Bloodstream Infections by AmpC-Producing Enterobacterales: Risk Factors and Therapeutic Outcome
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Bacterial Isolates
2.3. Antibiotic Susceptibility
3. Results
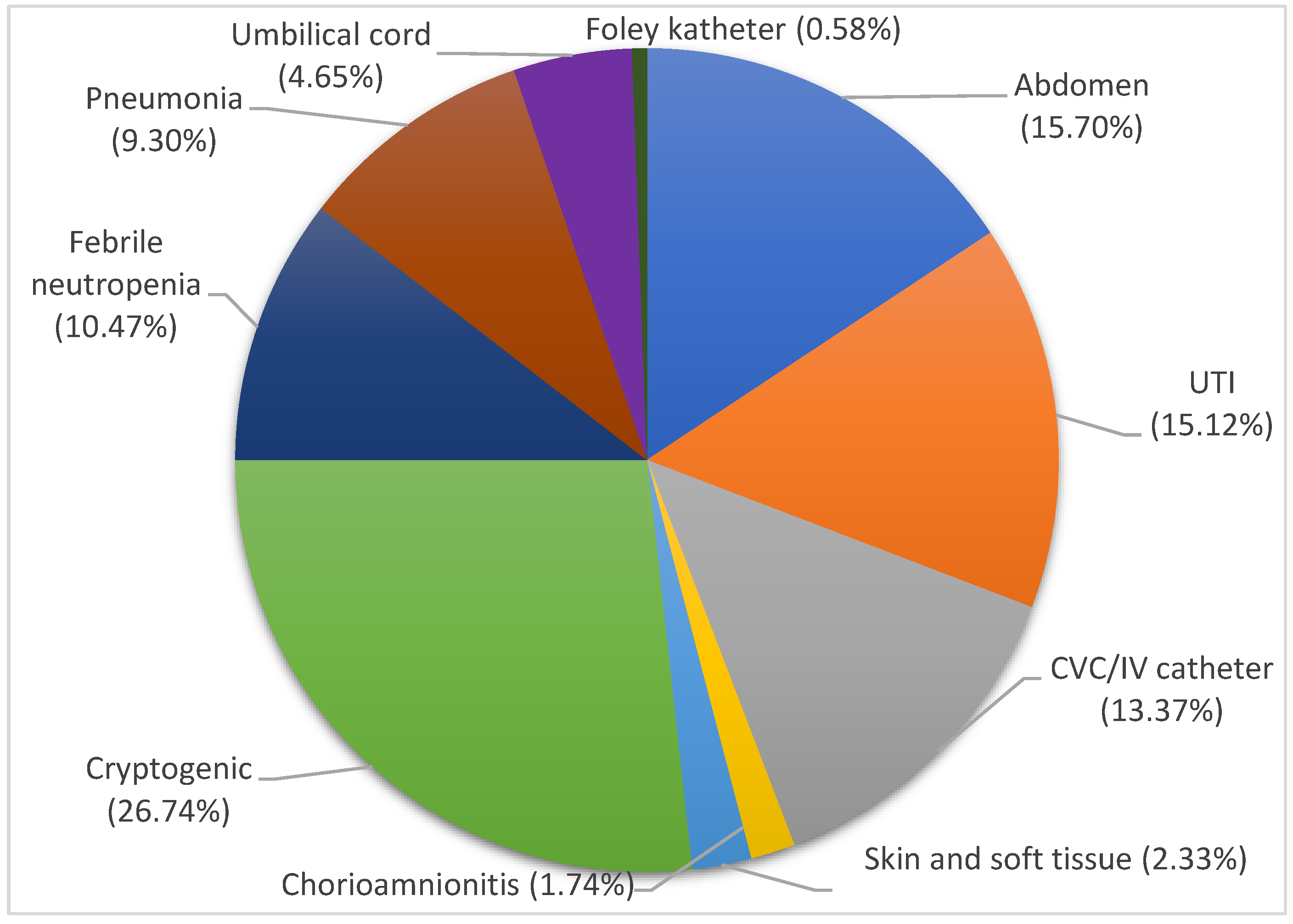
3.1. Patients
3.2. Bacterial Isolates
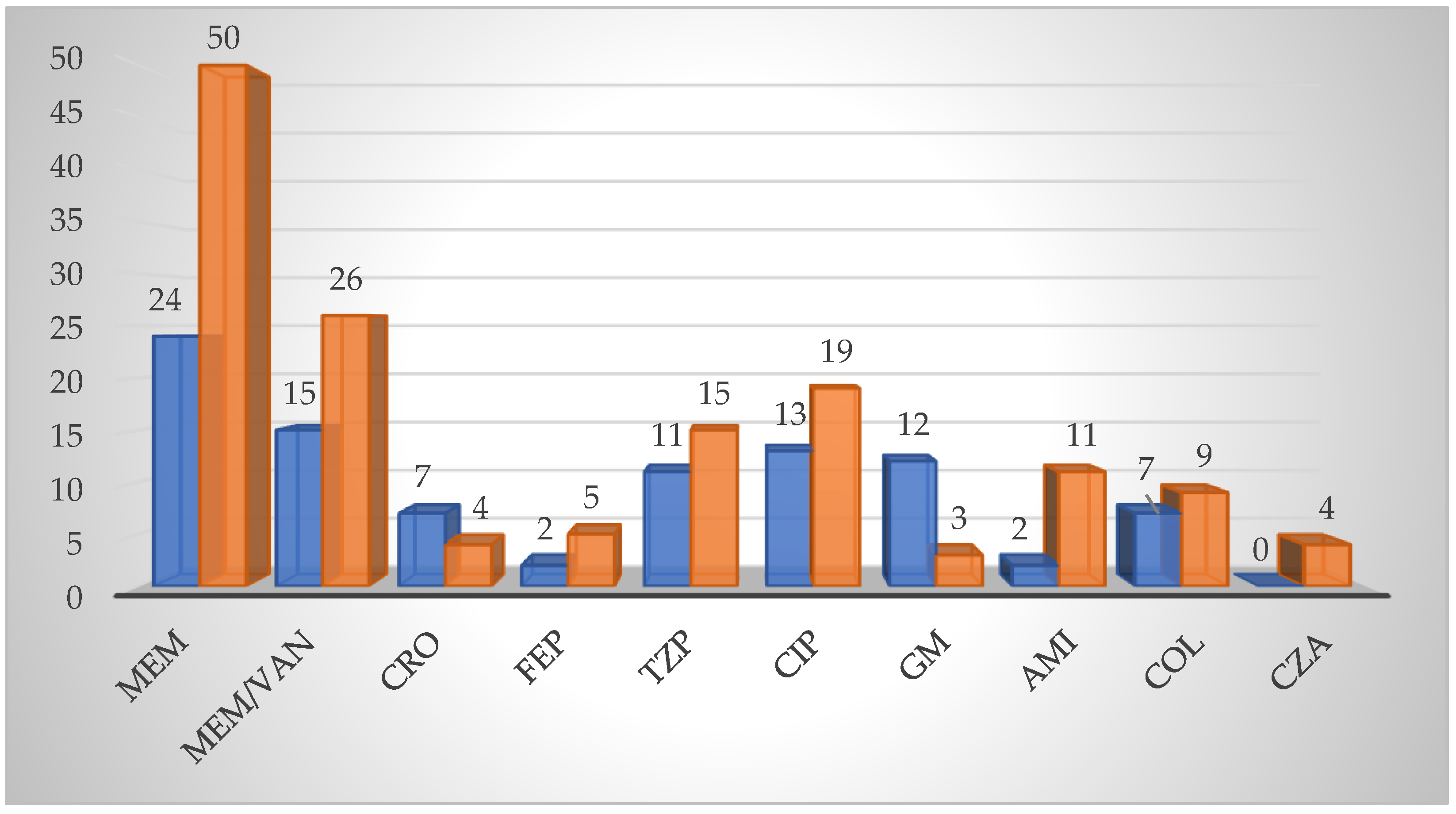
3.3. Antibiotic Resistance Patterns and Determinants
3.4. Antibiotic Therapy Outcome
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Perez-Palacios, P.; Girlich, D.; Soraa, N.; Lamrani, A.; Maoulainine, F.M.R.; Bennaoui, F.; Amri, H.; El Idrissi, N.S.; Bouskraoui, M.; Birer, A.; et al. Multidrug-resistant Enterobacterales responsible for septicaemia in a neonatal intensive care unit in Morocco. J. Glob. Antimicrob. Resist. 2023, 33, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Meini, S.; Tascini, C.; Cei, M.; Sozio, E.; Rossolini, G.M. AmpC β-lactamase-producing Enterobacterales: What a clinician should know. Infection 2019, 47, 363–375. [Google Scholar] [CrossRef]
- Jacoby, G.A. AmpC β-lactamases. J. Clin. Microbiol. 2009, 22, 161–182. [Google Scholar] [CrossRef]
