Central Nervous System Tuberculosis in a Murine Model: Neurotropic Strains or a New Pathway of Infection?
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Mycobacterial Strains
2.3. Experimental Murine Model
2.4. Histology Sample Preparation
2.5. Determination of Colony Forming Units
2.6. Statistical Analysis
3. Results
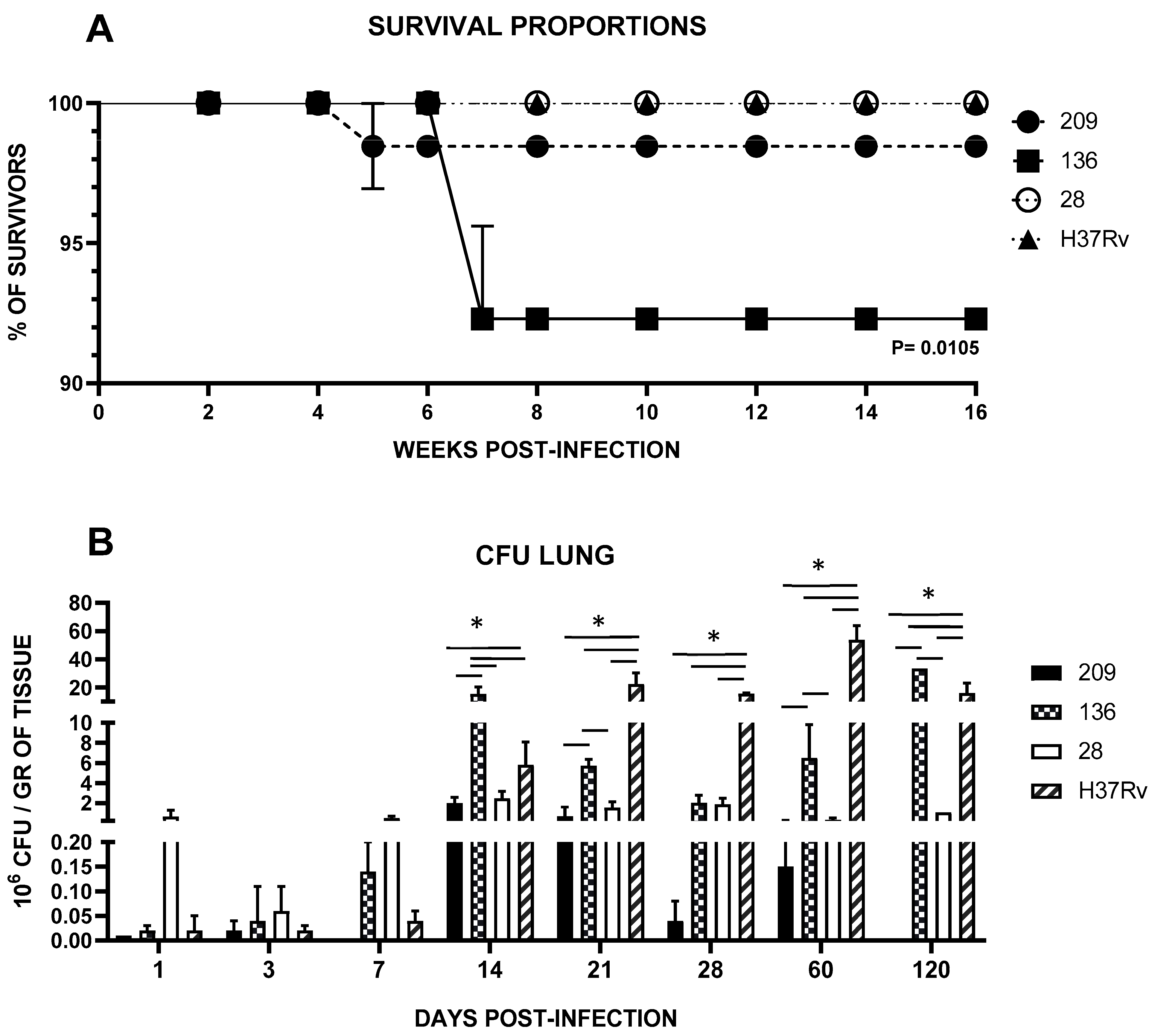
3.1. Superior Lethality, Virulence and Pulmonary Diseminationto of Neurotropic Strains
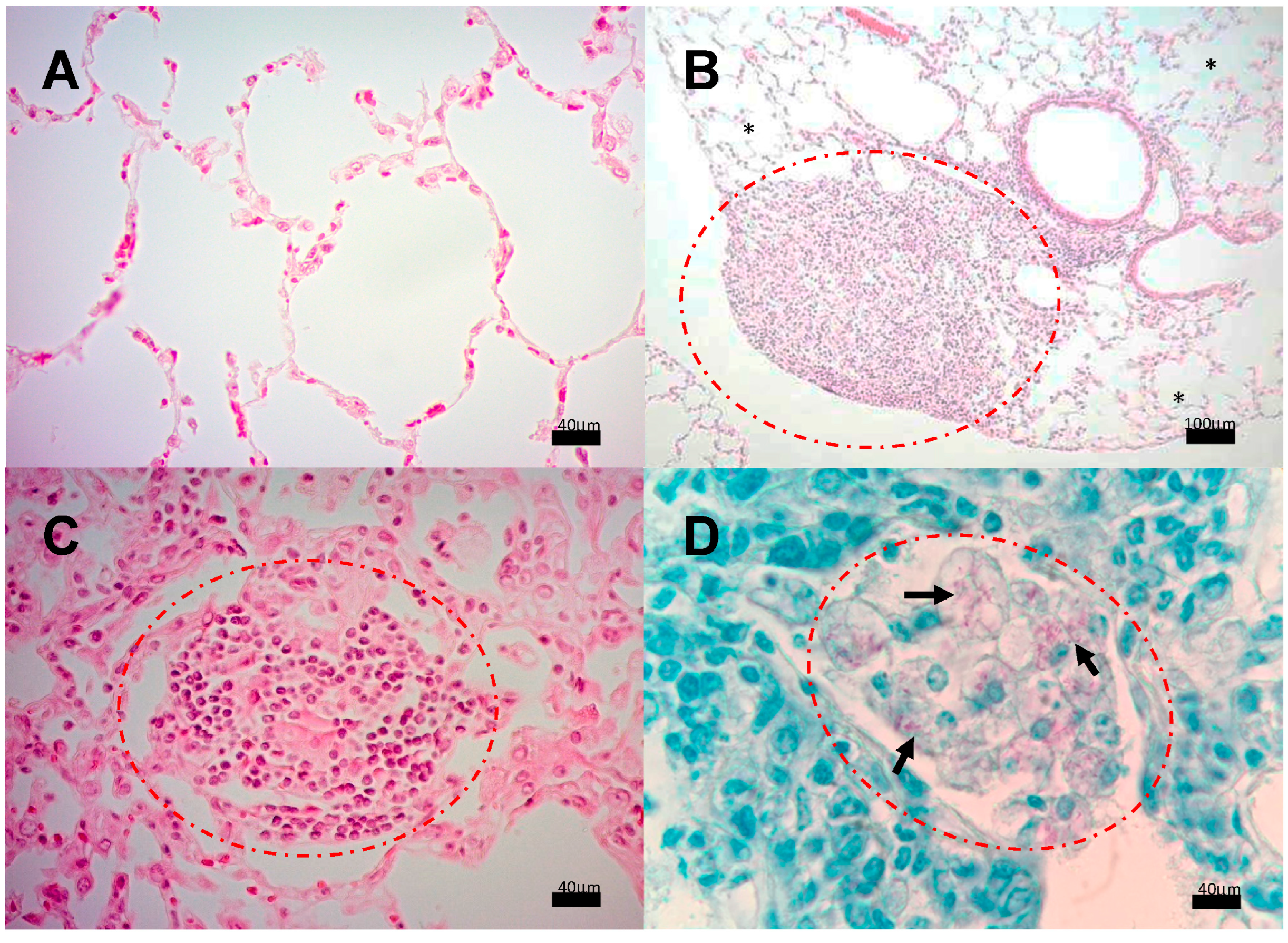
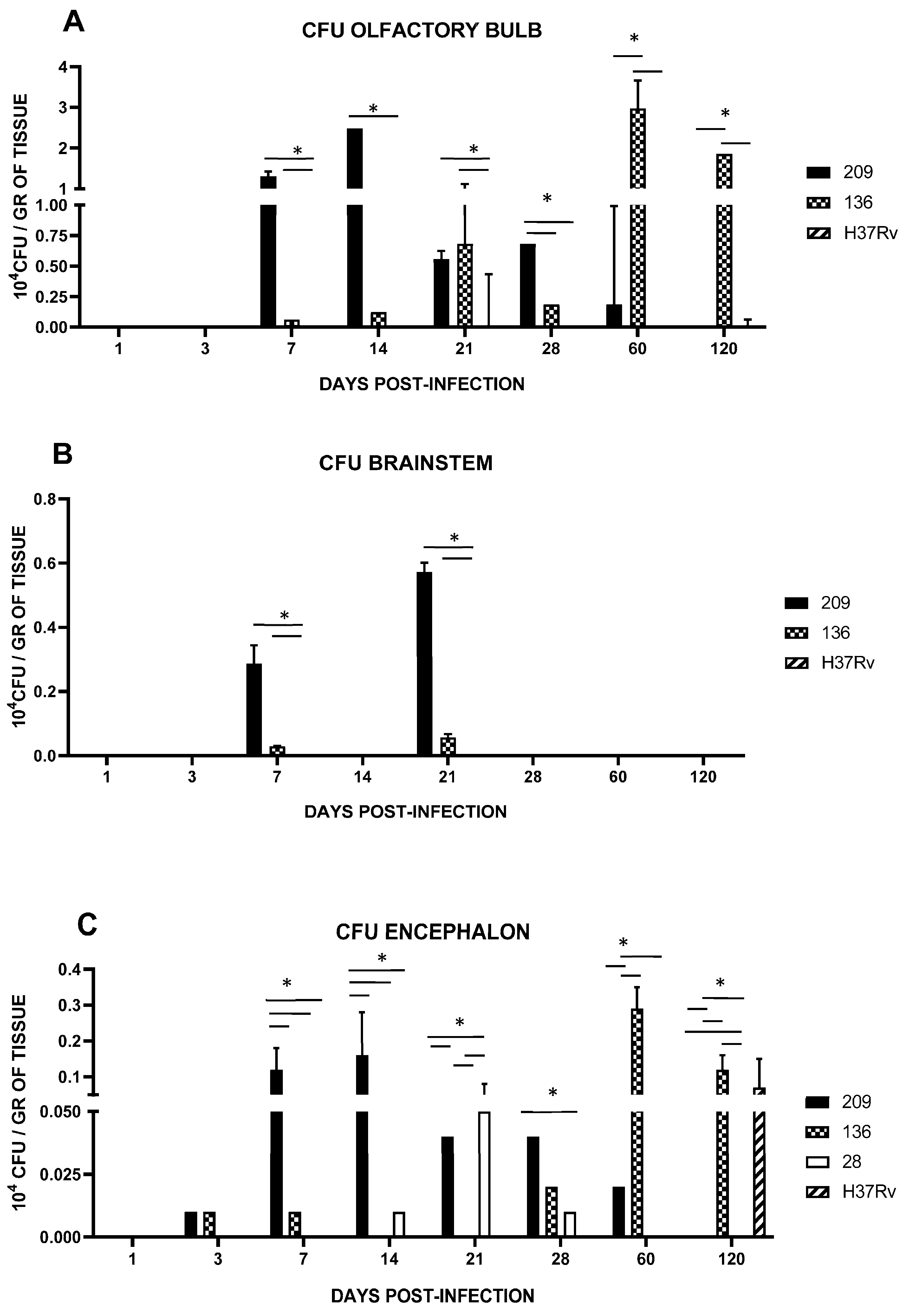
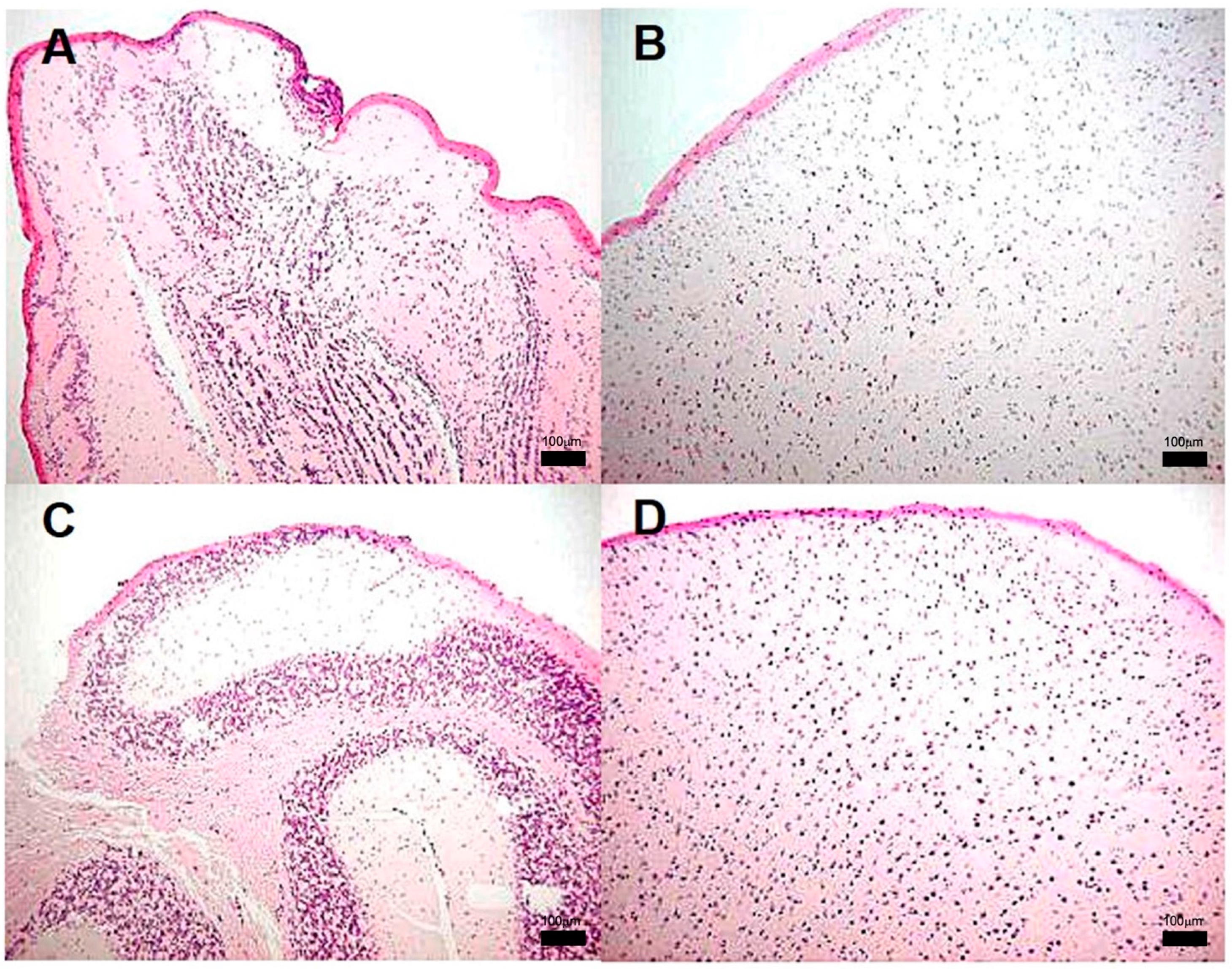
3.2. Encephalon Histology and Bacillary Load
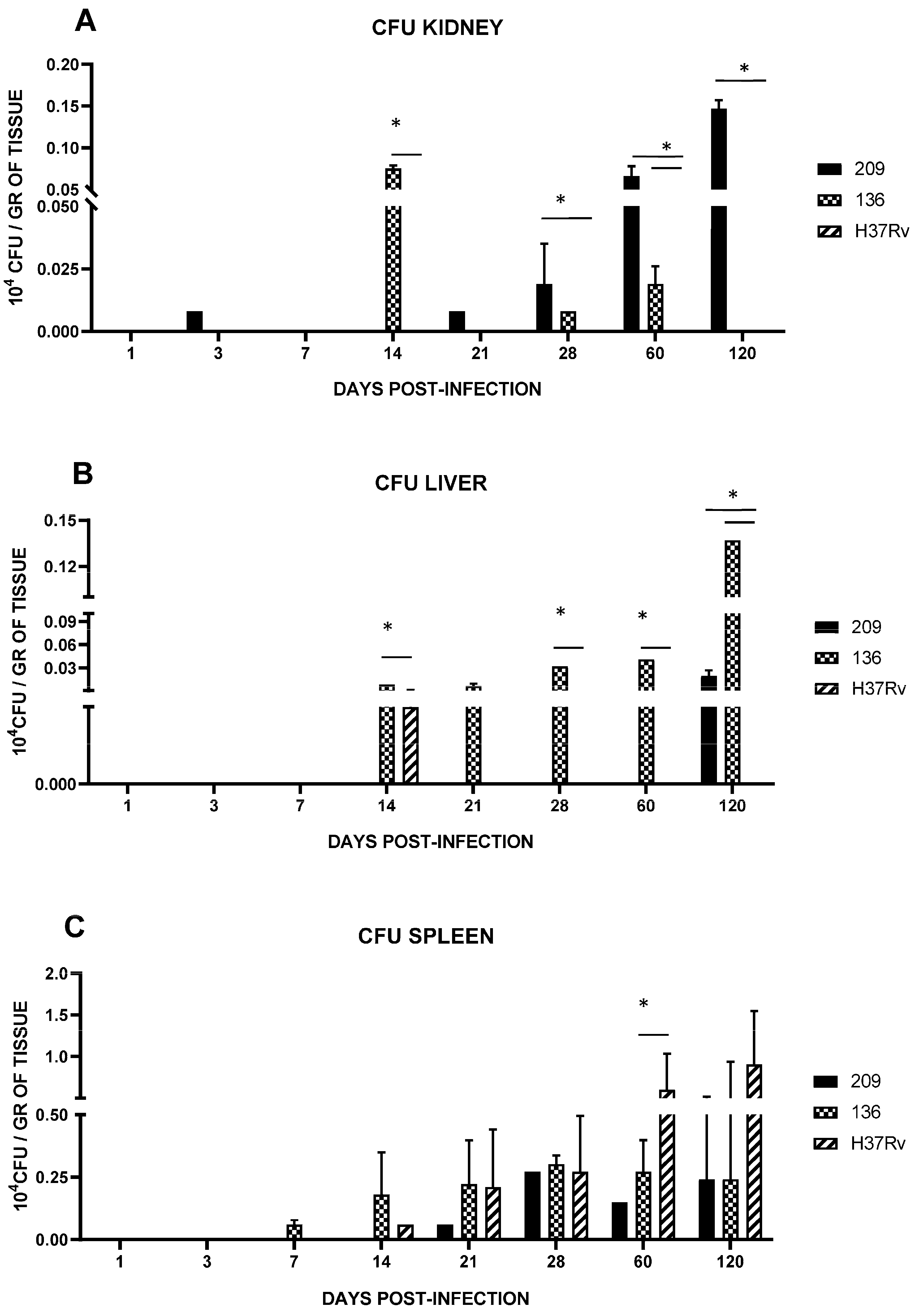
3.3. Spleen, Liver, and Kidney Mycobacterial Loads
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Batista, L.A.F.; Silva, K.J.S.; da Costa e Silva, L.M.; de Moura, Y.F.; Zucchi, F.C.R. Tuberculosis: A Granulomatous Disease Mediated by Epigenetic Factors. Tuberculosis 2020, 123, 101943. [Google Scholar] [CrossRef] [PubMed]
- Cherian, A.; Thomas, S.V. Central Nervous System Tuberculosis. Afr. Health Sci. 2011, 11, 116–127. [Google Scholar] [PubMed]
- Rock, R.B.; Olin, M.; Baker, C.A.; Molitor, T.W.; Peterson, P.K. Central Nervous System Tuberculosis: Pathogenesis and Clinical Aspects. Clin. Microbiol. Rev. 2008, 21, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Be, N.A.; Kim, K.S.; Bishai, W.R.; Jain, S.K. Pathogenesis of Central Nervous System Tuberculosis. Curr. Mol. Med. J. 2009, 9, 94–99. [Google Scholar] [CrossRef]
