Microorganisms and Breast Cancer: An In-Depth Analysis of Clinical Studies
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Analysis of Studies Reported in ClinicalTrials.gov and PubMed
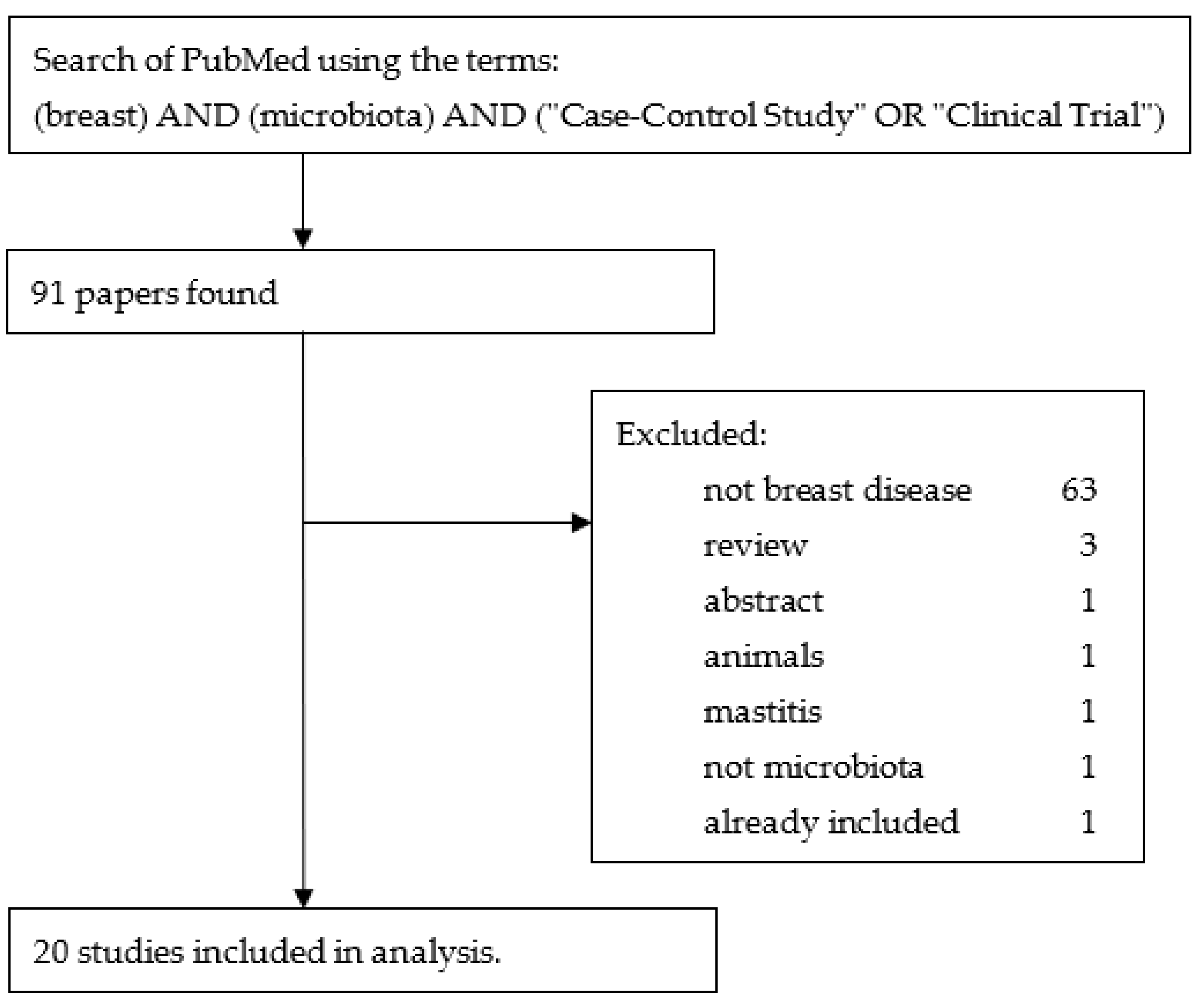
3.1.1. Selection of Relevant Terms and Studies
3.1.2. Geographic Distribution
3.1.3. Status of Studies
3.1.4. Start Date
3.1.5. Phases of Studies
3.1.6. Study Designs
3.1.7. Types of Intervention
3.1.8. Study Participants
3.2. Thematic Analysis
3.2.1. COVID-19
3.2.2. Treatment Delivery
3.2.3. Infection
3.2.4. Microbiome
3.2.5. Probiotics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bäckhed, F. Host Responses to the Human Microbiome. Nutr. Rev. 2012, 70, S14–S17. [Google Scholar] [CrossRef] [PubMed]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, R.; Wu, W.; Qin, H. The Role of Gut Microbiota in the Pathogenesis of Colorectal Cancer. Tumor Biol. 2013, 34, 1285–1300. Available online: https://link.springer.com/article/10.1007/s13277-013-0684-4 (accessed on 26 September 2023). [CrossRef] [PubMed]
- Hieken, T.J.; Chen, J.; Hoskin, T.L.; Walther-Antonio, M.; Johnson, S.; Ramaker, S.; Xiao, J.; Radisky, D.C.; Knutson, K.L.; Kalari, K.R.; et al. The Microbiome of Aseptically Collected Human Breast Tissue in Benign and Malignant Disease. Sci. Rep. 2016, 6, 30751. [Google Scholar] [CrossRef] [PubMed]
- Zackular, J.P.; Baxter, N.T.; Iverson, K.D.; Sadler, W.D.; Petrosino, J.F.; Chen, G.Y.; Schloss, P.D. The Gut Microbiome Modulates Colon Tumorigenesis. mBio 2013, 4, e00692-13. [Google Scholar] [CrossRef] [PubMed]
- Proctor, L.M.; Creasy, H.H.; Fettweis, J.M.; Lloyd-Price, J.; Mahurkar, A.; Zhou, W.; Buck, G.A.; Snyder, M.P.; Strauss, J.F.; Weinstock, G.M.; et al. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef]
- Kober, M.-M.; Bowe, W.P. The Effect of Probiotics on Immune Regulation, Acne, and Photoaging. Int. J. Women’s Dermatol. 2015, 1, 85–89. [Google Scholar] [CrossRef]
