Equine Herpesvirus Type 1 ORF76 Encoding US9 as a Neurovirulence Factor in the Mouse Infection Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Cells
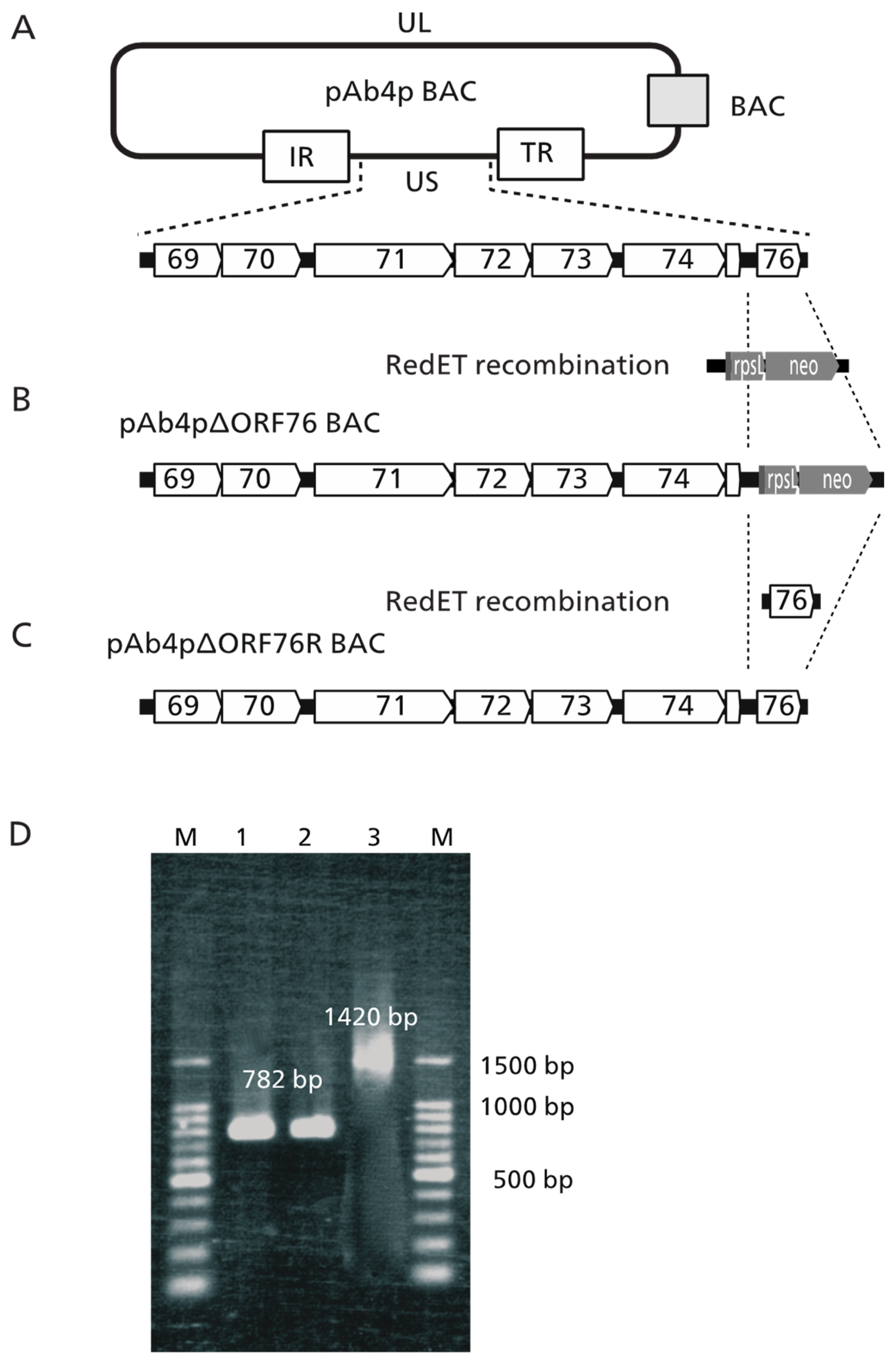
2.2. Construction of Ab4pΔORF76 (US9 Deletion) and Revertant BACs
2.3. Recovery of Infectious Ab4p attB, Ab4pΔORF76, and Ab4pΔORF76R Viruses from BAC DNA
2.4. Time Course of Viral Growth
2.5. Virus Growth Kinetics in Mouse Neurons
2.6. Analysis of Transcription Kinetics by Real-Time RT-PCR
2.7. Animal Experiments
2.8. Preparation of Tissues for Virus Titration
2.9. Preparation of Tissues for Virus DNA Detection by PCR
2.10. Histopathology and Immunohistochemistry
2.11. Immunofluorescence
2.12. Production of Anti-EHV-1 US9-Specific Polyclonal Antibody
2.13. Western Blotting
2.14. Statistical Analysis
3. Results
3.1. Construction of ORF76 (US9) Deletion Mutant and Its Revertant Virus
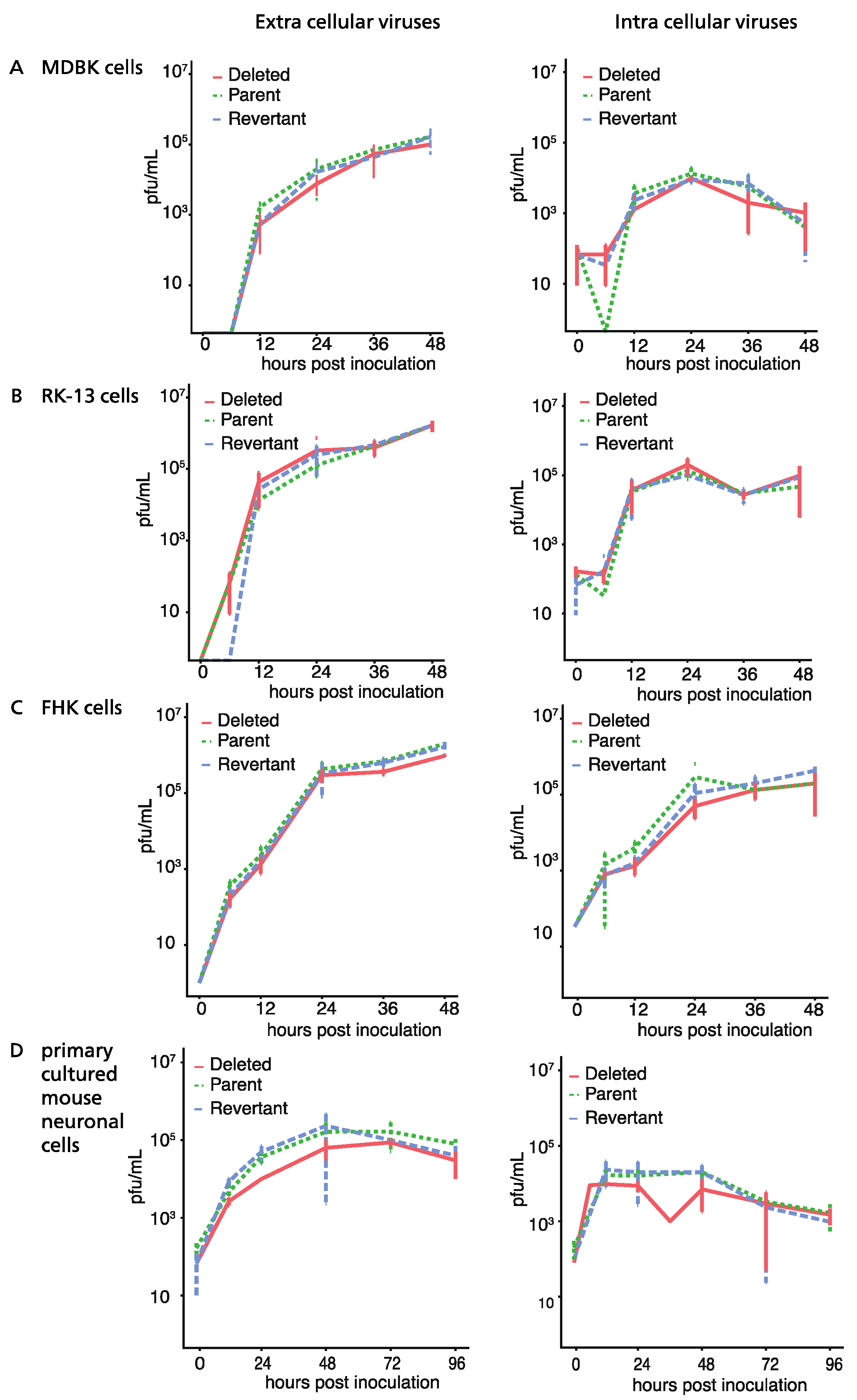
3.2. Growth of ORF76 (US9) Deletion Mutant in Cultured Cells and Mouse Neurons
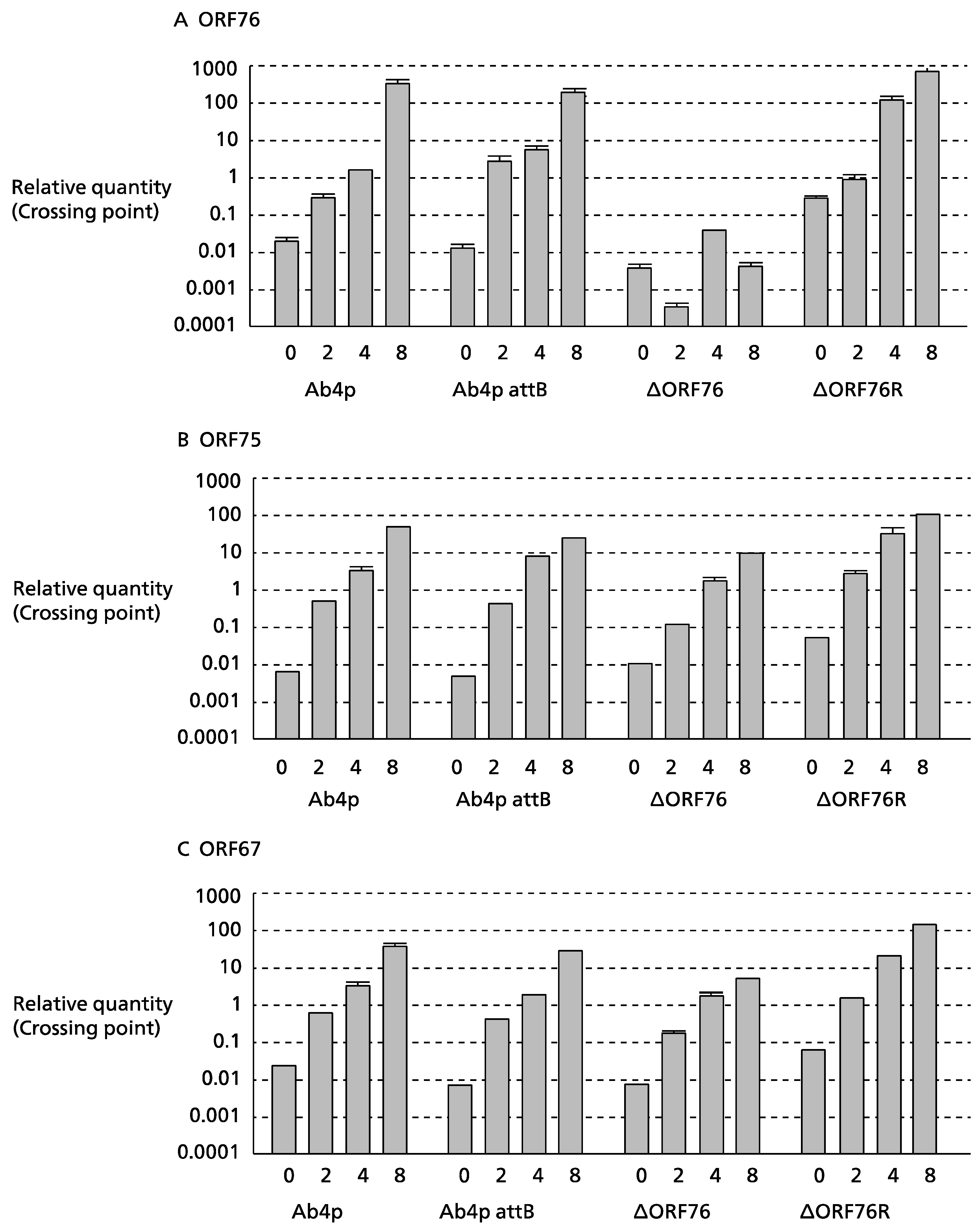
3.3. Effect of ORF76 Deletion on Transcription Activities of Other Genes
3.4. Identification and Initial Characterization of EHV-1 US9 Protein
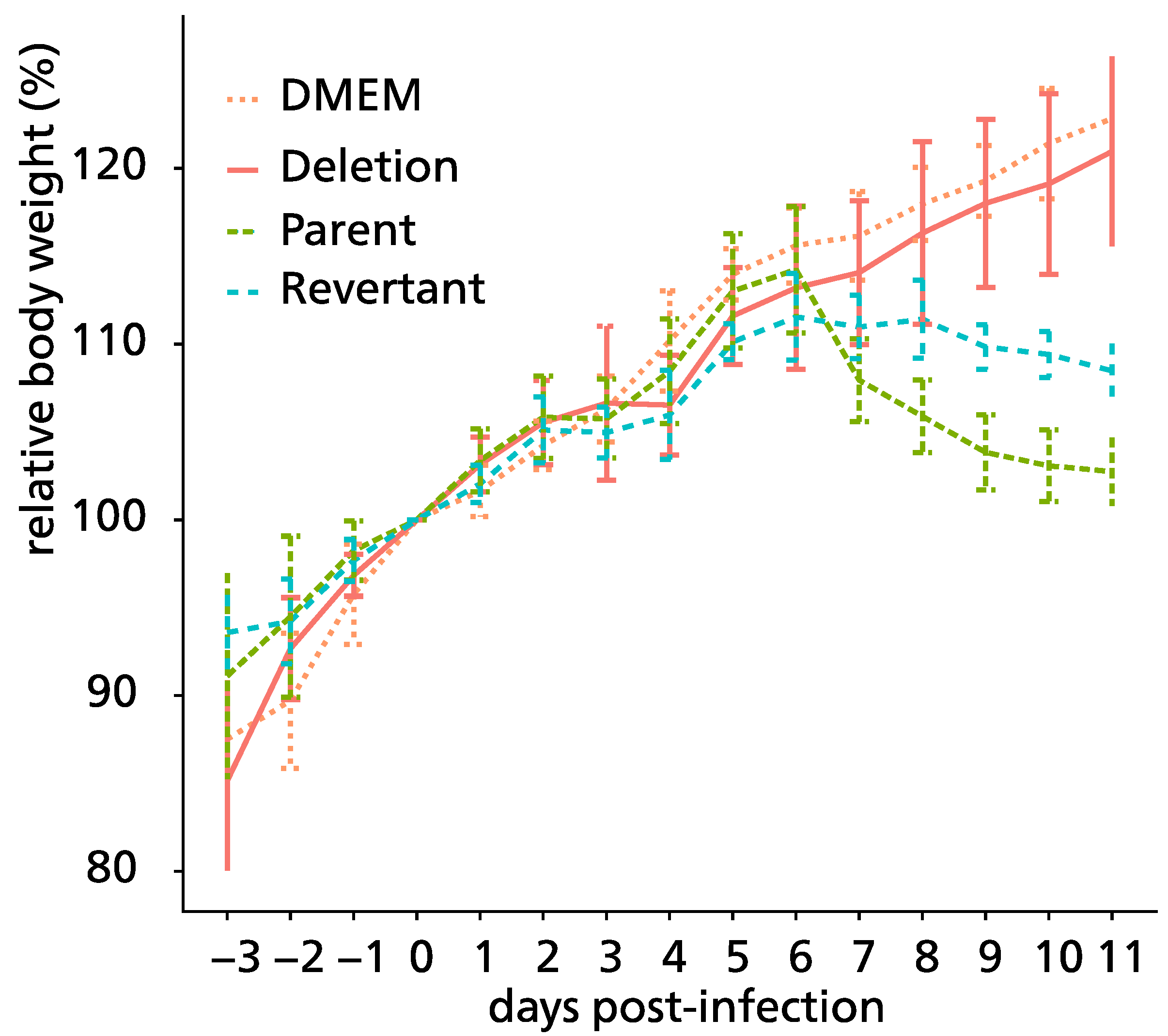
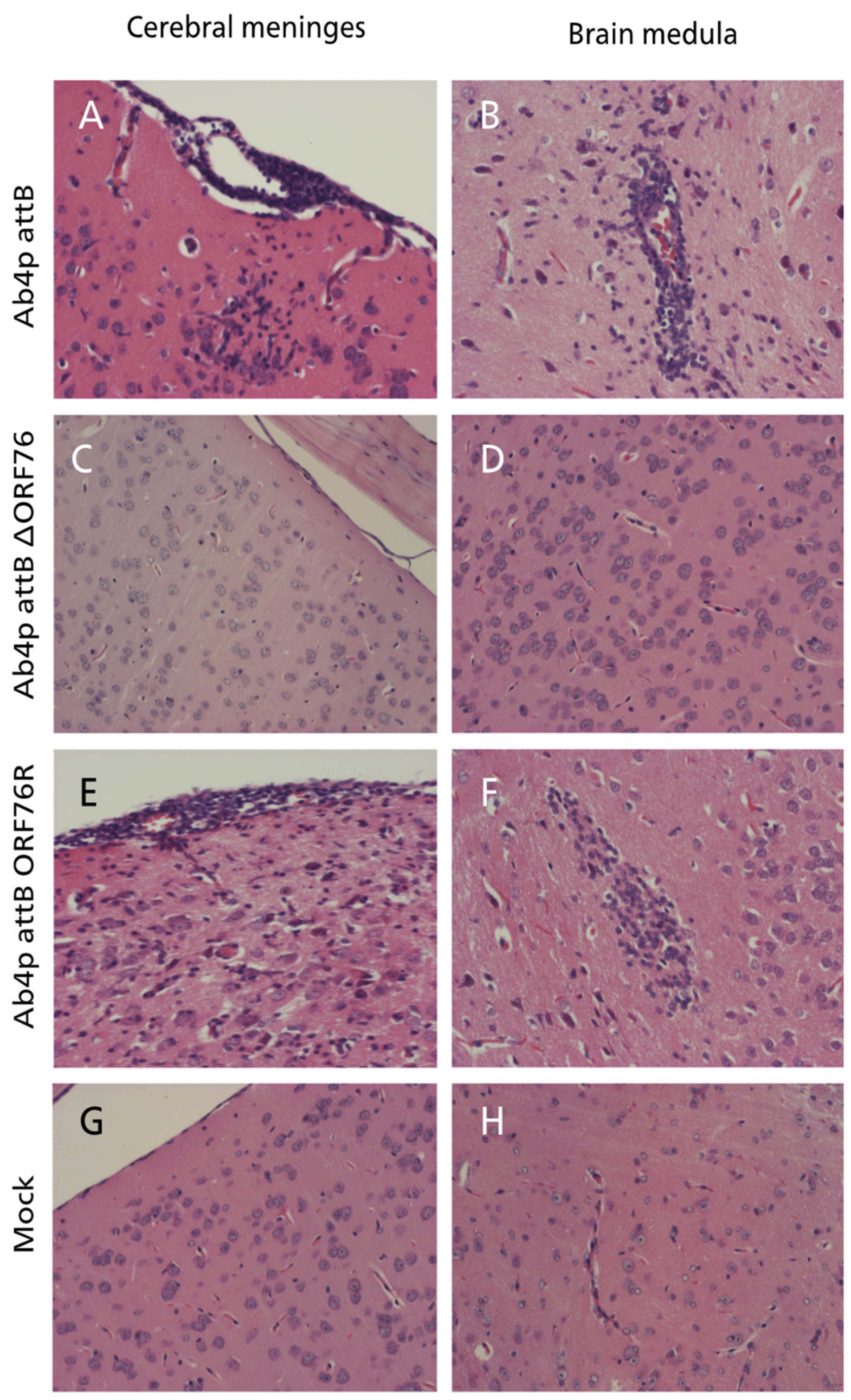
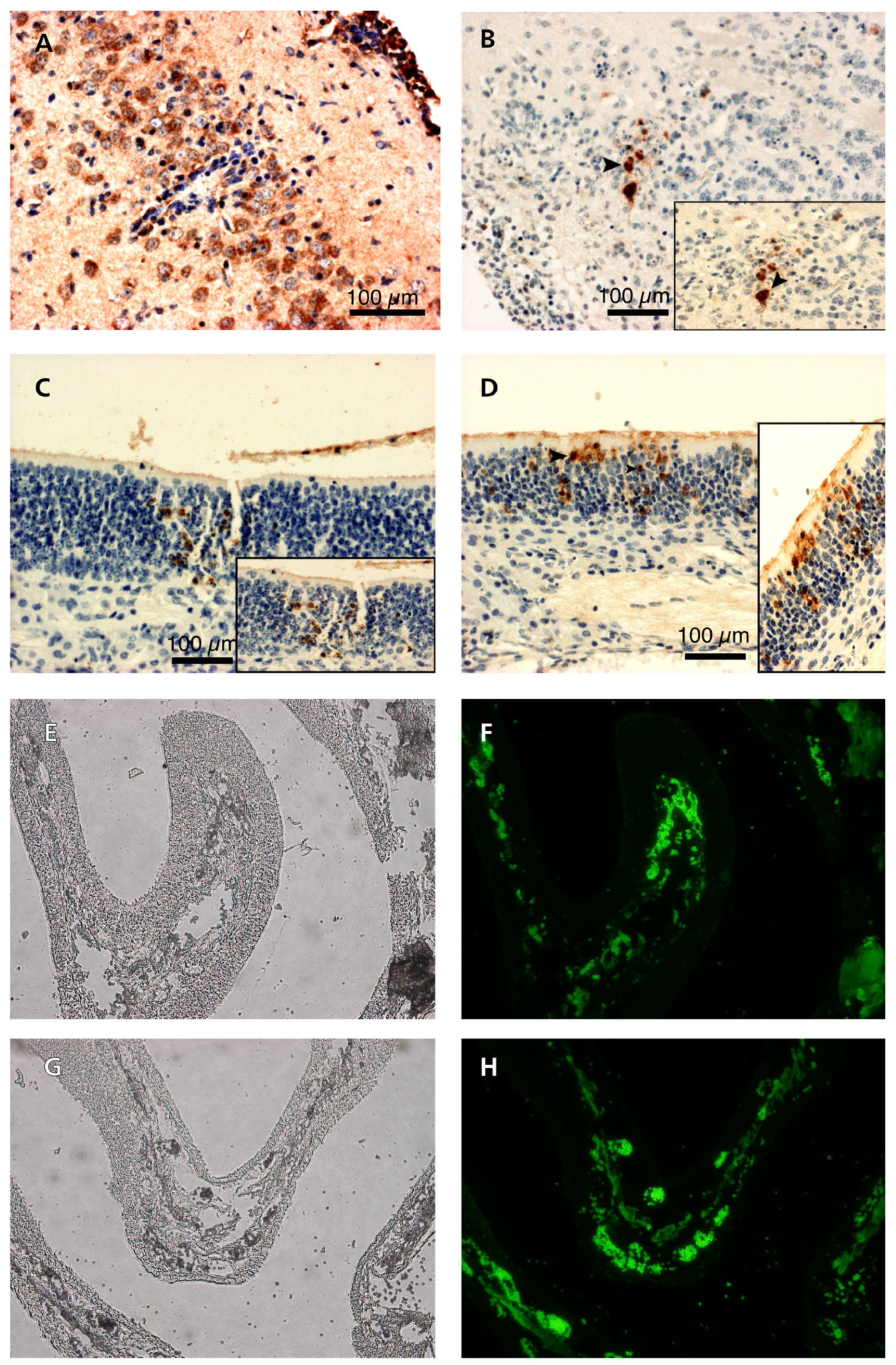
3.5. Pathogenicity of ORF76 Deletion Mutant Virus in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, J.; Heldens, J. Equine herpesviruses 1 (EHV-1) and 4 (EHV-4)—Epidemiology, disease and immunoprophylaxis: A brief review. Vet. J. 2005, 170, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Saxegaard, F. Isolation and identification of equine rhinopneumonitis virus (equine abortion virus) from cases of abortion and paralysis. Nordic Vet. Med. 1966, 18, 504–512. [Google Scholar]
- Allen, G.P. Development of a Real-Time Polymerase Chain Reaction Assay for Rapid Diagnosis of Neuropathogenic Strains of Equine Herpesvirus-1. J. Vet. Diagn. Investig. 2007, 19, 69–72. [Google Scholar] [CrossRef]
