Assessing Biofungicides and Host Resistance against Rhizoctonia Large Patch in Zoysiagrass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculum Preparation
2.2. Bio- and Synthetic Fungicides
2.3. In Vitro Bio- and Synthetic Fungicide Efficacy Assays
2.4. Efficacy of Bio- and Synthetic Fungicide Spray Programs in Growth Chamber Experiments
2.5. Efficacy of Bio- and Synthetic Fungicide Spray Programs in Field Experiments
2.6. Host Resistance Screening of Zoysiagrass against R. solani Isolates
2.6.1. Screening Zoysiagrass Genotypes
2.6.2. Screening Zoysiagrass Breeding Lines
3. Results
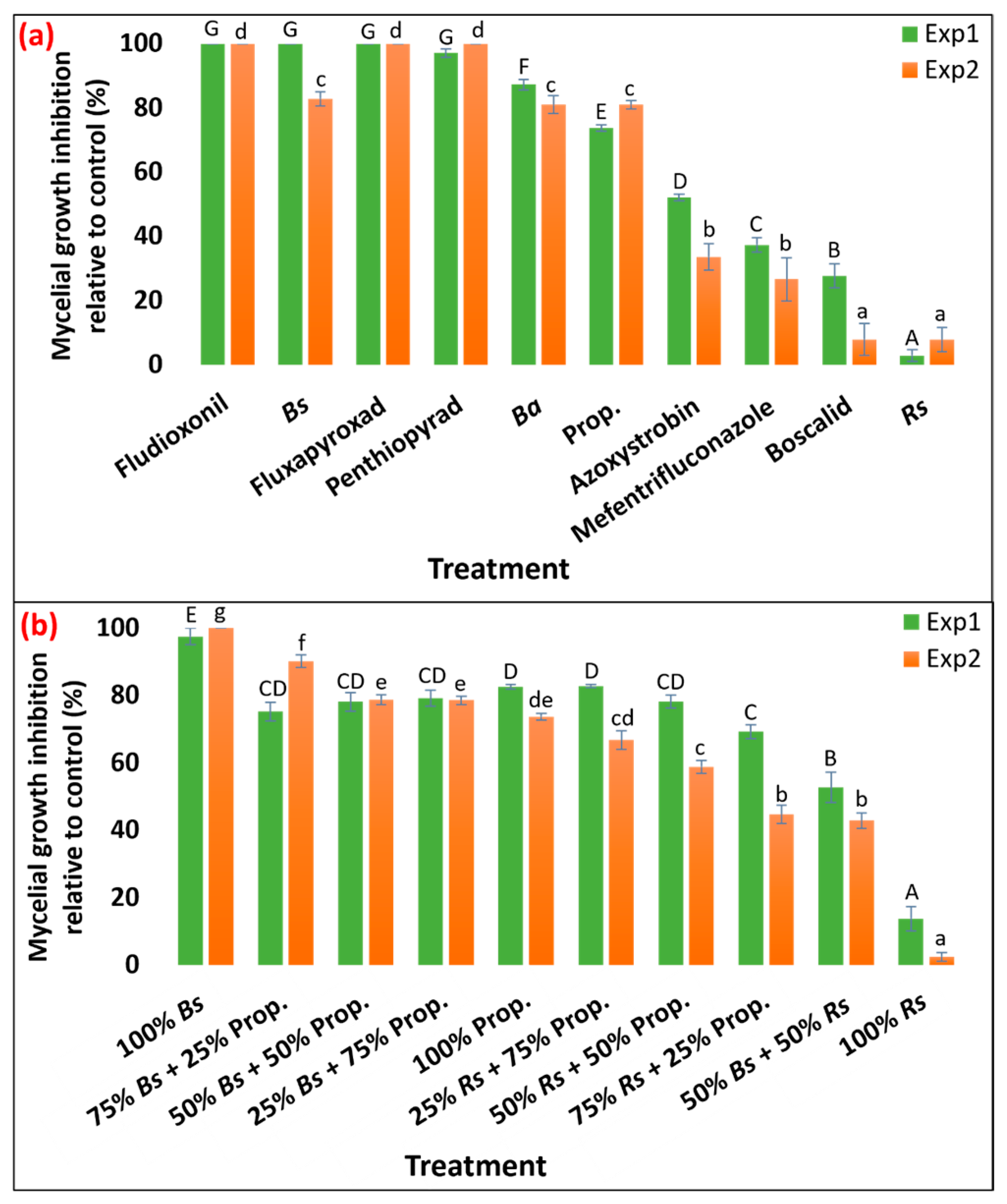
3.1. Efficacy of Bio- and Synthetic Fungicide In Vitro
3.2. Efficacy of Biofungicide-Inclusive Spray Program in Growth Chamber
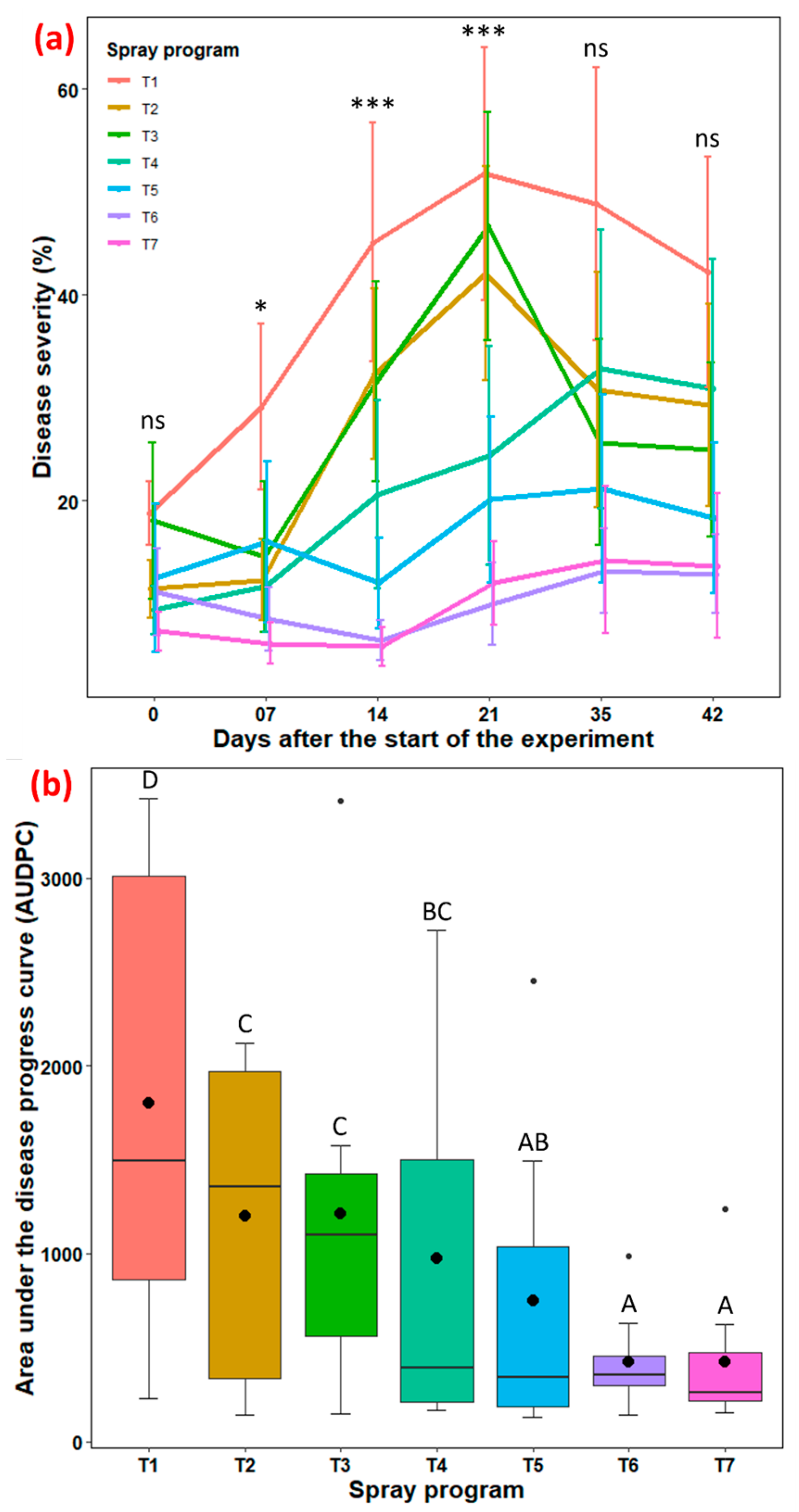
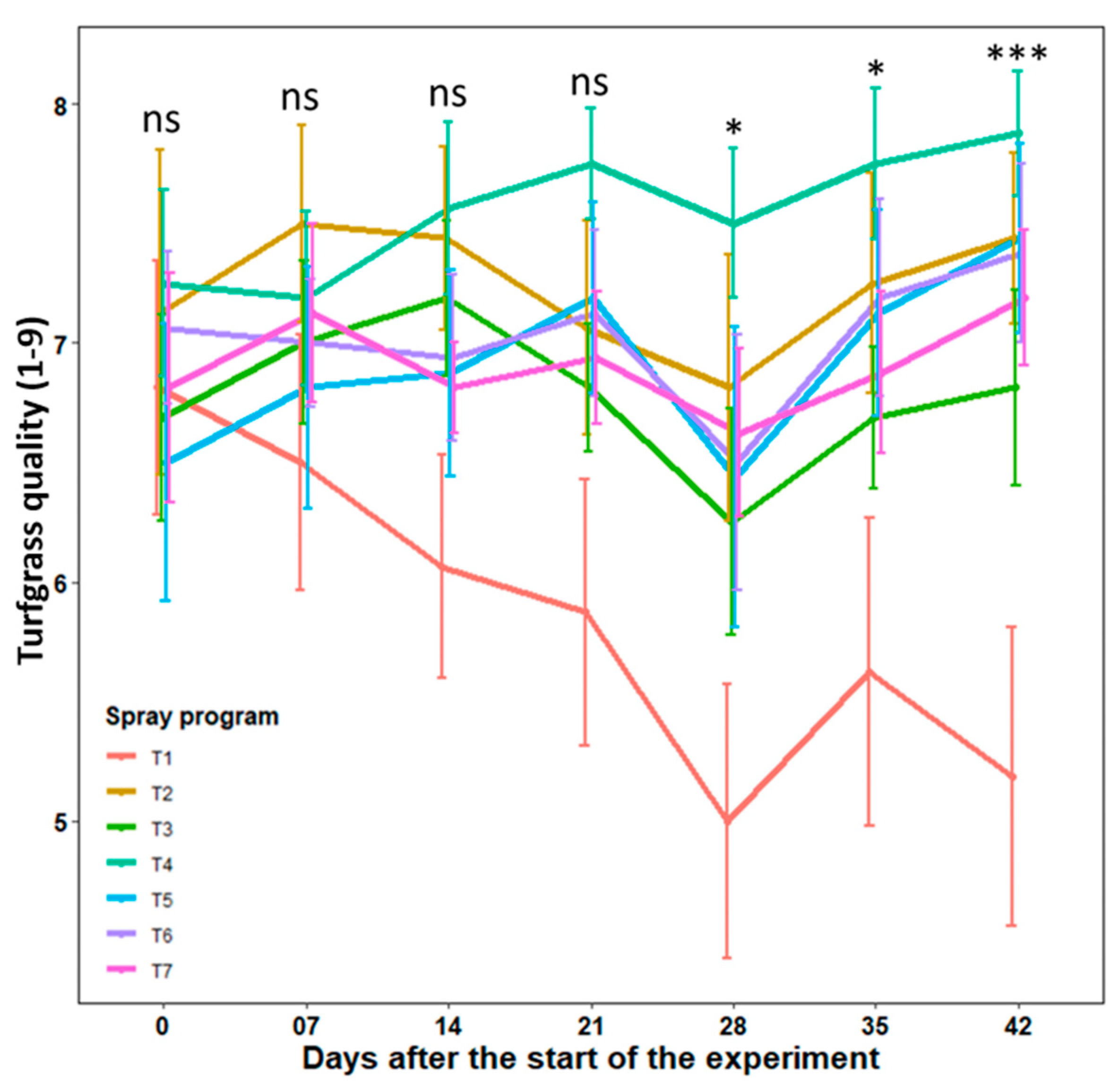
3.3. Efficacy of Biofungicide-Inclusive Spray Program in Field Experiments
3.3.1. University of Georgia in Griffin, GA Experimental Site
3.3.2. Rivermont Golf Club in Johns Creek, GA Experimental Site
3.4. Host Resistance Screening of Zoysiagrass Genotypes
3.5. Host Resistance Screening of Zoysiagrass Breeding Lines
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chawla, S.L.; Agnihotri, R.; Patel, M.A.; Patil, S.; Shah, H.P. Turfgrass: A Billion Dollar Industry. National Conference on Floriculture for Rural and Urban Prosperity in the Scenario of Climate Change; ICAR-National Research Centre for Orchids: Pakyong, India, 2018; pp. 30–35. [Google Scholar]
