Tumor Necrosis Factor Receptors and C-C Chemokine Receptor-2 Positive Cells Play an Important Role in the Intraerythrocytic Death and Clearance of Babesia microti
Abstract
1. Introduction
2. Methods and Materials
2.1. Mice
2.2. Parasites and Infection Model
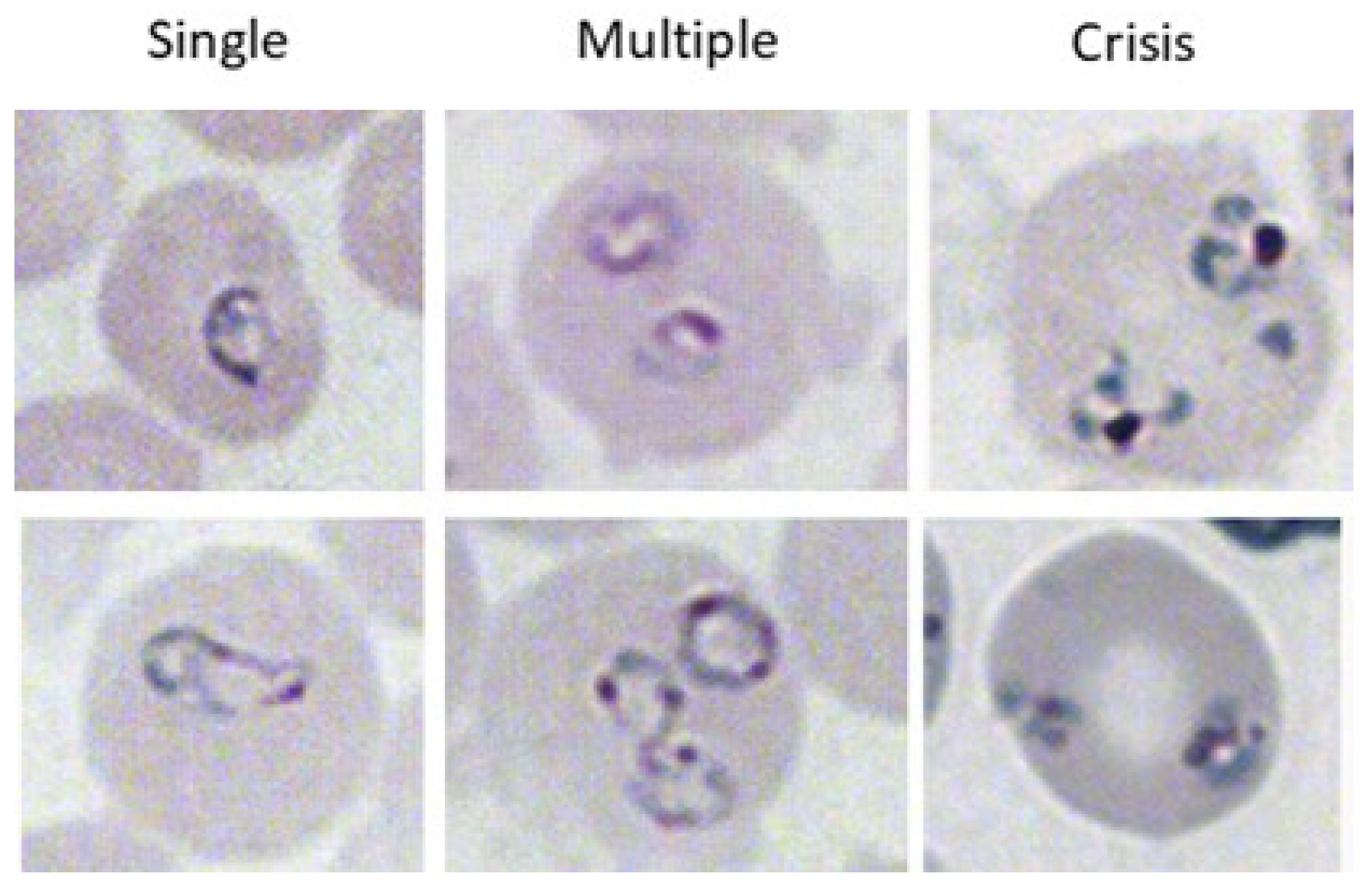
2.3. Evaluation of Parasitemia
2.4. D89E
2.5. Statistical Analysis
3. Results
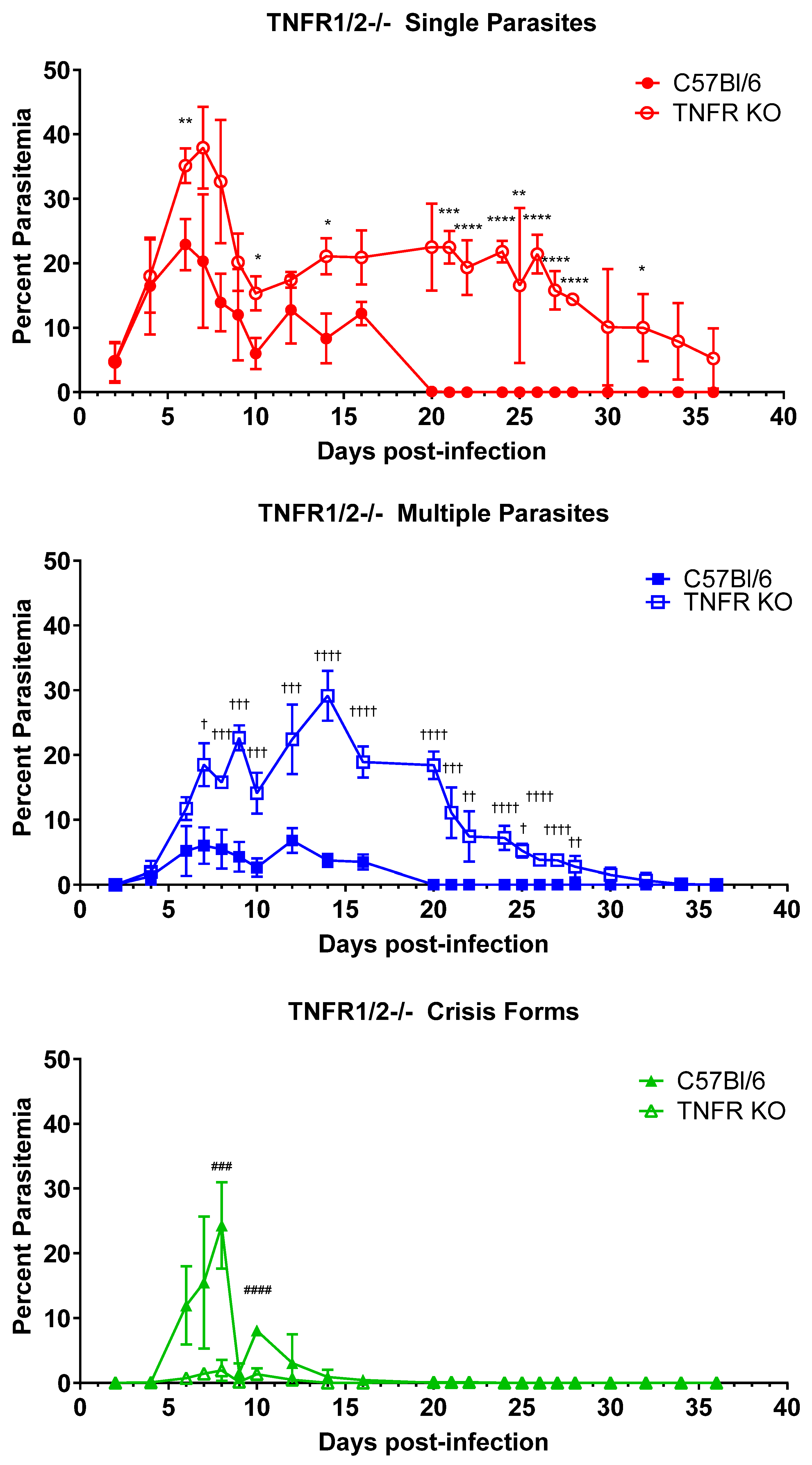
3.1. TNFα Plays a Key Role in Controlling and Clearing B. microti Infection
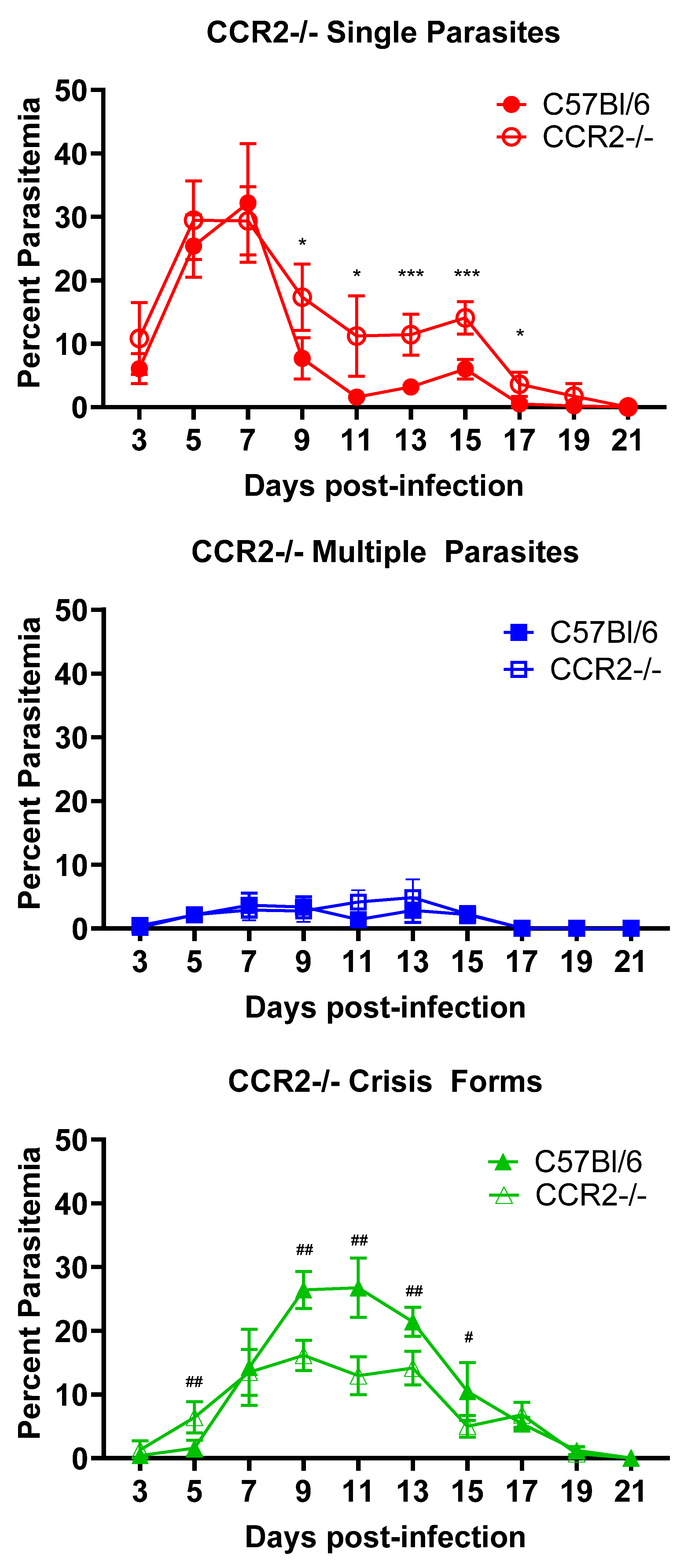
3.2. CCR2 Mobilization of Cells Impacts Intraerythrocytic Death of B. microti Parasites but Not Parasite Clearance
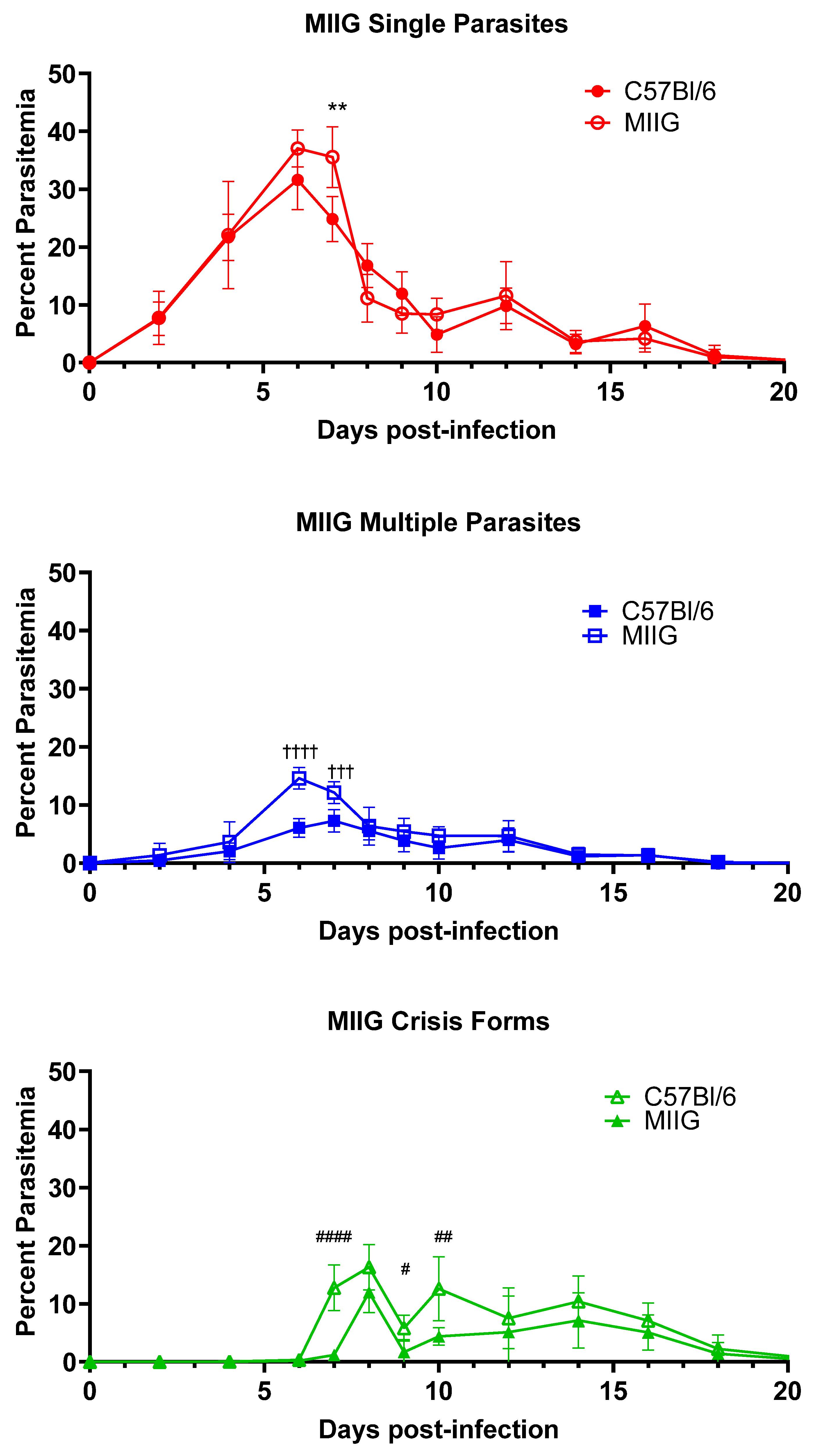
3.3. IFNγ Activation of Macrophages Is Not Required for Killing or Clearance of Babesia microti
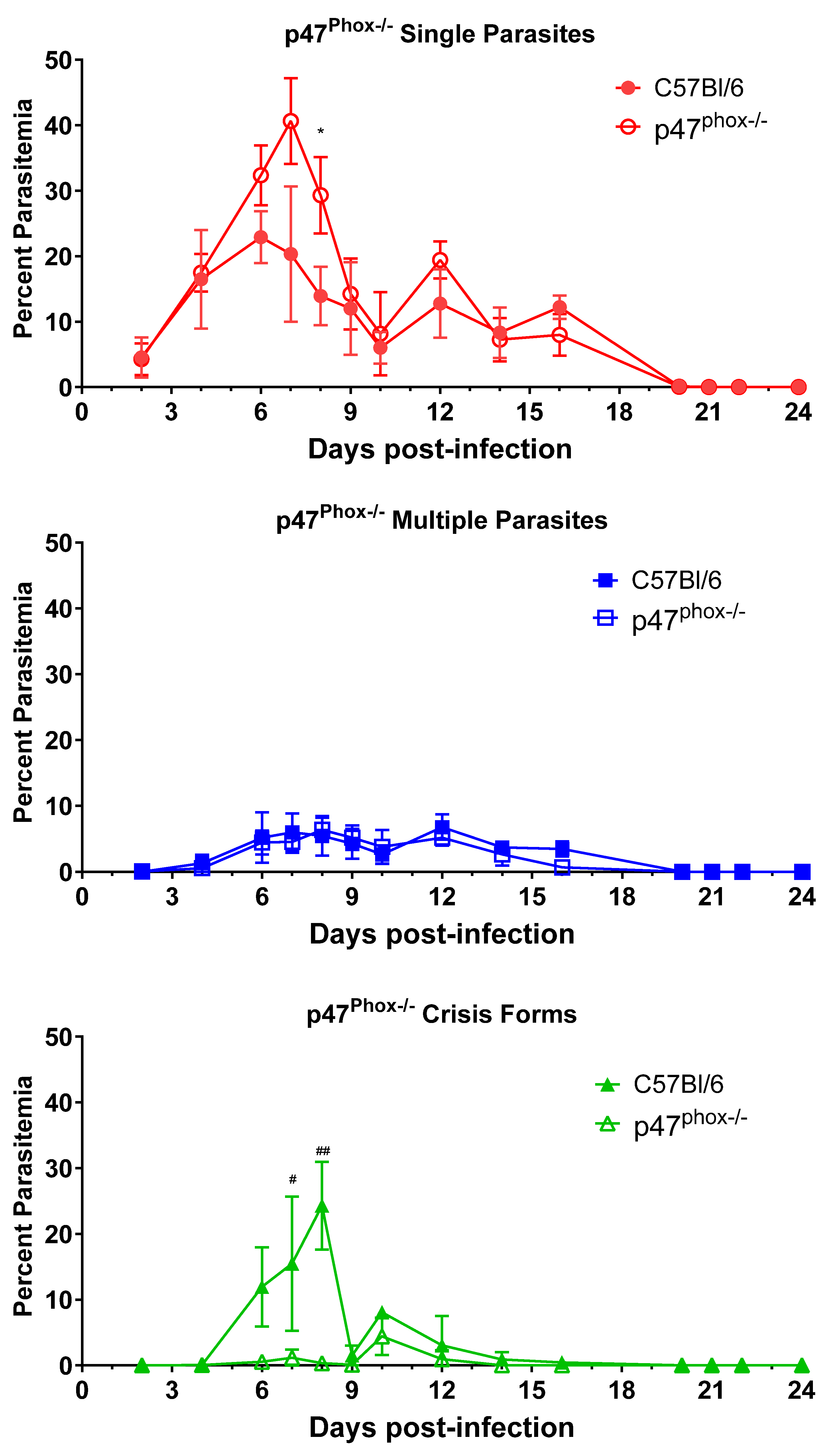
3.4. ROS Generation Is Critical for Intraerythrocytic Killing of B. microti Parasites
3.5. Phosphatidylserine-Mediated Recognition and Clearance of Damaged RBCs by Phagocytes Does Not Play a Role in Clearance of B. microti
