Avian Metapneumovirus Subtype B Circulation in Poultry and Wild Birds of Colombia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cross-Sectional Study and Samples
2.2. aMPV Detection Strategy
2.3. Sequencing and Phylogenetic Analyses
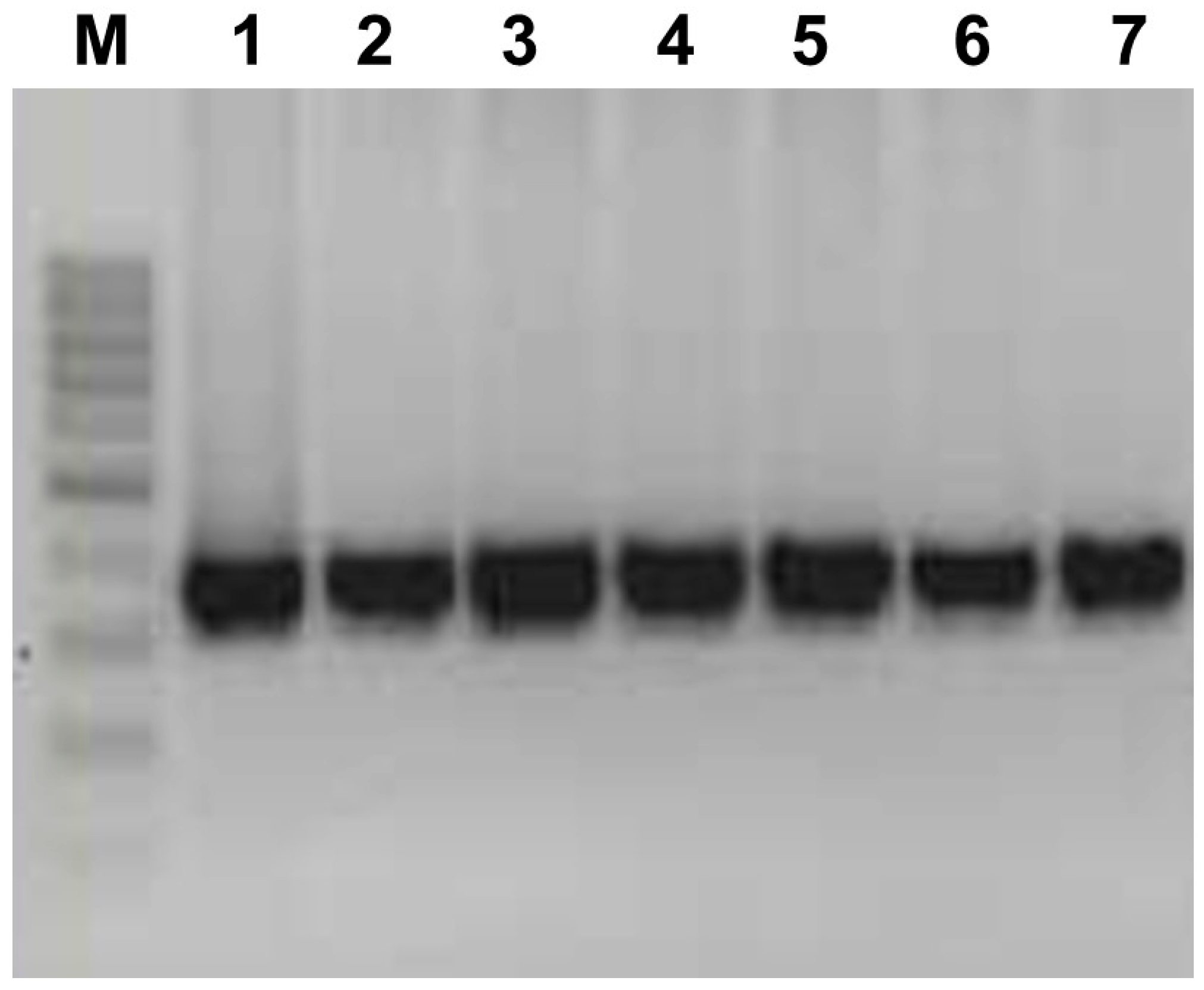
3. Results
3.1. Detection in Commercial Birds
3.2. Detection in Wild Birds
3.3. Phylogenetic Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Producción y Productos Avícolas. Available online: https://www.fao.org/poultry-production-products/production/es/ (accessed on 12 March 2024).
