. [Google Scholar] [CrossRef]
- Okabe, T.; Kamiya, Y.; Kikuchi, T.; Goto, H.; Umemura, M.; Suzuki, Y.; Sugita, Y.; Naiki, Y.; Hasegawa, Y.; Hayashi, J.I.; et al. Porphyromonas gingivalis Components/Secretions Synergistically Enhance Pneumonia Caused by Streptococcus pneumoniae in Mice. Int. J. Mol. Sci. 2021, 22, 2704. [Google Scholar] [CrossRef]
- Kamio, N.; Hayata, M.; Tamura, M.; Tanaka, H.; Imai, K. Porphyromonas gingivalis enhances pneumococcal adhesion to human alveolar epithelial cells by increasing expression of host platelet-activating factor receptor. FEBS Lett. 2021, 595, 1604–1612. [Google Scholar] [CrossRef]
- Walterscheid, J.P.; Ullrich, S.E.; Nghiem, D.X. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J. Exp. Med. 2002, 195, 171–179. [Google Scholar] [CrossRef]
- Iovino, F.; Brouwer, M.C.; van de Beek, D.; Molema, G.; Bijlsma, J.J. Signalling or binding: The role of the platelet-activating factor receptor in invasive pneumococcal disease. Cell. Microbiol. 2013, 15, 870–881. [Google Scholar] [CrossRef]
- Shukla, S.D.; Sohal, S.S.; Mahmood, M.Q.; Reid, D.; Muller, H.K.; Walters, E.H. Airway epithelial platelet-activating factor receptor expression is markedly upregulated in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2014, 9, 853–861. [Google Scholar] [CrossRef]
- Chauhan, S.J.; Thyagarajan, A.; Chen, Y.; Travers, J.B.; Sahu, R.P. Platelet-Activating Factor-Receptor Signaling Mediates Targeted Therapies-Induced Microvesicle Particles Release in Lung Cancer Cells. Int. J. Mol. Sci. 2020, 21, 8517. [Google Scholar] [CrossRef]
- Li, Q.; Pan, C.; Teng, D.; Lin, L.; Kou, Y.; Haase, E.M.; Scannapieco, F.A.; Pan, Y. Porphyromonas gingivalis modulates Pseudomonas aeruginosa-induced apoptosis of respiratory epithelial cells through the STAT3 signaling pathway. Microbes Infect. 2014, 16, 17–27. [Google Scholar] [CrossRef]
- Bromberg, J.; Darnell, J.E., Jr. The role of STATs in transcriptional control and their impact on cellular function. Oncogene 2000, 19, 2468–2473. [Google Scholar] [CrossRef]
- Wang, J.C.; Cordero, J.; Sun, Y.; Aranke, M.; Wolcott, R.; Colmer-Hamood, J.A.; Hamood, A.N. Planktonic Growth of Pseudomonas aeruginosa around a Dual-Species Biofilm Supports the Growth of Fusobacterium nucleatum within That Biofilm. Int. J. Otolaryngol. 2017, 2017, 3037191. [Google Scholar] [CrossRef]
- Eklöf, J.; Sørensen, R.; Ingebrigtsen, T.S.; Sivapalan, P.; Achir, I.; Boel, J.B.; Bangsborg, J.; Ostergaard, C.; Dessau, R.B.; Jensen, U.S.; et al. Pseudomonas aeruginosa and risk of death and exacerbations in patients with chronic obstructive pulmonary disease: An observational cohort study of 22,053 patients. Clin. Microbiol. Infect. 2020, 26, 227–234. [Google Scholar] [CrossRef]
- Kouanda, B.; Sattar, Z.; Geraghty, P. Periodontal Diseases: Major Exacerbators of Pulmonary Diseases? Pulm. Med. 2021, 2021, 4712406. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Özçaka, Ö.; Başoğlu, O.K.; Buduneli, N.; Taşbakan, M.S.; Bacakoğlu, F.; Kinane, D.F. Chlorhexidine decreases the risk of ventilator-associated pneumonia in intensive care unit patients: A randomized clinical trial. J. Periodontal. Res. 2012, 47, 584–592. [Google Scholar] [CrossRef]
- Cabov, T.; Macan, D.; Husedzinović, I.; Skrlin-Subić, J.; Bosnjak, D.; Sestan-Crnek, S.; Perić, B.; Kovac, Z.; Golubović, V. The impact of oral health and 0.2% chlorhexidine oral gel on the prevalence of nosocomial infections in surgical intensive-care patients: A randomized placebo-controlled study. Wien Klin Wochenschr 2010, 122, 397–404. [Google Scholar] [CrossRef]