- Goehring, L.S.; van Maanen, C.; van Sloet Oldruitenborgh-Oosterbaan, M.M. Neurological syndromes among horses in The Netherlands. A 5 year retrospective survey (1999–2004). Vet. Q. 2005, 27, 11–20. [Google Scholar] [CrossRef]
- Henninger, R.W.; Reed, S.M.; Saville, W.J.; Allen, G.P.; Hass, G.F.; Kohn, C.W.; Sofaly, C. Outbreak of neurologic disease caused by equine herpesvirus-1 at a university equestrian center. J. Vet. Int. Med. 2007, 21, 157–165. [Google Scholar]
- Lunn, D.; Davis-Poynter, N.; Flaminio, M.; Horohov, D.; Osterrieder, K.; Pusterla, N.; Townsend, H. Equine herpesvirus-1 consensus statement. J. Vet. Int. Med. 2009, 23, 450–461. [Google Scholar] [CrossRef]
- Kohn, C.W.; Reed, S.M.; Sofaly, C.D.; Henninger, R.W.; Saville, W.J.; Allen, G.P.; Premanadan, C. Transmission of EHV-1 by horses with EHV-1 myeloencephalopathy: Implications for biosecurity and review. Clin. Tech. Equine Pract. 2006, 5, 60–66. [Google Scholar] [CrossRef]
- Jackson, T.A.; Osburn, B.; Cordy, D.; Kendrick, J. Equine herpesvirus 1 infection of horses: Studies on the experimentally induced neurologic disease. Am. J. Vet. Res. 1977, 38, 709–719. [Google Scholar]
- Nugent, J.; Birch-Machin, I.; Smith, K.; Mumford, J.; Swann, Z.; Newton, J.; Bowden, R.; Allen, G.; Davis-Poynter, N. Analysis of equid herpesvirus 1 strain variation reveals a point mutation of the DNA polymerase strongly associated with neuropathogenic versus nonneuropathogenic disease outbreaks. J. Virol. 2006, 80, 4047–4060. [Google Scholar] [CrossRef]
- Leutenegger, C.; Madigan, J.; Mapes, S.; Thao, M.; Estrada, M.; Pusterla, N. Detection of EHV-1 neuropathogenic strains using real-time PCR in the neural tissue of horses with myeloencephalopathy. Vet. Rec. 2008, 162, 688. [Google Scholar] [CrossRef]
- Smith, K.L.; Allen, G.P.; Branscum, A.J.; Cook, R.F.; Vickers, M.L.; Timoney, P.J.; Balasuriya, U.B. The increased prevalence of neuropathogenic strains of EHV-1 in equine abortions. Vet. Microbiol. 2010, 141, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Vissani, M.; Becerra, M.; Perglione, C.O.; Tordoya, M.; Miño, S.; Barrandeguy, M. Neuropathogenic and non-neuropathogenic genotypes of Equid Herpesvirus type 1 in Argentina. Vet. Microbiol. 