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vannier, E.; Krause, P.J. Human Babesiosis. N. Engl. J. Med. 2012, 366, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- Saetre, K.; Godhwani, N.; Maria, M.; Patel, D.; Wang, G.; Li, K.I.; Wormser, G.P.; Nolan, S.M. Congenital Babesiosis after Maternal Infection with Borrelia burgdorferi and Babesia microti. J. Pediatric Infect. Dis. Soc. 2018, 7, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Herwaldt, B.L.; Linden, J.V.; Bosserman, E.; Young, C.; Olkowska, D.; Wilson, M. Transfusion-Associated Babesiosis in the United States: A Description of Cases. Ann. Intern. Med. 2011, 155, 509. [Google Scholar] [CrossRef] [PubMed]
- Lobo, C.A.; Cursino-Santos, J.; Alhassan, A.; Rodrigues, M. Babesia: An Emerging Infectious Threat in Transfusion Medicine. PLoS Pathogens 2013, 9, e1003387. [Google Scholar] [CrossRef]
- Vannier, E.G.; Diuk-Wasser, M.A.; Ben Mamoun, C.; Krause, P.J. Babesiosis. Infect. Dis. Clin. N. Am. 2015, 29, 357–370. [Google Scholar] [CrossRef]
- Bloch, E.M.; Kumar, S.; Krause, P.J. Persistence of Babesia microti Infection in Humans. Pathogens 2019, 8, 102. [Google Scholar] [CrossRef]