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reid, G. The Microbiota of Breast Tissue and Its Association with Breast Cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Ghazalpour, A.; Cespedes, I.; Bennett, B.J.; Allayee, H. Expanding Role of Gut Microbiota in Lipid Metabolism. Curr. Opin. Lipidol. 2016, 27, 141. [Google Scholar] [CrossRef]
- Lee, I.O.; Kim, J.H.; Choi, Y.J.; Pillinger, M.H.; Kim, S.-Y.; Blaser, M.J.; Lee, Y.C. Helicobacter Pylori CagA Phosphorylation Status Determines the Gp130-Activated SHP2/ERK and JAK/STAT Signal Transduction Pathways in Gastric Epithelial Cells. J. Biol. Chem. 2010, 285, 16042–16050. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, C.; Cummins, J.; Brackstone, M.; Macklaim, J.M.; Gloor, G.B.; Baban, C.K.; Scott, L.; O’Hanlon, D.M.; Burton, J.P.; Francis, K.P.; et al. Microbiota of Human Breast Tissue. Appl. Environ. Microbiol. 2014, 80, 3007–3014. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global Cancer Statistics. CA A Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Nakano, K.; Naderi, N.; Bajaj-Elliott, M.; Mosahebi, A. Is the Skin Microbiota a Modifiable Risk Factor for Breast Disease?: A Systematic Review. Breast 2021, 59, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Rainey, L.; van der Waal, D.; Jervaeus, A.; Wengström, Y.; Evans, D.G.; Donnelly, L.S.; Broeders, M.J.M. Are We Ready for the Challenge of Implementing Risk-Based Breast Cancer Screening and Primary Prevention? Breast 2018, 39, 24–32. [Google Scholar] [CrossRef] [PubMed]
- About ClinicalTrials.Gov—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/about-site/about-ctg (accessed on 2 September 2023).
- Trends, Charts, and Maps—ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/resources/trends (accessed on 2 October 2023).
- PubMed Overview. Available online: https://pubmed.ncbi.nlm.nih.gov/about/ (accessed on 17 November 2023).