2009, 139, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Matsumura, T.; Tsujimura, K.; Yamaguchi, T.; Ohya, K.; Fukushi, H. Comparison of the growth kinetics of neuropathogenic and nonneuropathogenic equid herpesvirus type 1 (EHV-1) strains in cultured murine neuronal cells and the relevance of the D/N752 coding change in DNA polymerase gene (ORF30). J. Vet. Med. Sci. 2008, 70, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, A.; Azab, W.; Damiani, A.M.; Baumgartner, K.; Will, H.; Osterrieder, N.; Greenwood, A.D. Zebra-borne equine herpesvirus type 1 (EHV-1) infection in non-African captive mammals. Vet. Microbiol. 2014, 169, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, A.D.; Tsangaras, K.; Ho, S.Y.W.; Szentiks, C.A.; Nikolin, V.M.; Ma, G.; Damiani, A.; East, M.L.; Lawrenz, A.; Hofer, H.; et al. A potentially fatal mix of herpes in zoos. Curr. Biol. 2012, 22, 1727–1731. [Google Scholar] [CrossRef]
- Kennedy, M.A.; Ramsay, E.; Diderrich, V.; Richman, L.; Allen, G.P.; Potgieter, L.N.D. Encephalitis associated with a variant of equine herpesvirus 1 in a Thomson’s Gazelle (Gazella thomsoni). J. Zoo Wildl. Med. 1996, 27, 533–538. [Google Scholar]
- Montali, R.J.; Allen, G.P.; Bryans, J.T.; Phillips, L.G.; Bush, M. Equine herpesvirus type 1 abortion in an onager and suspected herpesvirus myelitis in a zebra. J. Am. Vet. Med. Assoc. 1985, 187, 1248–1249. [Google Scholar]
- Wolff, P.L.; Meehan, T.P.; Basgall, E.J.; Allen, G.P.; Sundberg, J.P. Abortion and perinatal foal mortality associated with equine herpesvirus type 1 in a herd of Grevy’s zebra. J. Am. Vet. Med. Assoc. 1986, 189, 1185–1186. [Google Scholar]
- Gibson, J.; Slater, J.; Field, H. The pathogenicity of Ab4p, the sequenced strain of equine herpesvirus-1, in specific pathogen-free foals. Virology 1992, 189, 317–319. [Google Scholar] [CrossRef]
- Telford, E.A.; Watson, M.S.; McBride, K.; Davison, A.J. The DNA sequence of equine herpesvirus-1. Virology 1992, 189, 304–316. [Google Scholar] [CrossRef]