- Gray, E.B.; Herwaldt, B.L. Babesiosis Surveillance-United States, 2011–2015. MMWR Surveill. Summ. 2019, 68, 1–11. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Surveillance for Babesiosis—United States, 2020 Annual Summary; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/babesiosis/php/data-stats/index.html (accessed on 24 September 2024).
- Skariah, S.; Arnaboldi, P.; Dattwyler, R.J.; Sultan, A.A.; Gaylets, C.; Walwyn, O.; Mulhall, H.; Wu, X.; Dargham, S.R.; Mordue, D.G. Elimination of Babesia microti Is Dependent on Intraerythrocytic Killing and CD4+ T Cells. J. Immunol. 2017, 199, 633–642. [Google Scholar] [CrossRef]
- Krause, P.J.; Gewurz, B.E.; Hill, D.; Marty, F.M.; Vannier, E.; Foppa, I.M.; Furman, R.R.; Neuhaus, E.; Skowron, G.; Gupta, S.; et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin. Infect. Dis. 2008, 46, 370–376. [Google Scholar] [CrossRef]
- Wormser, G.P.; Prasad, A.; Neuhaus, E.; Joshi, S.; Nowakowski, J.; Nelson, J.; Mittleman, A.; Aguero-Rosenfeld, M.; Topal, J.; Krause, P.J. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clin. Infect. Dis. 2010, 50, 381–386. [Google Scholar] [CrossRef]