- Bennet, P.M.; Chopra, I. Molecular basis of β-lactamase induction in bacteria. Antimicrob. Agents Chemother. 1993, 37, 153–158. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: An update on their characteristics, epidemiology and detection. JAC Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef] [PubMed]
- Canton, R.; Akova, M.; Carmeli, Y.; Giske, C.G.; Glupczynski, Y.; Gniadkowski, M.; Miriagou, V.; Naas, T.; Rossolini, G.M.; Samuelsen, Ø.; et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 2012, 18, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile beta-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, V.P.; Pitout, J.D.; Dalton, B.; Gregson, D.B.; Ross, T.; Laupland, K.B. Clinical and microbiological characteristics of bloodstream infections due to AmpC β-lactamase producing Enterobacteriaceae: An active surveillance cohort in a large centralized Canadian region. BMC Infect. Dis. 2014, 14, 647. [Google Scholar] [CrossRef]
- Salamat, S.; Ejaz, H.; Zafar, A.; Javed, H. Detection of AmpC β-lactamase producing bacteria isolated in neonatal sepsis. Pak. J. Med. Sci. 2016, 32, 1512–1516. [Google Scholar] [CrossRef] [PubMed]
- Bedenić, B.; Firis, N.; Elveđi-Gašparović, V.; Krilanović, M.; Matanović, K.; Štimac, I.; Luxner, J.; Vraneš, J.; Meštrović, T.; Zarfel, G.; et al. Emergence of multidrug-resistant Proteus mirabilis in a long-term care facility in Croatia. Wien. Klin. Wochenschrift. 2016, 128, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Giakkoupi, P.; Tambic-Andrasevic, A.; Vourli, S.; Skrlin, J.; Sestan-Crnek, S.; Tzouvelekis, L.S.; Vatopoulos, A.C. Transferable DHA-1 cephalosporinase in Escherichia coli. Int. J. Antimicrob. Agents 2006, 27, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Franolić-Kukina, I.; Sardelić, S.; Beader, N.; Varda-Brkić, D.; Firis, N.; Čačić, M.; Šijak, D.; Frančula-Zaninović, S.; Elveđi-Gašparović, V.; Mareković, I.; et al. Evolution of resistance in Enterobacter spp. in Croatia. Liječ. Vjesn. 2016, 138, 240–249. [Google Scholar]
- Kleinpell, R.M.; Schorr, C.A.; Balk, R.A. The New Sepsis Definitions: Implications for Critical Care Practitioners. Am. J. Crit. Care 2016, 25, 457–464. [Google Scholar] [CrossRef]
- Dingle, T.C.; Butler-Wu, S.M. Maldi-tof mass spectrometry for microorganism identification. Clin. Lab. Med. 2013, 33, 589–609. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12. 2022. Available online: http://www.eucast.org (accessed on 1 October 2022).