- Awan, A.R.; Chong, Y.-C.; Field, H.J. The pathogenesis of equine herpesvirus type 1 in the mouse: A new model for studying host responses to the infection. J. Gen. Virol. 1990, 71, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.S.M.; Pagmajav, O.; Yamaguchi, T.; Matsumura, T.; Fukushi, H. Growth and virulence alterations of equine herpesvirus 1 by insertion of a green fluorescent protein gene in the intergenic region between ORFs 62 and 63. Microbiol. Immunol. 2004, 48, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Kasem, S.; Yu, M.H.H.; Yamada, S.; Kodaira, A.; Matsumura, T.; Tsujimura, K.; Madbouly, H.; Yamaguchi, T.; Ohya, K.; Fukushi, H. The ORF37 (UL24) is a neuropathogenicity determinant of equine herpesvirus 1 (EHV-1) in the mouse encephalitis model. Virology 2010, 400, 259–270. [Google Scholar] [CrossRef]
- Okada, A.; Izume, S.; Ohya, K.; Fukushi, H. Equine herpesvirus type 1 tegument protein VP22 is not essential for pathogenicity in a hamster model, but is required for efficient viral growth in cultured cells. J. Vet. Med. Sci. 2015, 77, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Gosztonyi, G.; Borchers, K.; Ludwig, H. Pathogenesis of equine herpesvirus-1 infection in the mouse model. APMIS 2009, 117, 10–21. [Google Scholar] [CrossRef]
- Frame, M.C.; McGeoch, D.J.; Rixon, F.J.; Orr, A.C.; Marsden, H.S. The 10K virion phosphoprotein encoded by gene US9 from herpes simplex virus type 1. Virology 1986, 150, 321–332. [Google Scholar] [CrossRef]
- McGeoch, D.J.; Dolan, A.; Donald, S.; Rixon, F.J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J. Mol. Biol. 1985, 181, 1–13. [Google Scholar] [CrossRef]
- McGraw, H.M.; Awasthi, S.; Wojcechowskyj, J.A.; Friedman, H.M. Anterograde spread of herpes simplex virus type 1 requires glycoprotein E and glycoprotein I but not Us9. J. Virol. 2009, 83, 8315–8326. [Google Scholar] [CrossRef][Green Version]
- Davison, A.J.; Scott, J.E. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 1986, 67, 1759–1816. [Google Scholar] [CrossRef]
- Van Zijl, M.; van der Gulden, H.; de Wind, N.; Gielkens, A.; Berns, A. Identification of two genes in the unique short region of pseudorabies virus; comparison with herpes simplex virus and varicella-zoster virus. J. Gen. Virol. 2001, 71, 1747–1755. [Google Scholar] [CrossRef]