- Bartzatt, R. Tuberculosis Infections of the Central Nervous System. Cent. Nerv. Syst. Agents Med. Chem. 2012, 11, 321–327. [Google Scholar] [CrossRef]
- Nguyen, L.; Pieters, J. The Trojan Horse: Survival Tactics of Pathogenic Mycobacteria in Macrophages. Trends Cell Biol. 2005, 15, 269–276. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Long, R.L.; Hershfield, E.S.; Manfreda, J.; Kabani, A.; Kunimoto, D.; Power, C.M. Tuberculosis Molecular Variation in CNS Infection Evidence for Strain-Dependent Neurovirulence. Neurology 1998, 50, 1827–1832. [Google Scholar] [CrossRef]
- Caws, M.; Thwaites, G.; Dunstan, S.; Hawn, T.R.; Lan, N.T.N.; Thuong, N.T.T.; Stepniewska, K.; Huyen, M.N.T.; Nguyen, D.B.; Tran, H.L.; et al. The Influence of Host and Bacterial Genotype on the Development of Disseminated Disease with Mycobacterium Tuberculosis. PLoS Pathog. 2008, 4, e1000034. [Google Scholar] [CrossRef]
- Hernandez Pando, R.; Aguilar, D.; Cohen, I.; Guerrero, M.; Ribon, W.; Acosta, P.; Orozco, H.; Marquina, B.; Salinas, C.; Rembao, D.; et al. Specific Bacterial Genotypes of Mycobacterium Tuberculosis Cause Extensive Dissemination and Brain Infection in an Experimental Model. Tuberculosis 2010, 90, 268–277. [Google Scholar] [CrossRef]