- National Golf Foundation. The 2016 U.S. Golf Economy Report; National Golf Foundation: Jupiter, FL, USA, 2016. [Google Scholar]
- Tredway, L.P.; Burpee, L.L. Rhizoctonia Diseases of Turfgrass. Plant Health Instr. 2001, 1. [Google Scholar] [CrossRef]
- Butler, E.L.; Galle, G.H.; Kerns, J.P. Influence of Nitrogen Rate and Timing, Fungicide Application Method, and Simulated Rainfall after Fungicide Application on Brown Patch Severity in Tall Fescue. Crop Forage Turfgrass Manag. 2019, 5, 190018. [Google Scholar] [CrossRef]
- Martinez-Espinoza, A.; Little, E.; Daly, T.; Vermeer, B. Identification and Control of Rhizoctonia Large Patch in Georgia. In UGA Cooperative Extension Circular 1088; The University of Georgia in Cooperation with Fort Valley State University, the U.S. Department of Agriculture, and Counties of the State: Garden City, CA, USA, 2022. [Google Scholar]
- Flor, N.C. Genetic Analysis of Large Patch Disease Resistance in Zoysia spp. Ph.D. Thesis, University of Florida, Gainsville, FL, USA, 2017. [Google Scholar]
- Kim, Y.S.; Lee, K.S.; Kim, H.G.; Lee, G.J. Biocontrol of Large Patch Disease in Zoysiagrass (Zoysia japonica) by Bacillus subtilis SA-15: Identification of Active Compounds and Synergism with a Fungicide. Horticulturae 2022, 8, 34. [Google Scholar] [CrossRef]
- Martinez-Espinoza, A. Turf Disease Control. In Georgia Pest Management Handbook—2024 Commercial Edition (vol. 2) UGA Extension Special Bulletin 28; The University of Georgia Press: Athens, GA, USA, 2024; pp. 436–462. [Google Scholar]
- Clarke, B.B.; Vincelli, P.; Koch, P.; Chou, M.-Y. Chemical Control of Turfgrass Diseases 2024. Agric. Nat. Resour. Publ. 2024, 185. [Google Scholar]