- Killeen, A.M.; Harrington, L.; Wall, L.V.; Kelly, D.C. Nucleotide sequence analysis of a homologue of herpes simplex virus type 1 gene US9 found in the genome of simian herpes B virus. J. Gen. Virol. 1992, 73, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Leung-Tack, P.; Audonnet, J.C.; Riviere, M. The complete DNA sequence and the genetic organization of the short unique region (US) of the bovine herpesvirus type 1 (ST strain). Virology 1994, 199, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Willemse, M.J.; Strijdveen, I.G.; van Schooneveld, S.H.; van den Berg, M.C.; Sondermeijer, P.J. Transcriptional analysis of the short segment of the feline herpesvirus type 1 genome and insertional mutagenesis of a unique reading frame. Virology 1995, 208, 704–711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haanes, E.J.; Tomlinson, C.C. Genomic organization of the canine herpesvirus US region. Virus Res. 1998, 53, 151–162. [Google Scholar] [CrossRef]
- Dolan, A.; Jamieson, F.E.; Cunningham, C.; Barnett, B.C.; McGeoch, D.J. The genome sequence of herpes simplex virus type 2. J. Virol. 1998, 72, 2010–2021. [Google Scholar] [CrossRef]
- Lyman, M.G.; Kemp, C.D.; Taylor, M.P.; Enquist, L.W. Comparison of the pseudorabies virus Us9 protein with homologs from other veterinary and human alphaherpesviruses. J. Virol. 2009, 83, 6978–6986. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mahmood, S.; Simon, J.; Al-Mubarak, A.; Zhou, Y. The Us9 gene of bovine herpesvirus 1 (BHV-1) effectively complements a Us9-null strain of BHV-5 for anterograde transport, neurovirulence, and neuroinvasiveness in a rabbit model. J. Virol. 2006, 80, 4396–4405. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chowdhury, S.; Onderci, M.; Bhattacharjee, P.; Al-Mubarak, A.; Weiss, M.; Zhou, Y. Bovine herpesvirus 5 (BHV-5) Us9 is essential for BHV-5 neuropathogenesis. J. Virol. 2002, 76, 3839–3851. [Google Scholar] [CrossRef]
- Ata, E.B.; Zaghawa, A.; Ghazy, A.A.; Elsify, A.; Abdelrahman, K.; Kasem, S.; Nayel, M. Development and characterization of ORF68 negative equine herpes virus type–1, Ab4p strain. J. Virol. Methods 2018, 261, 121–131. [Google Scholar] [CrossRef]