- Caws, M.; Thwaites, G.; Stepniewska, K.; Lan, N.T.N.; Duyen, N.T.H.; Phuong, N.T.; Huyen, M.N.T.; Duy, P.M.; Loc, T.H.; Chau, T.T.H.; et al. Beijing Genotype of Mycobacterium Tuberculosis Is Significantly Associated with Human Immunodeficiency Virus Infection and Multidrug Resistance in Cases of Tuberculous Meningitis. J. Clin. Microbiol. 2006, 44, 3934–3939. [Google Scholar] [CrossRef]
- Sánchez-Garibay, C.; Hernández-Campos, M.E.; Tena-Suck, M.L.; Salinas-Lara, C. Experimental Animal Models of Central Nervous System Tuberculosis: A Historical Review. Tuberculosis 2018, 110, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Tobin, D.M.; Tucker, E.W.; Venketaraman, V.; Ordonez, A.A.; Jayashankar, L.; Siddiqi, O.K.; Hammoud, D.A.; Prasadarao, N.V.; Sandor, M.; et al. Tuberculous Meningitis: A Roadmap for Advancing Basic and Translational Research. Nat. Immunol. 2018, 19, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Patrocínio, L.G.; Goulart, I.M.B.; Goulart, L.R.; Patrocínio, J.A.; Ferreira, F.R.; Fleury, R.N. Detection of Mycobacterium Leprae in Nasal Mucosa Biopsies by the Polymerase Chain Reaction. FEMS Immunol. Med. Microbiol. 2005, 44, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Naves, M.M.; Patrocinio, L.G.; Patrocinio, J.A.; Mota, F.M.N.; de Souza, A.D.; Fleury, R.N.; Goulart, I.M.B. Contribution of Nasal Biopsy to Leprosy Diagnosis. Am. J. Rhinol. Allergy 2009, 23, 177–180. [Google Scholar] [CrossRef] [PubMed]