- Bellissimo-Rodrigues, W.T.; Menegueti, M.G.; Gaspar, G.G.; Nicolini, E.A.; Auxiliadora-Martins, M.; Basile-Filho, A.; Martinez, R.; Bellissimo-Rodrigues, F. Effectiveness of a dental care intervention in the prevention of lower respiratory tract nosocomial infections among intensive care patients: A randomized clinical trial. Infect. Control Hosp. Epidemiol. 2014, 35, 1342–1348. [Google Scholar] [CrossRef]
- Akutsu, Y.; Matsubara, H.; Shuto, K.; Shiratori, T.; Uesato, M.; Miyazawa, Y.; Hoshino, I.; Murakami, K.; Usui, A.; Kano, M.; et al. Pre-operative dental brushing can reduce the risk of postoperative pneumonia in esophageal cancer patients. Surgery 2010, 147, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Han, J.; Liu, Z.; Song, Y.; Wang, Z.; Sun, Z. Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: A 2-year pilot randomized controlled trial. J. Clin. Periodontol. 2014, 41, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Kucukcoskun, M.; Baser, U.; Oztekin, G.; Kiyan, E.; Yalcin, F. Initial periodontal treatment for prevention of chronic obstructive pulmonary disease exacerbations. J. Periodontol. 2013, 84, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Shahoumi, L.A.; Saleh, M.H.A.; Meghil, M.M. Virulence Factors of the Periodontal Pathogens: Tools to Evade the Host Immune Response and Promote Carcinogenesis. Microorganisms 2023, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Tang, P.; Li, C.; Yang, Q.; Xu, Y.; Su, C.; Li, L. Fusobacterium nucleatum and its associated systemic diseases: Epidemiologic studies and possible mechanisms. J. Oral. Microbiol. 2023, 15, 2145729. [Google Scholar] [CrossRef]

| Periodontopathogen | Respiratory Disease | Correlation | References | Year |
|---|---|---|---|---|
| Porphyromonas gingivalis | Pneumonia | P. gingivalis was cultured from the lung aspirates of patients with aspiration pneumonia. | [62,63] | 1974, 2005 |
| P. gingivalis was detected in subglottic lavage samples from intubated and mechanically ventilated patients. | [64] | 2021 | ||
| COPD | Patients with higher IgG titers for P. gingivalis-related antibodies had fewer exacerbations and a lower rate of frequent exacerbations than those with normal IgG titers. | [65] | 2012 | |
| 1. P. gingivalis detected in tracheal aspirates from AECOPD patients was highly homologous to the strains present in the corresponding dental plaque. 2. The levels of P. gingivalis were higher in tracheal aspirates than in oral samples from AECOPD patients. | [66] | 2014 | ||
| The abundance of P. gingivalis was significantly higher in COPD than in non-COPD patients. | [67] | 2017 | ||
| Patients with COPD showed a statistically remarkable negative correlation between FEV1% and P. gingivalis content. | [68] | 2019 | ||
| Lung Cancer | 1. P. gingivalis-stained sections were significantly more frequent and intense in cancerous tissues of small cell lung cancer, lung adenocarcinoma, and lung squamous cell carcinoma, compared with adjacent lung tissues. 2. Lung cancer patients with P. gingivalis infection also had significantly lower survival rates and median survival times. | [69] | 2021 | |
| The risk of developing lung cancer shows a positive correlation with the serum levels of IgG antibodies directed against P. gingivalis. | [70,71] | 2023 | ||
| Asthma | Higher IgG concentrations of P. gingivalis-related antibodies were significantly associated with a diminished prevalence of asthma. | [72] | 2006 | |