- Badr, Y.; Okada, A.; Abo-Sakaya, R.; Beshir, E.; Ohya, K.; Fukushi, H. Equine herpesvirus type 1 ORF51 encoding UL11 as an essential gene for replication in cultured cells. Arch. Virol. 2018, 163, 599–607. [Google Scholar] [CrossRef]
- Matsumura, T.; Kondo, T.; Sugita, S.; Damiani, A.M.; O’Callaghan, D.J.; Imagawa, H. An equine herpesvirus type 1 recombinant with a deletion in the gE and gI genes is avirulent in young horses. Virology 1998, 242, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.H.; Kasem, S.G.; Tsujimura, K.; Matsumura, T.; Yanai, T.; Yamaguchi, T.; Ohya, K.; Fukushi, H. Diverse pathogenicity of equine herpesvirus 1 (EHV-1) isolates in CBA mouse model. J. Vet. Med. Sci. 2010, 72, 301–306. [Google Scholar] [CrossRef]
- Robertson, D.; Savage, K.; Reis-Filho, J.S.; Isacke, C.M. Multiple immunofluorescence labelling of formalin-fixed paraffin-embedded (FFPE) tissue. BMC Cell Biol. 2008, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Howard, P.W.; Howard, T.L.; Johnson, D.C. Herpes simplex virus membrane proteins gE/gI and US9 act cooperatively to promote transport of capsids and glycoproteins from neuron cell bodies into proximal axons. J. Virol. 2013, 87, 403–414. [Google Scholar] [CrossRef][Green Version]
- Brideau, A.; Card, J.; Enquist, L. Role of pseudorabies virus Us9, a type II membrane protein, in infection of tissue culture cells and the rat nervous system. J. Virol. 2000, 74, 834–845. [Google Scholar] [CrossRef]
- Brandimarti, R.; Roizman, B. Us9, a stable lysine-less herpes simplex virus 1 protein, is ubiquitinated before packaging into virions and associates with proteasomes. Proc. Natl. Acad. Sci. USA 1997, 94, 13973–13978. [Google Scholar] [CrossRef]
- Brideau, A.; Banfield, B.W.; Enquist, L. The Us9 gene product of pseudorabies virus, an alphaherpesvirus, is a phosphorylated, tail-anchored type II membrane protein. J. Virol. 1998, 72, 4560–4570. [Google Scholar] [CrossRef] [PubMed]