- Heritage Fungicide. Available online: https://www.greencastonline.com/products/heritage-fungicide/turf (accessed on 16 August 2024).
- Martinez, A.; Burpee, L.; Allen, T. Guide to Turfgrass Fungicides; Bulletin 1316; University of Georgia: Athens, GA, USA, 2010. [Google Scholar]
- Amaradasa, B.S.; Lakshman, D.; McCall, D.S.; Horvath, B.J. In Vitro Fungicide Sensitivity of Rhizoctonia and Waitea Isolates Collected from Turfgrasses. J. Environ. Hortic. 2014, 32, 126–132. [Google Scholar] [CrossRef]
- Gong, C.; Liu, M.; Liu, D.; Wang, Q.; Hasnain, A.; Zhan, X.; Pu, J.; Liang, Y.; Liu, X.; Wang, X. Status of Fungicide Resistance and Physiological Characterization of Tebuconazole Resistance in Rhizocotonia solani in Sichuan Province, China. Curr. Issues Mol. Biol. 2022, 44, 4859–4876. [Google Scholar] [CrossRef]
- Djébali, N.; Elkahoui, S.; Taamalli, W.; Hessini, K.; Tarhouni, B.; Mrabet, M. Tunisian Rhizoctonia solani AG3 Strains Affect Potato Shoot Macronutrients Content, Infect Faba Bean Plants and Show in Vitro Resistance to Azoxystrobin. Australas. Plant Pathol. 2014, 43, 347–358. [Google Scholar] [CrossRef]
- Li, B.; Li, Q.; Xu, Z.; Zhang, N.; Shen, Q.; Zhang, R. Responses of Beneficial Bacillus amyloliquefaciens SQR9 to Different Soilborne Fungal Pathogens through the Alteration of Antifungal Compounds Production. Front. Microbiol. 2014, 5, 636. [Google Scholar] [CrossRef]