- Krause, P.J.; Spielman, A.; Telford, S.R., 3rd; Sikand, V.K.; McKay, K.; Christianson, D.; Pollack, R.J.; Brassard, P.; Magera, J.; Ryan, R.; et al. Persistent parasitemia after acute babesiosis. N. Engl. J. Med. 1998, 339, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, J.C.; Greenberg, P.D.; Antique, J.; Jimenez-Lucho, V.E. Severe babesiosis in Long Island: Review of 34 cases and their complications. Clin. Infect. Dis. 2001, 32, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Lykens, J.E.; Terrell, C.E.; Zoller, E.E.; Divanovic, S.; Trompette, A.; Karp, C.L.; Aliberti, J.; Flick, M.J.; Jordan, M.B. Mice with a selective impairment of IFN-gamma signaling in macrophage lineage cells demonstrate the critical role of IFN-gamma-activated macrophages for the control of protozoan parasitic infections in vivo. J. Immunol. 2010, 184, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Western, K.A.; Benson, G.D.; Gleason, N.N.; Healy, G.R.; Schultz, M.G. Babesiosis in a Massachusetts resident. N. Engl. J. Med. 1970, 283, 854–856. [Google Scholar] [CrossRef]
- Hanayama, R.; Tanaka, M.; Miwa, K.; Shinohara, A.; Iwamatsu, A.; Nagata, S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002, 417, 182–187. [Google Scholar] [CrossRef]
- Murai, K.; Murakami, H.; Nagata, S. Myeloid-specific transcriptional activation by murine myeloid zinc-finger protein 2. Proc. Natl. Acad. Sci. USA 1998, 95, 3461–3466. [Google Scholar] [CrossRef]
- Asano, K.; Miwa, M.; Miwa, K.; Hanayama, R.; Nagase, H.; Nagata, S.; Tanaka, M. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J. Exp. Med. 2004, 200, 459–467. [Google Scholar] [CrossRef]
- Calmon-Hamaty, F.; Combe, B.; Hahne, M.; Morel, J. Lymphotoxin α revisited: General features and implications in rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, 232. [Google Scholar] [CrossRef]
- Peschon, J.J.; Torrance, D.S.; Stocking, K.L.; Glaccum, M.B.; Otten, C.; Willis, C.R.; Charrier, K.; Morrissey, P.J.; Ware, C.B.; Mohler, K.M. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 1998, 160, 943–952. [Google Scholar] [CrossRef]
- Boring, L.; Gosling, J.; Chensue, S.W.; Kunkel, S.L.; Farese, R.V., Jr.; Broxmeyer, H.E.; Charo, I.F. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Investig. 1997, 100, 2552–2561. [Google Scholar] [CrossRef]