- Jarlier, V.; Nicolas, M.H.; Fournier, G.; Philippon, A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: Hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 1988, 10, 867–878. [Google Scholar] [CrossRef]
- Coudron, P.E. Inhibitor-based methods for detection of plasmid-mediated AmpC β-lactamases in Klebsiella spp., Escherichia coli and Proteus mirabilis. J. Clin. Microbiol. 2005, 43, 4163–4167. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.W.; Chibabhai, V. Evaluation of the RESIST-4 O.K.N.V immunochromatographic lateral flow assay for the rapid detection of OXA-48, KPC, NDM and VIM carbapenemases from cultured isolates. Access Microbiol. 2019, 1, e000031. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Navarro, M.D.; Romero, L.; Muniain, M.A.; de Cueto, M.; Ríos, M.J.; Hernández, J.R.; Pascual, A. Bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli in the CTX-M era: A new clinical challenge. Clin. Infect. Dis. 2006, 43, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha Ferreira, T.; Martins, I.S. Risk Factors of Death in Bloodstream Infections Caused by AmpC β-Lactamase-Producing Enterobacterales in Patients with Neoplasia. Infect. Drug Resist. 2021, 14, 3083–3097. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, S.; Nguyen, Q.; Nguyen Thi Nguyen, T.; Pham, D.T.; Rabaa, M.A.; Dongol, S.; Basnyat, B.; Dixit, S.M.; Baker, S.; Karkey, A. Genomic epidemiology, antimicrobial resistance and virulence factors of Enterobacter cloacae complex causing potential community-onset bloodstream infections in a tertiary care hospital of Nepal. JAC Antimicrob. Resist. 2022, 4, dlac050. [Google Scholar] [CrossRef] [PubMed]
- Intra, J.; Carcione, D.; Sala, R.M.; Siracusa, C.; Brambilla, P.; Leoni, V. Antimicrobial Resistance Patterns of Enterobacter cloacae and Klebsiella aerogenes Strains Isolated from Clinical Specimens: A Twenty-Year Surveillance Study. Antibiotics 2023, 12, 775. [Google Scholar] [CrossRef]
- Soltani, J.; Poorabbas, B.; Miri, N.; Mardaneh, J. Health care associated infections, antibiotic resistance and clinical outcome: A surveillance study from Sanandaj, Iran. World J. Clin. Cases 2016, 4, 63–70. [Google Scholar] [CrossRef]
- Simsek, M. Determination of the antibiotic resistance rates of Serratia marcescens isolates obtained from various clinical specimens. Niger. J. Clin. Pract. 2019, 22, 125–130. [Google Scholar] [CrossRef]
- Laupland, K.B.; Paterson, D.L.; Edwards, F.; Stewart, A.G.; Harris, P.N.A. Morganella morganii, an Emerging Cause of Bloodstream Infections. Microbiol. Spectr. 2022, 10, e0056922. [Google Scholar] [CrossRef]
- Liu, J.; Wang, R.; Fang, M. Clinical and drug resistance characteristics of Providencia stuartii infections in 76 patients. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef]
- Liu, L.; Chen, D.; Liu, L.; Lan, R.; Hao, S.; Jin, W.; Sun, H.; Wang, Y.; Liang, Y.; Xu, J. Genetic Diversity, Multidrug Resistance, and Virulence of Citrobacter freundii From Diarrheal Patients and Healthy Individuals. Front. Cell Infect. Microbiol. 2018, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Bedenić, B.; Slade, M.; Starčević, L.Ž.; Sardelić, S.; Vranić-Ladavac, M.; Benčić, A.; Zujić Atalić, V.; Bogdan, M.; Bubonja-Šonje, M.; Tomić-Paradžik, M.; et al. Epidemic spread of OXA-48 β-lactamase in Croatia. J. Med. Microbiol. 2018, 67, 1031–1041. [Google Scholar] [CrossRef]