- Ghimire, B.; Aktaruzzaman, M.; Chowdhury, S.R.; Spratling, W.T.; Vermeer, C.B.; Buck, J.W.; Martinez-Espinoza, A.D.; Bahri, B.A. Sensitivity of Clarireedia spp. to Benzimidazoles and Dimethyl Inhibitors Fungicides and Efficacy of Biofungicides on Dollar Spot of Warm Season Turfgrass. Front. Plant Sci. 2023, 14, 1155670. [Google Scholar] [CrossRef]
- Pandin, C.; Le Coq, D.; Deschamps, J.; Védie, R.; Rousseau, T.; Aymerich, S.; Briandet, R. Complete Genome Sequence of Bacillus velezensis QST713: A Biocontrol Agent That Protects Agaricus bisporus Crops against the Green Mould Disease. J. Biotechnol. 2018, 278, 10–19. [Google Scholar] [CrossRef]
- Jeon, C.W.; Kim, D.R.; Kwak, Y.S. Valinomycin, Produced by Streptomyces sp. S8, a Key Antifungal Metabolite in Large Patch Disease Suppressiveness. World J. Microbiol. Biotechnol. 2019, 35, 128. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Morgan, C.; Campbell, B.; Huang, H.; Hernandez, J.; Koivunen, M.E.; Marrone, P.G. Efficacy of New Formulations of Milsana®, Conventional and Organic Regalia®, in Controlling Cucumber Powdery Mildew (Sphaerotheca fuliginea). Phytopathology 2009, 99, S125. [Google Scholar]
- Su, H. Regalia® Bioprotectant in Plant Disease Management. Outlooks Pest Manag. 2012, 23, 30–34. [Google Scholar] [CrossRef]
- Patton, A.J.; Schwartz, B.M.; Kenworthy, K.E. Zoysiagrass (Zoysia spp.) History, Utilization, and Improvement in the United States: A Review. Crop Sci. 2017, 57, S-37–S-72. [Google Scholar] [CrossRef]
- Moore, K.A.; Zuleta, M.C.; Patton, A.J.; Schwartz, B.M.; Aranaz, G.; Milla-Lewis, S.R. SSR Allelic Diversity Shifts in Zoysiagrass (Zoysia spp.) Cultivars Released from 1910 to 2016. Crop Sci. 2017, 57, S-1–S-12. [Google Scholar] [CrossRef]