- Marschalek, J.; Farr, A.; Marschalek, M.-L.; Domig, K.J.; Kneifel, W.; Singer, C.F.; Kiss, H.; Petricevic, L. Influence of Orally Administered Probiotic Lactobacillus Strains on Vaginal Microbiota in Women with Breast Cancer during Chemotherapy: A Randomized Placebo-Controlled Double-Blinded Pilot Study. Breast Care 2017, 12, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.; Ross, J.S.; Miller, J.E. Transparency of Results Reporting in Cancer Clinical Trials. JAMA Netw. Open 2023, 6, e2328117. [Google Scholar] [CrossRef]
- Jones, C.W.; Safferman, M.R.; Adams, A.C.; Platts-Mills, T.F. Discrepancies between ClinicalTrials.Gov Recruitment Status and Actual Trial Status: A Cross-Sectional Analysis. BMJ Open 2017, 7, e017719. [Google Scholar] [CrossRef]
- Becker, J.E.; Krumholz, H.M.; Ben-Josef, G.; Ross, J.S. Reporting of results in ClinicalTrials.gov and high-impact journals. JAMA 2014, 311, 1063–1065. [Google Scholar] [CrossRef]
- Begg, C.B.; Berlin, J.A. Publication Bias: A Problem in Interpreting Medical Data. J. R. Stat. Soc. Ser. A Stat. Soc. 1988, 151, 419–463. [Google Scholar] [CrossRef]
- Coughlin, S.S.; Paxton, R.J.; Moore, N.; Stewart, J.L.; Anglin, J. Survivorship Issues in Older Breast Cancer Survivors. Breast Cancer Res Treat 2019, 174, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Hoving, J.; Broekhuizen, M.; Frings-Dresen, M. Return to Work of Breast Cancer Survivors: A Systematic Review of Intervention Studies. BMC Cancer 2009, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shigaki, C.L.; Armer, J.M. Return to Work among Breast Cancer Survivors: A Literature Review. Support Care Cancer 2017, 25, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Shamonki, J.M.; Chung, A.; DiNome, M.L.; Chung, M.; Sieling, P.A.; Lee, D.J. Microbial Dysbiosis Is Associated with Human Breast Cancer. PLoS ONE 2014, 9, e83744. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.; Glenn, W.K.; Ye, Y.; Tran, B.; Delprado, W.; Lutze-Mann, L.; Whitaker, N.J.; Lawson, J.S. Human Papilloma Virus Is Associated with Breast Cancer. Br. J. Cancer 2009, 101, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Yang, J.; Lee, W.-H.; Kim, J.B.; Yang, Y.; Kim, J.; Kim, H.; Paek, S.H.; Lee, J.W.; Woo, J.; et al. Diagnostic Kit of Breast Cancer via Urine Microbiome. Eur. J. Surg. Oncol. 2020, 46, e33. [Google Scholar] [CrossRef]
- Fernández, L.; Langa, S.; Martín, V.; Maldonado, A.; Jiménez, E.; Martín, R.; Rodríguez, J.M. The Human Milk Microbiota: Origin and Potential Roles in Health and Disease. Pharmacol. Res. 2013, 69, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Rodríguez, J.M. The Origin of Human Milk Bacteria: Is There a Bacterial Entero-Mammary Pathway during Late Pregnancy and Lactation? Adv. Nutr. 2014, 5, 779–784. [Google Scholar] [CrossRef]
- Urbaniak, C.; Burton, J.P.; Reid, G. Breast, Milk and Microbes: A Complex Relationship That Does Not End with Lactation. Womens Health 2012, 8, 385–398. [Google Scholar] [CrossRef]





| Term | Count |
|---|---|
| vaccination | 18 |
| microbiome | 16 |
| covid | 14 |
| infection | 10 |
| infections | 10 |
| virus | 10 |
| vaccinia | 6 |
| flora | 5 |
| microbiota | 5 |
| antibiotics | 3 |
| probiotics | 3 |
| yeast | 3 |
| adenoviral | 2 |
| bacterial | 2 |
| botulinum | 2 |
| lactobacillus | 2 |
| probiotic | 2 |
| retroviral | 2 |
| viral | 2 |
| antisepsis | 1 |
| antiseptics | 1 |
| aspergillosis | 1 |
| biotics | 1 |
| clostridium | 1 |
| coxsackie | 1 |
| fecal | 1 |
| infected | 1 |