- Jelić, M.; Škrlin, J.; Bejuk, D.; Košćak, I.; Butić, I.; Gužvinec, M.; Tambić-Andrašević, A. Characterization of isolates associated with emergence of OXA-48-producing Klebsiella pneumoniae in Croatia. Microb. Drug Resist. 2018, 24, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Tomić Paradžik, M.; Drenjančević, D.; Presečki-Stanko, A.; Kopić, J.; Talapko, J.; Zarfel, G.; Bedenić, B. Hidden Carbapenem Resistance in OXA-48 and Extended-Spectrumβ-Lactamase-Positive Escherichia coli. Microb. Drug Resist. 2019, 25, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Šuto, S.; Bedenić, B.; Likić, S.; Kibel, S.; Anušić, M.; Tičić, V.; Zarfel, G.; Grisold, A.; Barišić, I.; Vraneš, J. Diffusion of OXA-48 carbapenemase among urinary isolates of Klebsiella pneumoniae in non-hospitalized elderly patients. BMC Microbiol. 2022, 22, 30. [Google Scholar] [CrossRef]
- Bedenić, B.; Sardelić, S.; Luxner, J.; Bošnjak, Z.; Varda-Brkić, D.; Lukić-Grlić, A.; Mareković, I.; Frančula-Zaninović, S.; Krilanović, M.; Šijak, D.; et al. Molecular characterization of class B carbapenemases in advanced stage of dissemination and emergence of class D carbapenemases in Enterobacteriaceae from Croatia. Infect. Genet. Evol. 2016, 43, 74–82. [Google Scholar] [CrossRef]
- Atalić, V.Z.; Bedenić, B.; Kocsis, E.; Mazzariol, A.; Sardelić, S.; Barišić, M.; Plečko, V.; Bošnjak, Z.; Mijač, M.; Jajić, I.; et al. Diversity of carbapenemases in clinical isolates of Enterobacteriaceae in Croatia—The results of a multicentre study. Clin. Microbiol. Infect. 2014, 20, O894–O903. [Google Scholar] [CrossRef][Green Version]
- Serefhanoglu, K.; Turan, H.; Timurkaynak, F.E.; Arslan, H. Bloodstream infections caused by ESBL-producing E. coli and K. pneumoniae: Risk factors for multidrug-resistance. Braz. J. Infect. Dis. 2009, 13, 403–407. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; González-Rico, C.; Gozalo-Margüello, M.; Marco, F.; Gracia-Ahufinger, I.; Aranzamendi, M.; Sánchez-Díaz, A.M.; Vicente-Rangel, T.; Chaves, F.; Calvo Montes, J.; et al. Molecular characterization of multidrug resistant Enterobacterales strains isolated from liver and kidney transplant recipients in Spain. Sci. Rep. 2021, 11, 11875. [Google Scholar] [CrossRef]
- Mashaly, G.E.; Mashaly, M.E. Colistin-heteroresistance in carbapenemase-producing Enterobacter species causing hospital-acquired infections among Egyptian patients. J. Glob. Antimicrob. Resist. 2021, 24, 108–113. [Google Scholar] [CrossRef]
- Carlsen, L.; Büttner, H.; Christner, M.; Franke, G.; Indenbirken, D.; Knobling, B.; Lütgehetmann, M.; Knobloch, J. High burden and diversity of carbapenemase-producing Enterobacterales observed in wastewater of a tertiary care hospital in Germany. Int. J. Hyg. Env. Environ. Health 2022, 242, 113968. [Google Scholar] [CrossRef] [PubMed]
- Bedenić, B.; Luxner, J.; Car, H.; Sardelić, S.; Bogdan, M.; Varda-Brkić, D.; Šuto, S.; Grisold, A.; Beader, N.; Zarfel, G. Emergence and Spread of Enterobacterales with Multiple Carbapenemases after COVID-19 Pandemic. Pathogens 2023, 12, 677. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Antimicrobial-Resistant Treatment Guidance: Gram-Negative Bacterial Infections; Version 3.0; Infectious Diseases Society of America: Arlington, VA, USA, 2023; Available online: https://www.idsociety.org/practice-guideline/amr-guidance/ (accessed on 27 August 2023).