- da Costa Martins, A.C.; Miranda, A.; de Oliveira, M.L.W.d.R.; Bührer-Sékula, S.; Martinez, A. Nasal Mucosa Study of Leprosy Contacts with Positive Serology for the Phenolic Glycolipid 1 Antigen. Braz. J. Otorhinolaryngol. 2010, 76, 579–587. [Google Scholar] [CrossRef]
- Rambukkana, A.; Salzer, J.L.; Yurchenco, P.D.; Tuomanen, E.I. Neural Targeting of Mycobacterium Leprae Mediated by the G Domain of the Laminin-α2 Chain. Cell 1997, 88, 811–821. [Google Scholar] [CrossRef]
- Rambukkana, A.; Yamada, H.; Zanani, G.; Mathus, T.; Salzer, J.L.; Yurchenco, P.D. Role of A-Dystroglycan as a Schwann Cell Receptor for Mycobacterium Leprae. Science 1998, 282, 2076–2079. [Google Scholar] [CrossRef]
- Shimoji, Y.; Ng, V.; Matsumura, K.; Fischetti, V.A.; Rambukkana, A. A 21-KDa Surface Protein of Mycobacterium Leprae Binds Peripheral Nerve Laminin-2 and Mediates Schwann Cell Invasion. Proc. Natl. Acad. Sci. USA 1999, 96, 9857–9862. [Google Scholar] [CrossRef]
- Ng, V.; Zanazzi, G.; Timpl, R.; Talts, J.F.; Salzer, J.L.; Brennan, P.J.; Rambukkana, A. Role of the Cell Wall Phenolic Glycolipid-1 in the Peripheral Nerve Predilection of Mycobacterium Leprae. Cell 2000, 103, 511–524. [Google Scholar] [CrossRef]