| infectious | 1 |
| influenza | 1 |
| microbe | 1 |
| microbiotaã 1 | 1 |
| mycobiome | 1 |
| mycograb | 1 |
| mycosis | 1 |
| pneumococcus | 1 |
| pneumonia | 1 |
| poxviral | 1 |
| saccharomyces | 1 |
| Country | Studies |
|---|---|
| USA | 75 |
| China | 18 |
| Canada | 7 |
| France | 5 |
| Italy | 4 |
| Germany | 3 |
| Spain | 3 |
| Brazil | 2 |
| Egypt | 2 |
| Ghana | 2 |
| Russia | 2 |
| Australia | 1 |
| Austria | 1 |
| Belgium | 1 |
| Colombia | 1 |
| Greece | 1 |
| Mexico | 1 |
| Netherlands | 1 |
| Pakistan | 1 |
| Philippines | 1 |
| Poland | 1 |
| Portugal | 1 |
| Singapore | 1 |
| Switzerland | 1 |
| United Kingdom | 1 |
| Study Status | Number of Studies |
|---|---|
| Not yet recruiting | 3 |
| Recruiting | 31 |
| Active not recruiting | 10 |
| Completed | 70 |
| Approved for marketing | 1 |
| Terminated | 8 |
| Unknown | 8 |
| Withdrawn | 6 |
| Total | 137 |
| Results Available? | Number of Studies |
|---|---|
| No | 108 |
| Yes | 29 |
| Total | 137 |
| Study Design and Topic | Status * | Planned Enrollment | NCT Number |
|---|---|---|---|
| Randomized | |||
| Novel Probiotics and the bacteriome and mycobiome | NYR | 100 | NCT04362826 |
| Diagnostic evaluation during the COVID-19 pandemic | Recruiting | 196 | NCT05181722 |
| Randomized (cluster) | |||
| Cancer screening and HPV vaccination | NYR | 2000 | NCT05524480 |
| Non-randomized | |||
| Intratumoral oncolytic virus | NYR | 24 | NCT05600582 |
| Oncolytic virus injection | Recruiting | 20 | NCT05860374 |
| Recombinant herpes simplex virus I | Recruiting | 24 | NCT05886075 |
| MEM-288 oncolytic virus | Recruiting | 18 | NCT05076760 |
| Vaccinia virus VV-GMCSF-Lact | Recruiting | 73 | NCT05376527 |
| WOKVAC vaccine | Recruiting | 16 | NCT04329065 |
| Observational | |||
| Oral Aromatase Inhibitors and Gut Microbiome | Recruiting | 25 | NCT05030038 |
| COVID-19 related financial hardship | ANR | 14 | NCT05076266 |
| Gut microbiome components | Recruiting | 100 | NCT05444647 |
| Intestinal microbiota | Recruiting | 35 | NCT05580887 |
| Phase of Study | Number of Studies |
|---|---|
| Early Phase 1 | 5 |
| Phase 1 | 28 |
| Phase 1/Phase 2 | 8 |
| Phase 2 | 13 |
| Phase 3 | 7 |
| Phase 4 | 5 |
| NA 1 | 22 |
| Not specified 2 | 49 |
| Total | 137 |
| Design of Study | Number of Studies |
|---|---|
| Randomized with masking | 23 |
| Randomized without masking | 17 |
| Non-randomized | 54 |
| Observational | 42 |
| Not specified | 1 |
| Total | 137 |
| Intervention | Number of Studies |
|---|---|
| Behavioral | 3 |
| Biological | 38 |
| Device | 2 |
| Diagnostic test | 1 |
| Dietary supplement | 10 |
| Drug | 49 |
| Genetic | 4 |
| Other | 21 |
| Procedure | 8 |
| Radiation | 3 |
| Not applicable (observational study) | 22 |
| Theme | Number of Studies |
|---|---|
| COVID-19 | 15 |
| Treatment delivery | 44 |
| Infection | 29 |
| Microbiome | 37 |
| Probiotic supplements | 12 |
| 137 |
| Study Description | Location | Start Date | Identifier * |
|---|---|---|---|
| Impact of the pandemic on patient economic factors | USA | 2019 | NCT04169542 |
| Impact of COVID-19 infection in women with cancer | France | 2020 | NCT04351139 |
| Cancer screening and prevention during the pandemic | USA | 2020 | NCT04587258 |
| Nutritional care in oncology patients during the pandemic | Greece | 2020 | NCT04876560 |
| Effect of the pandemic on management of patients with breast cancer | Pakistan | 2020 | NCT04929964 |
| Effect of one preoperative fraction of radiation during the pandemic | Canada | 2020 | NCT05037019 |