- Chandra, A.; Fry, J.D.; Genovesi, A.D.; Meeks, M.; Engelke, M.C.; Zhang, Q.; Okeyo, D.; Moss, J.Q.; Ervin, E.; Xiong, X.; et al. Registration of ‘KSUZ 0802’ Zoysiagrass. J. Plant Regist. 2017, 11, 100–106. [Google Scholar] [CrossRef]
- Chandra, A.; Genovesi, A.D.; Fry, J.D.; Patton, A.J.; Meeks, M.; Braun, R.C.; Xiang, M.; Chhetri, M.; Kennelly, M. ‘DALZ 1701’, a Third-Generation Interspecific Zoysiagrass Hybrid. J. Plant Regist. 2023, 17, 499–511. [Google Scholar] [CrossRef]
- Braun, R.C.; Patton, A.J.; Chandra, A.; Fry, J.D.; Genovesi, A.D.; Meeks, M.; Kennelly, M.M.; Xiang, M.; Chhetri, M.; Richardson, M.D.; et al. Development of Winter Hardy, Fine-Leaf Zoysiagrass Hybrids for the Upper Transition Zone. Crop Sci. 2022, 62, 2486–2505. [Google Scholar] [CrossRef]
- Grover, R.K.; Moore, J.D. Toximetric Studies of Fungicides against the Brown Rot Organisms, Sclerotinia fructicola and S. laxa. Phytopathology 1962, 52, 876–880. [Google Scholar]
- Zuleta, M.C.; Gomez, S.; Arellano, C.; Flor, N.; Harmon, P.F.; Kenworthy, K.E.; Milla-lewis, S.R. Identification of Zoysiagrass Genotypes with Resistance to Large Patch under Controlled Environmental Conditions. In Proceedings of the 5th ETS Conference 2016, Albufeira, Portugal, 5–8 June 2016. [Google Scholar]
- Horsfall, J.G.; Barratt, R.W. An Improved Grading System for Measuring Plant Disease. Phytopathology 1945, 35, 655. [Google Scholar]
- Agriculture Research Management (ARM) Software. Available online: https://www.gdmdata.com/ (accessed on 16 August 2024).