| Subcutaneously injected P. gingivalis reduces airway responsiveness in ovalbumin-induced asthma mice. | [73] | 2010 | ||
| Other Respiratory Diseases | The presence of P. gingivalis has been detected in the BALF of certain emphysema patients. | [74] | 2011 | |
| A case of subcutaneous chest wall abscess caused by P. gingivalis infection is reported. | [75] | 2022 | ||
| Fusobacterium nucleatum | Pneumonia | A total of 54.8% of lower respiratory tract specimens of patients with bacterial pneumonia tested positive for F. nucleatum by real-time PCR. | [76] | 2017 |
| F. nucleatum was found in the blood culture of patients with orotracheal intubation and demonstrated higher values in mini-bronchoalveolar lavage, potentially contributing to VAP. | [77] | 2018 | ||
| Reports the case of a patient who suffered from pneumonia with chest wall invasion by F. nucleatum. | [78] | 2021 | ||
| Reports four cases of F. nucleatum bacteremia associated with coronavirus pneumonia. | [79] | 2021 | ||
| The pharyngeal F. nucleatum was significantly increased in COVID-19 patients and was higher in male than female patients. | [80] | 2021 | ||
| COPD | 1. A total of 60.8% of individuals with AECOPD had F. nucleatum present in their tracheal aspirates. 2. FEV1% of AECOPD patients gradually decreased as the number of F. nucleatum rose. | [81] | 2020 | |
| Lung Cancer | The presence of F. nucleatum is associated with the risk of developing systemic cancers, especially lung cancer among postmenopausal females. | [82] | 2016 | |
| Airway-enriched F. nucleatum before anti-PD-1 treatment was associated with resistance to anti-PD-1 response in lung cancer. | [83] | 2022 | ||
| F. nucleatum was detected within pleural effusions of lung cancer patients. | [84] | 2023 | ||
| The risk of developing lung cancer shows a positive correlation with the serum levels of IgG antibodies directed against F. nucleatum. | [71] | 2023 | ||
| Other Respiratory Diseases | IgA antibody levels against F. nucleatum in the sputum of patients with acute exacerbations of chronic bronchitis were on average 3.5 times higher than in healthy controls. | [85] | 2003 | |
| Examination of sputum from cystic fibrosis patients utilizing 16S rRNA gene sequencing illuminated that the relative abundance of F. nucleatum exceeded 50%. | [86] | 2015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Wang, J.; Dong, J.; Hu, P.; Guo, Q. Periodontopathogens Porphyromonas gingivalis and Fusobacterium nucleatum and Their Roles in the Progression of Respiratory Diseases. Pathogens 2023, 12, 1110. https://doi.org/10.3390/pathogens12091110
Shi T, Wang J, Dong J, Hu P, Guo Q. Periodontopathogens Porphyromonas gingivalis and Fusobacterium nucleatum and Their Roles in the Progression of Respiratory Diseases. Pathogens. 2023; 12(9):1110. https://doi.org/10.3390/pathogens12091110
Chicago/Turabian StyleShi, Tao, Jiale Wang, Jiajia Dong, Pingyue Hu, and Qiang Guo. 2023. "Periodontopathogens Porphyromonas gingivalis and Fusobacterium nucleatum and Their Roles in the Progression of Respiratory Diseases" Pathogens 12, no. 9: 1110. https://doi.org/10.3390/pathogens12091110
APA StyleShi, T., Wang, J., Dong, J., Hu, P., & Guo, Q. (2023). Periodontopathogens Porphyromonas gingivalis and Fusobacterium nucleatum and Their Roles in the Progression of Respiratory Diseases. Pathogens, 12(9), 1110. https://doi.org/10.3390/pathogens12091110