- Peck, S.C. Analysis of protein phosphorylation: Methods and strategies for studying kinases and substrates. Plant Journal 2006, 45, 512–522. [Google Scholar] [CrossRef]
- Lee, B.J.; Weiss, M.L.; Mosier, D.; Chowdhury, S.I. Spread of bovine herpesvirus type 5 (BHV-5) in the rabbit brain after intranasal inoculation. J. Neurovirol. 1999, 5, 474–484. [Google Scholar] [CrossRef][Green Version]
- Fukushi, H.; Taniguchi, A.; Yasuda, K.; Yanai, T.; Masegi, T.; Yamaguchi, T.; Hirai, K. A hamster model of equine herpesvirus 9 induced encephalitis. J. Neurovirol. 2000, 6, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Uchimura, A.; Kawanabe, M.; Fukushi, H.; Hirai, K. Invasion and spread of equine herpesvirus 9 in the olfactory pathway of pigs after intranasal inoculation. J. Comp. Pathol. 2001, 124, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Enquist, L.; Tomishima, M.; Gross, S.; Smith, G. Directional spread of an α-herpesvirus in the nervous system. Vet. Microbiol. 2002, 86, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Tomishima, M.; Enquist, L. A conserved α-herpesvirus protein necessary for axonal localization of viral membrane proteins. J. Cell Biol. 2001, 154, 741–752. [Google Scholar] [CrossRef]
- Tomishima, M.; Smith, G.; Enquist, L. Sorting and transport of alpha herpesviruses in axons. Traffic 2001, 2, 429–436. [Google Scholar] [CrossRef]


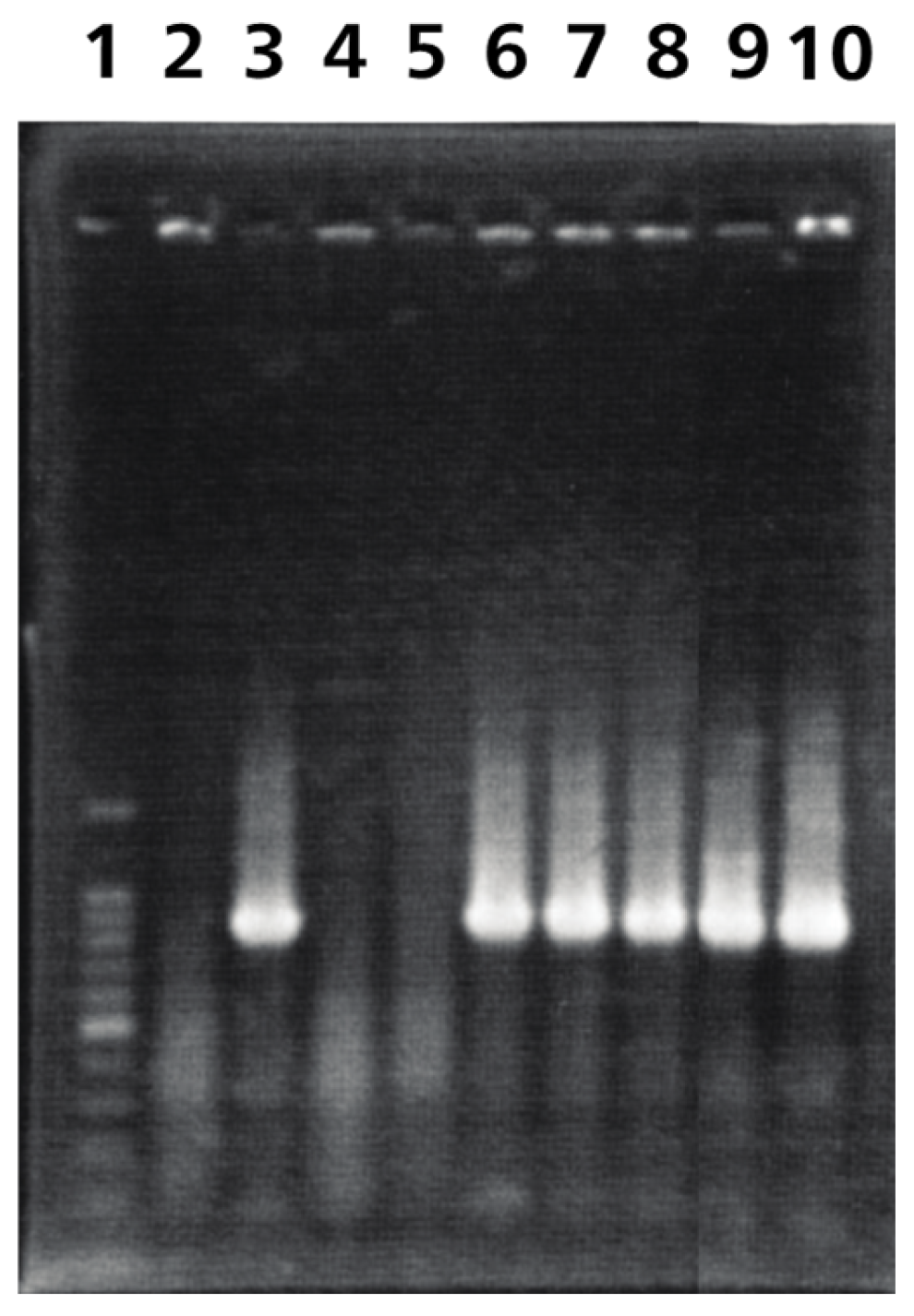
| Target | Primer | Sequence |
|---|---|---|
| rpsL-neo cassette | 1 | 5′-TTT CCC TCT CAG CGA TCA CTT TTC ACC ACC GAA GAA CAG GCC CTC ATC GGG GCC TGG TGA TGA TGG CGG GAT CG-3′ |
| 2 | 5′-GGG CTG TTG TGG GGT AAA AGG TGG TGT TAC GGA AAC ACG CGT GCC AAG AAT CAG AAG AAC TCG TCA AGA AGG CG-3′ | |
| rpsL-ORF76 | 3 | 5′-TTT CCC TCT CAG CGA TCA CTT TTC ACC ACC GAA GAA CAG GCC CTC ATC GG-3′ |
| 4 | 5′-GGG CTG TTG TGG GGT AAA AGG TGG TGT TAC GGA AAC ACG CGT GCC AAG AA-3′ | |
| ORF76 with EcoRI and NotI | 5 | 5′-ccg gaa ttc ATG GAG AAG GCG GAG GCT GCC GCA-3′ |
| 6 | 5′-aag gaa aaa agc ggc cgc TTA CGG AAA CAC GCG TGC CAA GAA-3′ | |
| ORF76 | 7 | 5′-CTA CCG TGG AAG CGG TAT GT-3′ |
| 8 | 5′-ATT CTC AGA AGC AGC GGT GT-3′ | |
| ORF75 | 9 | 5′-CAA CCC TGT CAG AAA CAG CA-3′ |