- McKamey, L.; Venugopalan, V.; Cherabuddi, K.; Borgert, S.; Voils, S.; Shah, K.; Klinker, K.P. Assessing antimicrobial stewardship initiatives: Clinical evaluation of cefepime or piperacillin/tazobactam in patients with bloodstream infections secondary to AmpC-producing organisms. Int. J. Antimicrob. Agents 2018, 52, 719–723. [Google Scholar] [CrossRef]
- Cheng, L.; Nelson, B.C.; Mehta, M.; Seval, N.; Park, S.; Giddin, M.J.; Shi, Q.; Whitter, S.; Gomez Simonds, A.; Uhlemann, A.C. Piperacillin-tazobactam versus other antibacterial agents for treatment of bloodstream infections due to AmpC β-lactamase producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61, e00276-17. [Google Scholar] [CrossRef] [PubMed]
- Negri, M.C.; Baquero, F. In vitro selective concentrations of cefepime and ceftazidime for AmpC beta-lactamase hyperproducer Enterobacter cloacae variants. Clin. Microbiol. Infect. 1999, 5 (Suppl. S1), S25–S28. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, K.H.; Chung, J.W.; Sung, H.; Choi, S.H.; Choi, S.H. Prevalence and impact of extended-spectrum β-lactamase production on clinical outcomes in cancer patients with Enterobacter species bacteremia. Korean J. Intern. Med. 2014, 29, 637–646. [Google Scholar] [CrossRef]
- de Jonge, B.L.; Karlowsky, J.A.; Kazmierczak, K.M.; Biedenbach, D.J.; Sahm, D.F.; Nichols, W.W. In Vitro Susceptibility to Ceftazidime-Avibactam of Carbapenem-Nonsusceptible Enterobacteriaceae Isolates Collected during the INFORM Global Surveillance Study (2012 to 2014). Antimicrob. Agents Chemother. 2016, 60, 3163–3169. [Google Scholar] [CrossRef]
- Tot, T.; Kibel, S.; Sardelić, S.; Nemer, K.; Benčić, A.; Vraneš, J.; Krilanović, M.; Jelić, M.; Tripković, M.; Bubonja-Šonje, M.; et al. Polyclonal spread of colistin resistant Klebsiella pneumoniae in Croatian hospitals and outpatient setting. Germs 2021, 11, 163–178. [Google Scholar] [CrossRef]
- D’Onofrio, V.; Conzemius, R.; Varda-Brkić, D.; Bogdan, M.; Grisold, A.; Gyssens, I.C.; Bedenić, B.; Barišić, I. Epidemiology of Colistin-Resistant, Carbapenemase-Producing Enterobacteriaceae and Acinetobacter Baumannii in Croatia. Infect. Genet. Evol. 2020, 81, 104263. [Google Scholar] [CrossRef]
- Wesevich, A.; Sutton, G.; Ruffin, F.; Park, L.P.; Fouts, D.E.; Fowler, V.G., Jr.; Thaden, J.T. Newly Named Klebsiella aerogenes (formerly Enterobacter aerogenes) Is Associated with Poor Clinical Outcomes Relative to Other Enterobacter Species in Patients with Bloodstream Infection. J. Clin. Microbiol. 2020, 58, e00582-20. [Google Scholar] [CrossRef]

| TZP | CAZ | CTX | CRO | FEP | ETP | IMI | MEM | GM | AMI | CIP | COL | ESBL | AmpC Hyper-Production | Carbapenemase | Type of Carbapenemase | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enterobacter cloacae n = 87 | 24.14% (21/87) | 34.48% (30/87) | 35.63% (31/87) | 34.48% (30/87) | 27.59% (24/87) | 21.84% (19/87) | 5.75% (5/87) | 5.75% (5/87) | 20.69% (18/87) | 1.15% (1/87) | 20.69% (18/87) | 0.00% (0/87) | 11.49% (10/87) | 22.99% (20/87) | 4.60% (4/87) | VIM (n = 2) NDM (n = 2) |