- R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 16 August 2024).
- Golf Course Superintendents Association of America (GCSAA). Best Management Practices for Georgia Golf Courses; Golf Course Superintendents Association of America: Lawrence, KS, USA, 2018. [Google Scholar]
- Morris, K.N.; Shearman, R.C. NTEP Turfgrass Evaluation Guidelines. In NTEP Turfgrass Evaluation Workshop; NTEP: Beltsville, MD, USA, 1998. [Google Scholar]
- Lee, H.; Bremer, D.J.; Su, K.; Keeley, S.J. Relationships between Normalized Difference Vegetation Index and Visual Quality in Turfgrasses: Effects of Mowing Height. Crop Sci. 2011, 51, 323–332. [Google Scholar] [CrossRef]
- Houting, K.P. Gaining Insight into the Polygenic Inheritance of Large Patch Resistance in Zoysiagrass. Master’s Thesis, North Carolina State University, Raleigh, NC, USA, 2023. [Google Scholar]
- Cheng, X.; Man, X.; Wang, Z.; Liang, L.; Zhang, F.; Wang, Z.; Liu, P.; Lei, B.; Hao, J.; Liu, X. Fungicide SYP-14288 Inducing Multidrug Resistance in Rhizoctonia solani. Plant Dis. 2020, 104, 2563–2570. [Google Scholar] [CrossRef]
- Arabiat, S.; Khan, M.F.R. Sensitivity of Rhizoctonia solani AG-2-2 from Sugar Beet to Fungicides. Plant Dis. 2016, 100, 2427–2433. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.-Y.; Kwark, S.N.; Lee, G.-J. Isolation and Selection of Antagonistic Microbes for Biological Control of Zoysiagrass Large Patch Disease. Korean J. Hortic. Sci. Technol. 2013, 31, 657–665. [Google Scholar]
- Lee, G.; Choi, H.; Liu, H.; Han, Y.H.; Paul, N.C.; Han, G.H.; Kim, H.; Kim, P., II; Seo, S., II; Song, J.; et al. Biocontrol of the Causal Brown Patch Pathogen Rhizoctonia solani by Bacillus velezensis GH1-13 and Development of a Bacterial Strain Specific Detection Method. Front. Plant Sci. 2023, 13, 1091030. [Google Scholar] [CrossRef] [PubMed]
- Beckerman, J.; Palmer, C.; Tedford, E.; Ypema, H. Fifty Years of Fungicide Development, Deployment, and Future Use. Phytopathology 2023, 113, 694–706. [Google Scholar] [CrossRef]
- Amiri, A.; Zuniga, A.I.; Cordova, L.G.; Peres, N.A. The Importance of Selecting Appropriate Rotation and Tank-Mix Partners for Novel SDHIs to Enhance Botrytis Fruit Rot Control in Strawberry. Plant Dis. 2019, 103, 729–736. [Google Scholar] [CrossRef]
- Wyenandt, A. Theories on Managing Fungicide Resistance Development by Tank Mixing or Rotating Fungicides. Available online: https://plant-pest-advisory.rutgers.edu/theories-on-managing-fungicide-resistance-development-by-tank-mixing-or-rotating-fungicides/ (accessed on 16 August 2024).