| A survey of cancer patient perspectives during the pandemic | USA | 2020 | NCT05062538 |
| Remote rehabilitation in women with breast cancer during the pandemic | Brazil | 2020 | NCT05530876 |
| Changes in gut microbiota composition after 12 weeks in lockdown | Italy | 2020 | PMID37727203 |
| Immunogenicity of COVID-19 vaccine in patients receiving cancer treatment | USA | 2021 | NCT04821570 |
| Evaluating treatment for COVID-19 infection in breast cancer patients | Egypt | 2021 | NCT04871854 |
| Patient experiences with COVID-19 vaccination after breast cancer treatment | USA | 2021 | NCT04872738 |
| Impact of the pandemic on patient economic factors | USA | 2022 | NCT05076266 |
| Timely diagnostic evaluation during the pandemic | USA | 2022 | NCT05181722 |
| At-home administration of chemotherapy during the COVID-19 pandemic | USA | NS | NCT04395508 |
| Investigation Type | Number of Studies | NCT Number |
|---|---|---|
| Oncolytic virus | 15 | NCT00574977, NCT00636558, NCT01152398, NCT01846091, NCT02179515, NCT03004183, NCT03110445, NCT03740256, NCT04215146, NCT05076760, NCT05180851, NCT05376527, NCT05600582, NCT05860374, NCT05886075 |
| Vaccination | 17 | NCT00027131, NCT00197522, NCT00317603, NCT00485277, NCT00622401, NCT00880464, NCT00924092, NCT01127074, NCT01291420, NCT02276300, NCT02938442, NCT03632941, NCT03789097, NCT04105582, NCT04329065, NCT01390064, NCT02395614 |
| Viral vector gene transfer | 6 | NCT00001493, NCT00307229, NCT00451022, NCT01703754, NCT02140996, NCT02576665 |
| Study Description | PI Location | Start Date | NCT Number |
|---|---|---|---|
| Use of botulinum toxin A in breast reconstruction | Canada | 2011 | NCT01427400 |
| Botulinum toxin A in tissue expander breast reconstruction | USA | 2012 | NCT01591746 |
| Pembrolizumab with intratumoral injection of Clostridium novyi-NT | USA | 2018 | NCT03435952 |
| Bacterial cellulose-monolaurin hydrogel for acute radiation dermatitis | Philippines | 2021 | NCT05079763 |
| Investigation Type | Number of Studies | Identifier * |
|---|---|---|
| Infection Prevention | 18 | NCT00003883, NCT00005590, NCT00045292, NCT00064311, NCT00079222, NCT00324324, NCT00378781, NCT00536081, NCT00741039, NCT01286168, NCT01899690, NCT02395614, NCT02479347, NCT02816112, NCT03229824, NCT03742908, NCT04818931, PMID37754546 |
| Infection Treatment | 4 | NCT00014391, NCT00110045, NCT00509691, NCT00769613 |
| Study Description | PI Location | Start Date | Identifier * |
|---|---|---|---|
| Intratumoral microbiome is driven by metastatic site | France | 2012 | PMID36868056 |
| Analysis of gut microbiome predicts risk of diarrhea associated with neratinib | USA | 2015 | PMID33796451 |
| Gut microbiome and gastrointestinal toxicities after neoadjuvant chemotherapy | USA | 2016 | NCT02696759 |
| Effect of radiotherapy on circulating immune cells and effect on microbiome | USA | 2018 | NCT03383107 |
| The role of the skin microbiome in post-mastectomy radiation dermatitis | USA | 2018 | NCT03519438 |
| Gut and intratumoral microbiome effect on neoadjuvant chemotherapy | USA | 2017 | NCT03586297 |
| Relationship between gut microbiome and adjuvant chemotherapy | China | 2018 | NCT03702868 |
| Breast cancer and its relationship with the microbiota | Spain | 2018 | NCT03885648 |
| Effects of exercise on gut microbe composition in breast cancer survivors | USA | 2020 | NCT04088708 |
| Study of skin microbiome after chemotherapy for breast cancer | China | 2019 | NCT04132713 |