- Esposito, C.; Marasco, D.; Delogu, G.; Pedone, E.; Berisio, R. Heparin-Binding Hemagglutinin HBHA from Mycobacterium Tuberculosis Affects Actin Polymerisation. Biochem. Biophys. Res. Commun. 2011, 410, 339–344. [Google Scholar] [CrossRef]
- Finlay, B.B.; Falkow, S. Common Themes in Microbial Pathogenicity Revisited. Microbiol. Mol. Biol. Rev. 1997, 61, 136–169. [Google Scholar] [PubMed]
- Skerry, C.; Pokkali, S.; Pinn, M.; Be, N.A.; Harper, J.; Karakousis, P.C.; Jain, S.K. Vaccination with Recombinant Mycobacterium Tuberculosis PknD Attenuates Bacterial Dissemination to the Brain in Guinea Pigs. PLoS ONE 2013, 8, e66310. [Google Scholar] [CrossRef] [PubMed]
- Be, N.A.; Klinkenberg, L.G.; Bishai, W.R.; Karakousis, P.C.; Jain, S.K. Strain-Dependent CNS Dissemination in Guinea Pigs after Mycobacterium Tuberculosis Aerosol Challenge. Tuberculosis 2011, 91, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Chaves, A.T.; Ribeiro-Junior, A.F.; Lyon, S.; Medeiros, N.I.; Cassirer-Costa, F.; Paula, K.S.; Alecrim, E.S.; Menezes, C.A.S.; Correa-Oliveira, R.; Rocha, M.O.C.; et al. Regulatory T Cells: Friends or Foe in Human Mycobacterium Leprae Infection? Immunobiology 2018, 223, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Froes, L.A.R.; Sotto, M.N.; Trindade, M.A.B. Leprosy: Clinical and Immunopathological Characteristics. An. Bras. Dermatol. 