- Jackson, S.H.; Gallin, J.I.; Holland, S.M. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 1995, 182, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Terkawi, M.A.; Cao, S.; Herbas, M.S.; Nishimura, M.; Li, Y.; Moumouni, P.F.; Pyarokhil, A.H.; Kondoh, D.; Kitamura, N.; Nishikawa, Y.; et al. Macrophages are the determinant of resistance to and outcome of nonlethal Babesia microti infection in mice. Infect. Immun. 2015, 83, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Cursino-Santos, J.R.; Singh, M.; Pham, P.; Rodriguez, M.; Lobo, C.A. Babesia divergens builds a complex population structure composed of specific ratios of infected cells to ensure a prompt response to changing environmental conditions. Cell Microbiol. 2016, 18, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mordue, D.G.; Katseff, A.S.; Galeota, A.J.; Hale, S.J.; Rezaee, S.; Schwartz, I.; Sambir, M.; Arnaboldi, P.M. Tumor Necrosis Factor Receptors and C-C Chemokine Receptor-2 Positive Cells Play an Important Role in the Intraerythrocytic Death and Clearance of Babesia microti. Pathogens 2024, 13, 858. https://doi.org/10.3390/pathogens13100858
Mordue DG, Katseff AS, Galeota AJ, Hale SJ, Rezaee S, Schwartz I, Sambir M, Arnaboldi PM. Tumor Necrosis Factor Receptors and C-C Chemokine Receptor-2 Positive Cells Play an Important Role in the Intraerythrocytic Death and Clearance of Babesia microti. Pathogens. 2024; 13(10):858. https://doi.org/10.3390/pathogens13100858
Chicago/Turabian StyleMordue, Dana G., Adiya S. Katseff, Andrew J. Galeota, Synthia J. Hale, Shaaf Rezaee, Ilana Schwartz, Mariya Sambir, and Paul M. Arnaboldi. 2024. "Tumor Necrosis Factor Receptors and C-C Chemokine Receptor-2 Positive Cells Play an Important Role in the Intraerythrocytic Death and Clearance of Babesia microti" Pathogens 13, no. 10: 858. https://doi.org/10.3390/pathogens13100858
APA StyleMordue, D. G., Katseff, A. S., Galeota, A. J., Hale, S. J., Rezaee, S., Schwartz, I., Sambir, M., & Arnaboldi, P. M. (2024). Tumor Necrosis Factor Receptors and C-C Chemokine Receptor-2 Positive Cells Play an Important Role in the Intraerythrocytic Death and Clearance of Babesia microti. Pathogens, 13(10), 858. https://doi.org/10.3390/pathogens13100858