| Intestinal microbiota of patients with breast cancer undergoing chemotherapy | China | 2019 | NCT04138979 |
| A study of modern therapies on flora in body fluids and blood | China | 2019 | NCT04202848 |
| Assessing the impact of the microbiome on breast cancer radiotherapy toxicity | USA | 2019 | NCT04245150 |
| Microbiota as a non-invasive tool to predict postoperative depression | China | 2019 | PMID33211236 |
| Breast microbiome associations with breast tumor characteristics | China | 2019 | PMID36172155 |
| The role of gut microbiota in women treated with aromatase inhibitors | Italy | 2019 | PMID36558756 |
| Preoperative gut microbiota and chronic postoperative pain | China | 2019 | PMID34403381 |
| Effect of an anaesthetic given during breast cancer surgery on gut microbiota | China | 2021 | NCT04303325 |
| Exercise, gut microbiome, and breast cancer in underserved populations | USA | 2021 | NCT05000502 |
| Microbiome and association with breast implant infections | USA | 2021 | NCT05020574 |
| Oral aromatase inhibitors and the gut microbiome | USA | 2022 | NCT05030038 |
| A study of diarrhea and intestinal flora changes after a breast cancer therapy | China | 2021 | NCT05030519 |
| The association between radiation dermatitis and skin microbiome | China | 2021 | NCT05032768 |
| The gut microbiome and immune-checkpoint-inhibitor therapy | USA | 2021 | NCT05037825 |
| Changes in the gut microbiome and chemotherapy-induced nausea | USA | 2021 | NCT05417867 |
| Mechanism of acupuncture on cancer-related fatigue | China | 2021 | PMID35578688 |
| A study of gut microbiome components and response to neoadjuvant therapy | China | 2022 | NCT05444647 |
| Intestinal microbiota impact on prognosis and treatment outcomes | Russia | 2022 | NCT05580887 |
| Breast cancer survivors and healthy women: “BiotaCancerSurvivors” study | Portugal | NS | PMID36765550 |
| Fecal microbiota composition in patients with breast cancer | France | NS | PMID34444865 |
| The oral microbiome and breast cancer in the Ghana Breast Health Study | Ghana | NS | PMID35657343 |
| Fecal microbial profiles and breast cancer in the Ghana Breast Health Study | Ghana | NS | PMID33460452 |
| Diet alters entero-mammary signaling to regulate the breast microbiome | USA | NS | PMID34083249 |
| Plasma metabolomic signatures associated with long-term breast cancer risk | France | NS | PMID31164347 |
| Diet-related metabolomic signature of long-term breast cancer risk | France | NS | PMID31767565 |
| Health-related quality of life is associated with fecal microbial composition | USA | NS | PMID36512109 |
| Potential antiproliferative activity of polyphenol metabolites | Brazil | NS | PMID28541359 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naderi, N.; Mosahebi, A.; Williams, N.R. Microorganisms and Breast Cancer: An In-Depth Analysis of Clinical Studies. Pathogens 2024, 13, 6. https://doi.org/10.3390/pathogens13010006
Naderi N, Mosahebi A, Williams NR. Microorganisms and Breast Cancer: An In-Depth Analysis of Clinical Studies. Pathogens. 2024; 13(1):6. https://doi.org/10.3390/pathogens13010006
Chicago/Turabian StyleNaderi, Naghmeh, Afshin Mosahebi, and Norman R. Williams. 2024. "Microorganisms and Breast Cancer: An In-Depth Analysis of Clinical Studies" Pathogens 13, no. 1: 6. https://doi.org/10.3390/pathogens13010006
APA StyleNaderi, N., Mosahebi, A., & Williams, N. R. (2024). Microorganisms and Breast Cancer: An In-Depth Analysis of Clinical Studies. Pathogens, 13(1), 6. https://doi.org/10.3390/pathogens13010006