| Klebsiella aerogenes n = 16 | 62.50% (10/16) | 62.50% (10/16) | 62.50% (10/16) | 62.50% (10/16) | 31.25% (5/16) | 43.75% (7/16) | 6.25% (1/16) | 6.25% (1/16) | 25.00% (4/16) | 12.50% (2/16) | 18.75% (3/16) | 0.00% (0/16) | 18.75% (3/16) | 31.25% (5/16) | 12.50% (2/16) | OXA-48 (n = 2) |
| Serratia marcescens n = 47 | 8.51% (4/47) | 27.66% (13/47) | 25.53% (12/47) | 27.66% (13/47) | 23.40% (11/47) | 6.38% (3/47) | 4.25% (2/47) | 4.25% (2/47) | 25.53% (12/47) | 14.89% (7/47) | 8.51% (4/47) | 0.00% (0/47) | 19.15% (9/47) | 17.02% (8/47) | 2.13% (1/47) | OXA-48 (n = 1) |
| Morganella morganii n = 9 | 22.22% (2/9) | 11.11% (1/9) | 11.11% (1/9) | 11.11% (1/9) | 22.22% (2/9) | 22.22% (2/9) | 0.00% (0/9) | 0.00% (0/9) | 0.00% (0/9) | 0.00% (0/9) | 0.00% (0/9) | 0.00% (0/9) | 0.00% (0/9) | 0.00% (0/9) | 22.22% (2/9) | OXA-48 (n = 2) |
| Providencia spp. n = 11 | 27.27% (3/11) | 63.64% (7/11) | 63.64% (7/11) | 63.64% (7/11) | 36.36% (4/11) | 27.27% (3/11) | 27.27% (3/11) | 0.00% (0/11) | 63.64% (7/11) | 27.27% (3/11) | 72.73% (8/11) | 0.00% (0/11) | 18.18% (2/11) | 45.45% (5/11) | 27.27% (3/11) | OXA-48 (n = 3) |
| Citrobacter freundii n = 9 | 33.33% (3/9) | 33.33% (3/9) | 33.33% (3/9) | 33.33% (3/9) | 22.22% (2/9) | 11.11% (1/9) | 11.11% (1/9) | 11.11% (1/9) | 33.33% (3/9) | 11.11% (1/9) | 33.33% (3/9) | 0.00% (0/9) | 11.11% (1/9) | 22.22% (2/9) | 11.11% (1/9) | KPC (n = 1) NDM (n = 1) |
| Enterobacter spp. n = 18 | 16.67% (3/18) | 16.67% (3/18) | 16.67% (3/18) | 16.67% (3/18) | 11.11% (2/18) | 11.11% (2/18) | 0.00% (0/18) | 0.00% (0/18) | 5.56% (1/18) | 0.00% (0/18) | 5.56% (1/18) | 0.00% (0/18) | 5.56% (1/18) | 16.67% (3/18) | 0.00% (0/18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pospišil, M.; Car, H.; Elveđi-Gašparović, V.; Beader, N.; Herljević, Z.; Bedenić, B. Bloodstream Infections by AmpC-Producing Enterobacterales: Risk Factors and Therapeutic Outcome. Pathogens 2023, 12, 1125. https://doi.org/10.3390/pathogens12091125
Pospišil M, Car H, Elveđi-Gašparović V, Beader N, Herljević Z, Bedenić B. Bloodstream Infections by AmpC-Producing Enterobacterales: Risk Factors and Therapeutic Outcome. Pathogens. 2023; 12(9):1125. https://doi.org/10.3390/pathogens12091125
Chicago/Turabian StylePospišil, Mladen, Haris Car, Vesna Elveđi-Gašparović, Nataša Beader, Zoran Herljević, and Branka Bedenić. 2023. "Bloodstream Infections by AmpC-Producing Enterobacterales: Risk Factors and Therapeutic Outcome" Pathogens 12, no. 9: 1125. https://doi.org/10.3390/pathogens12091125
APA StylePospišil, M., Car, H., Elveđi-Gašparović, V., Beader, N., Herljević, Z., & Bedenić, B. (2023). Bloodstream Infections by AmpC-Producing Enterobacterales: Risk Factors and Therapeutic Outcome. Pathogens, 12(9), 1125. https://doi.org/10.3390/pathogens12091125