2022, 97, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Mazzolla, R.; Puliti, M.; Barluzzi, R.; Neglia, R.; Bistoni, F.; Barbolini, G.; Blasi, E. Di¡erential Microbial Clearance and Immunoresponse of Balb/c (Nramp1 Susceptible) and DBA2 (Nramp1 Resistant) Mice Intracerebrally Infected with Mycobacterium Bovis BCG (BCG). Immunol. Med. Microbiol. 2002, 14, 149–158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Well, G.T.J.; Wieland, C.W.; Florquin, S.; Roord, J.J.; Van Der Poll, T.; Van Furth, A.M. A New Murine Model to Study the Pathogenesis of Tuberculous Meningitis. J. Infect. Dis. 2007, 195, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Be, N.A.; Bishai, W.R.; Jain, S.K. Role of Mycobacterium Tuberculosis PknD in the Pathogenesis of Central Nervous System Tuberculosis. BMC Microbiol. 2012, 12, 7. [Google Scholar] [CrossRef]
- Be, N.A.; Lamichhane, G.; Grosset, J.; Tyagi, S.; Cheng, Q.J.; Kim, K.S.; Bishai, W.R.; Jain, S.K. Murine Model to Study the Invasion and Survival of Mycobacterium Tuberculosis in the Central Nervous System. J. Infect. Dis. 2008, 198, 1520–1528. [Google Scholar] [CrossRef]
- Mcshane, H.; Shane, C. Susceptibility to Tuberculosis-the Importance of the Pathogen as Well as the Host. Clin. Exp. Immunol. 2003, 133, 20–21. [Google Scholar] [CrossRef]