- Nicot, P.C. Classical and Augmentative Biological Control against Diseases and Pests: Critical Status Analysis and Review of Factors Influencing Their Success. In IOBC—International Organisation for Biological and Integrated Control of Noxious Animals and Plants; West Palaearctic Regional Section: Bern, Switzerland, 2011; 194p, 978–92-9067-243-2; hal–02809014. [Google Scholar]
- Bardin, M.; Ajouz, S.; Comby, M.; Lopez-Ferber, M.; Graillot, B.; Siegwart, M.; Nicot, P.C. Is the Efficacy of Biological Control against Plant Diseases Likely to Be More Durable than That of Chemical Pesticides? Front. Plant Sci. 2015, 6, 566. [Google Scholar] [CrossRef]
- Anderson, J.P.; Gleason, C.A.; Foley, R.C.; Thrall, P.H.; Burdon, J.B.; Singh, K.B. Plants versus Pathogens: An Evolutionary Arms Race. Funct. Plant Biol. 2010, 37, 499–512. [Google Scholar] [CrossRef]
- Morris, K.N. 2019 National Zoysiagrass Test—2023 Data. In Progress Report NTEP No. 24-6; National Turfgrass Evaluation Program (NTEP): Beltsville, MD, USA, 2019. [Google Scholar]
- Morris, K.N. 2019 National Zoysiagrass Test—2022 Data. In Progress Report NTEP No. 24-6; National Turfgrass Evaluation Program (NTEP): Beltsville, MD, USA, 2022. [Google Scholar]
- Morris, K.N. 2019 National Zoysiagrass Test—2020 Data. In Progress Report NTEP No. 24-6; National Turfgrass Evaluation Program (NTEP): Beltsville, MD, USA, 2020. [Google Scholar]
- Obasa, K.; Fry, J.; Kennelly, M. Susceptibility of Zoysiagrass Germplasm to Large Patch Caused by Rhizoctonia solani. HortScience 2012, 47, 1252–1256. [Google Scholar] [CrossRef]


| Genotypes | Rhizoctonia solani Isolates | Mean | ||||||
|---|---|---|---|---|---|---|---|---|
| Rs_Meyer2019 | Rs_SeaStar2022 | LPZM2 | ||||||
| Cashmere | 1706.2 ± 366.5 abcdefghijkl | S | 900.5 ± 136.4 abcde | I | 698.1 ± 412.8 abcde | I | 1101.6 ± 6.3 BCD | S |
| Cavalier | 1308.8 ± 556.6 abcdefgh | S | 1708.9 ± 160.0 abcdefghijkl | S | 700.8 ± 185.0 abcde | I | 1239.5 ± 6.6 CD | S |
| Diamond | 998.0 ± 462.2 abcde | I | 1185.7 ± 546.7 abcdefg | S | 183 ± 29.0 ab | R | 788.9 ± 7.5 ABCD | I |
| El Toro | 2794.6 ± 88.9 ghijklm | S | 3018.8 ± 207.5 hijklm | S | 1637.9 ± 158.1 abcdefghijk | S | 2483.7 ± 6.3 FG | S |
| Emerald | 3204.7 ± 115.7 jklm | S | 3456.3 ± 11.9 m | S | 2850.2 ± 246.5 ghijklm | S | 3170.4 ± 3.7 G | S |
| Empire | 1730 ± 384.7 abcdefghijklm | S | 1184.9 ± 217.2 abcdefg | S | 607.8 ± 19.1 abcde | I | 1174.2 ± 5.4 CD | S |
| Geo | 3322.3 ± 38.3 klm | S | 153.0 ± 27.7 ab | R | 1009.0 ± 306.1 abcdef | I | 1494.7 ± 13.6 CDE | S |
| Innovation | 3363.3 ± 64.5 klm | S | 1186.7 ± 281.1 abcdefg | S | 3177.3 ± 142.3 jklm | S | 2575.8 ± 10 FG | S |
| JaMur | 1977.9 ± 251.0 cdefghijklm | S | 430.1 ± 40.6 abcd | I | 1538.5 ± 471.4 abcdefghij | S | 1315.5 ± 7.3 CD | S |
| Matrella | 114.8 ± 37.0 a | R | 397.3 ± 244.5 abc | I | 62.8 ± 39.4 a | R | 191.6 ± 2.4 A | R |