| 10 | 5′-GGG GGA GGT AGA GTT TCC AG-3′ | |
| ORF67 | 11 | 5′-TCG GCC CTT ATG TAA TAG CG-3′ |
| 12 | 5′-CTC CTA CTT CAG GCG GTG TC-3′ | |
| ORF30 | 13 | 5′-gtc agg ccc aca aac ttg at-3′ |
| 14 | 5′-act cgg ttt acg gat tca cg-3′ |
| Virus | Organ 1 | Virus DNA Detection on Days Post-Infection | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Ab4p attB | O | - | + | + | + | + | + | + | + | + | + | + |
| B | - | + | + | + | + | + | + | + | + | + | + | |
| L | - | + | + | + | + | + | + | + | + | + | + | |
| Ab4p ΔORF76 | O | - | - | - | - | - | - | - | - | - | - | - |
| B | - | - | - | - | - | - | - | - | - | - | - | |
| L | - | - | + | + | + | + | + | + | + | - | - | |
| Ab4p ΔORF76R | O | - | + | + | + | + | + | + | + | + | + | + |
| B | - | + | + | + | + | + | + | + | + | + | + | |
| L | - | + | + | + | + | + | + | + | + | + | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayel, M.; Kasem, S.; Fukushi, N.; El-Habashi, N.; Elsify, A.; Salama, A.; Hassan, H.; Yanai, T.; Ohya, K.; Fukushi, H. Equine Herpesvirus Type 1 ORF76 Encoding US9 as a Neurovirulence Factor in the Mouse Infection Model. Pathogens 2024, 13, 865. https://doi.org/10.3390/pathogens13100865
Nayel M, Kasem S, Fukushi N, El-Habashi N, Elsify A, Salama A, Hassan H, Yanai T, Ohya K, Fukushi H. Equine Herpesvirus Type 1 ORF76 Encoding US9 as a Neurovirulence Factor in the Mouse Infection Model. Pathogens. 2024; 13(10):865. https://doi.org/10.3390/pathogens13100865
Chicago/Turabian StyleNayel, Mohamed, Samy Kasem, Noriko Fukushi, Nagwan El-Habashi, Ahmed Elsify, Akram Salama, Hany Hassan, Tokuma Yanai, Kenji Ohya, and Hideto Fukushi. 2024. "Equine Herpesvirus Type 1 ORF76 Encoding US9 as a Neurovirulence Factor in the Mouse Infection Model" Pathogens 13, no. 10: 865. https://doi.org/10.3390/pathogens13100865
APA StyleNayel, M., Kasem, S., Fukushi, N., El-Habashi, N., Elsify, A., Salama, A., Hassan, H., Yanai, T., Ohya, K., & Fukushi, H. (2024). Equine Herpesvirus Type 1 ORF76 Encoding US9 as a Neurovirulence Factor in the Mouse Infection Model. Pathogens, 13(10), 865. https://doi.org/10.3390/pathogens13100865