- Zucchi, F.C.R.; Pelegrini-da-Silva, A.; Neder, L.; Silva, C.L.; Tsanaclis, A.M.C.; Takayanagui, O.M. The Contribution of a Murine CNS-TB Model for the Understanding of the Host-Pathogen Interactions in the Formation of Granulomas. J. Neurosci. Methods 2012, 206, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Aurangzeb, S.; Badshah, M.; Khan, R.S. Chest Radiographic Findings in Neurotuberculosis without Pulmonary Signs and Symptoms. J. Coll. Physicians Surg.-Pak. 2008, 18, 27–30. [Google Scholar] [PubMed]
- Luo, M.; Wang, W.; Zeng, Q.; Luo, Y.; Yang, H.; Yang, X. Tuberculous Meningitis Diagnosis and Treatment in Adults: A Series of 189 Suspected Cases. Exp. Ther. Med. 2018, 16, 2770–2776. [Google Scholar] [CrossRef] [PubMed]
- Yasar, K.; Pehlivanoglu, F.; Cicek, G.; Sengoz, G. The Evaluation of the Clinical, Laboratory and the Radiological Findings of the Fifty-Five Cases Diagnosed with Tuberculous, Brucellar and Pyogenic Spondylodiscitis. J. Neurosci. Rural Pract. 2012, 3, 17–20. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rembao-Bojórquez, D.; Sánchez-Garibay, C.; Salinas-Lara, C.; Marquina-Castillo, B.; Letechipía-Salcedo, A.; Castillón-Benavides, O.J.; Galván-Arzate, S.; Gómez-López, M.; Jiménez-Zamudio, L.A.; Soto-Rojas, L.O.; et al. Central Nervous System Tuberculosis in a Murine Model: Neurotropic Strains or a New Pathway of Infection? Pathogens 2024, 13, 37. https://doi.org/10.3390/pathogens13010037