| Meyer | 692.6 ± 100.7 abcde | I | 1121.0 ± 339.6 abcdefg | S | 798.4 ± 142.2 abcde | I | 870.7 ± 3.6 ABCD | I |
| Palisades | 1222.2 ± 429.5 abcdefg | S | 2748.1 ± 222.6 fghijklm | S | 721.8 ± 180.8 abcde | I | 1564 ± 9.5 DE | S |
| PI 231146 | 261.5 ± 171.0 abc | R | 382.7 ± 107.7 abc | I | 60.0 ± 10.9 a | R | 234.7 ± 2.3 AB | R |
| PI 553020 | 1563.1 ± 228.2 abcdefghij | S | 3128.1 ± 80.1 ijklm | S | 1875.8 ± 612.4 bcdefghijklm | S | 2189 ± 8.3 EF | S |
| Pristine Flora | 257.0 ± 42.5 abc | R | 1797.4 ± 267.3 abcdefghijklm | S | 144.8 ± 69.0 ab | R | 733 ± 7.9 ABCD | I |
| Rollmaster | 747.3 ± 414.4 abcde | I | 359.9 ± 182.4 abc | I | 806.5 ± 366.8 abcde | I | 637.9 ± 5.3 ABC | I |
| Shadow Turf | 1169.3 ± 581.1 abcdefg | S | 994.3 ± 481.8 abcde | I | 1392.6 ± 399.7 abcdefghi | S | 1185.4 ± 7 CD | S |
| Trinity (formerly L1F) | 3148.2 ± 73.5 jklm | S | 2203.0 ± 112.2 efghijklm | S | 3401.6 ± 0.02 lm | S | 2917.6 ± 5.4 FG | S |
| Zenith | 2993.2 ± 159.1 hijklm | S | 2154.7 ± 320.1 defghijklm | S | 3317.8 ± 113.2 klm | S | 2821.9 ± 5.8 FG | S |
| Zeon | 951.5 ± 422.0 abcde | I | 712.6 ± 335.6 abcde | I | 668.0 ± 365.4 abcde | I | 777.4 ± 5.4 ABCD | I |
| Zorro | 668.9 ± 342.2 abcde | I | 876.7 ± 480.2 abcde | I | 1570.4 ± 643.8 abcdefghij | S | 1038.7 ± 7.8 ABCD | I |
| Mean | 1628.4 ± 146.7 B | 1433.4 ± 133.5 AB | 1296.3 ± 144.0 A | 1452.3 | ||||
| Breeding Lines | Day 16 | Day 36 | ||
|---|---|---|---|---|
| GZP 09.03.12 | 16.4 ± 4.8 b | I | 53.9 ± 15.7 bc | S |
| GZP 10.12.70.23 | 3.5 ± 0.5 a | R | 74.4 ± 9.8 c | S |
| GZP 10.5.029.56 | 1.2 ± 0.5 a | R | 5.9 ± 1.2 a | R |
| GZZ LG3.20 | 0.0 ± 0.0 a | R | 11.7 ± 3.3 a | I |
| HN 17-4 | 1.6 ± 0.8 a | R | 9.8 ± 5.7 a | R |
| Zeon | 30.7 ± 7.8 c | S | 27.3 ± 6.5 ab | I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimire, B.; Orellana, R.; Chowdhury, S.R.; Vermeer, C.B.; Patel, P.; Raymer, P.; Milla-Lewis, S.; Buck, J.W.; Martinez-Espinoza, A.D.; Bahri, B.A. Assessing Biofungicides and Host Resistance against Rhizoctonia Large Patch in Zoysiagrass. Pathogens 2024, 13, 864. https://doi.org/10.3390/pathogens13100864
Ghimire B, Orellana R, Chowdhury SR, Vermeer CB, Patel P, Raymer P, Milla-Lewis S, Buck JW, Martinez-Espinoza AD, Bahri BA. Assessing Biofungicides and Host Resistance against Rhizoctonia Large Patch in Zoysiagrass. Pathogens. 2024; 13(10):864. https://doi.org/10.3390/pathogens13100864
Chicago/Turabian StyleGhimire, Bikash, Rolando Orellana, Shukti R. Chowdhury, Christopher Brian Vermeer, Paige Patel, Paul Raymer, Susana Milla-Lewis, James W. Buck, Alfredo D. Martinez-Espinoza, and Bochra A. Bahri. 2024. "Assessing Biofungicides and Host Resistance against Rhizoctonia Large Patch in Zoysiagrass" Pathogens 13, no. 10: 864. https://doi.org/10.3390/pathogens13100864