Rembao-Bojórquez D, Sánchez-Garibay C, Salinas-Lara C, Marquina-Castillo B, Letechipía-Salcedo A, Castillón-Benavides OJ, Galván-Arzate S, Gómez-López M, Jiménez-Zamudio LA, Soto-Rojas LO, et al. Central Nervous System Tuberculosis in a Murine Model: Neurotropic Strains or a New Pathway of Infection? Pathogens. 2024; 13(1):37. https://doi.org/10.3390/pathogens13010037
Chicago/Turabian StyleRembao-Bojórquez, Daniel, Carlos Sánchez-Garibay, Citlaltepetl Salinas-Lara, Brenda Marquina-Castillo, Adriana Letechipía-Salcedo, Omar Jorge Castillón-Benavides, Sonia Galván-Arzate, Marcos Gómez-López, Luis Antonio Jiménez-Zamudio, Luis O. Soto-Rojas, and et al. 2024. "Central Nervous System Tuberculosis in a Murine Model: Neurotropic Strains or a New Pathway of Infection?" Pathogens 13, no. 1: 37. https://doi.org/10.3390/pathogens13010037
APA StyleRembao-Bojórquez, D., Sánchez-Garibay, C., Salinas-Lara, C., Marquina-Castillo, B., Letechipía-Salcedo, A., Castillón-Benavides, O. J., Galván-Arzate, S., Gómez-López, M., Jiménez-Zamudio, L. A., Soto-Rojas, L. O., Tena-Suck, M. L., Nava, P., Fernández-Vargas, O. E., Coria-Medrano, A., & Hernández-Pando, R. (2024). Central Nervous System Tuberculosis in a Murine Model: Neurotropic Strains or a New Pathway of Infection? Pathogens, 13(1), 37. https://doi.org/10.3390/pathogens13010037