- OECD-FAO. OECD-FAO Agricultural Outlook 2023–2032. In OECD-FAO Agricultural Outlook; OECD: Paris, France, 2023; ISBN 9789264619333. [Google Scholar]
- Salles, G.B.C.; Pilati, G.V.T.; Muniz, E.C.; de Lima Neto, A.J.; Vogt, J.R.; Dahmer, M.; Savi, B.P.; Padilha, D.A.; Fongaro, G. Trends and Challenges in the Surveillance and Control of Avian Metapneumovirus. Viruses 2023, 15, 1960. [Google Scholar] [CrossRef]
- Croville, G.; Foret, C.; Heuillard, P.; Senet, A.; Delpont, M.; Mouahid, M.; Ducatez, M.F.; Kichou, F.; Guerin, J.L. Disclosing Respiratory Co-Infections: A Broad-Range Panel Assay for Avian Respiratory Pathogens on a Nanofluidic PCR Platform. Avian Pathol. 2018, 47, 253–260. [Google Scholar] [CrossRef]
- Samy, A.; Naguib, M.M. Avian Respiratory Coinfection and Impact on Avian Influenza Pathogenicity in Domestic Poultry: Field and Experimental Findings. Vet. Sci. 2018, 5, 23. [Google Scholar] [CrossRef]
- Naylor, C.J.; Al-Ankari, A.R.; Al-Afaleq, A.I.; Bradbury, J.M.; Jones, R.C. Exacerbation of Mycoplasma Gallisepticum Infection in Turkeys by Rhinotracheitis Virus. Avian Pathol. 1992, 21, 295–305. [Google Scholar] [CrossRef]
- Rüger, N.; Sid, H.; Meens, J.; Szostak, M.P.; Baumgärtner, W.; Bexter, F.; Rautenschlein, S. New Insights into the Host–Pathogen Interaction of Mycoplasma Gallisepticum and Avian Metapneumovirus in Tracheal Organ Cultures of Chicken. Microorganisms 2021, 9, 2407. [Google Scholar] [CrossRef]
- Cook, J.K. Avian Rhinotracheitis. Rev. Sci. Tech. 2000, 19, 602–613. [Google Scholar] [CrossRef]
- Rautenschlein, S. Newcastle Disease, Other Avian Paramyxoviruses, and Avian Metapneumovirus Infections. In Diseases of Poultry; Swayne, D.E., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 135–143. [Google Scholar]
- Jirjis, F.F.; Noll, S.L.; Halvorson, D.A.; Nagaraja, K.V.; Shaw, D.P. Pathogenesis of Avian Pneumovirus Infection in Turkeys. Vet. Pathol. 2002, 39, 300–310. [Google Scholar] [CrossRef]
- Salles, G.B.C.; Pilati, G.V.T.; Savi, B.P.; Muniz, E.C.; Dahmer, M.; Vogt, J.R.; de Lima Neto, A.J.; Fongaro, G. Surveillance of Avian Metapneumovirus in Non-Vaccinated Chickens and Co-Infection with Avian Pathogenic Escherichia Coli. Microorganisms 2024, 12, 56. [Google Scholar] [CrossRef]
- Gharaibeh, S.M.; Algharaibeh, G.R. Serological and Molecular Detection of Avian Pneumovirus in Chickens with Respiratory Disease in Jordan. Poult. Sci. 2007, 86, 1677–1681. [Google Scholar] [CrossRef]
- Ball, C.; Manswr, B.; Herrmann, A.; Lemiere, S.; Ganapathy, K. Avian Metapneumovirus Subtype B Vaccination in Commercial Broiler Chicks: Heterologous Protection and Selected Host Transcription Responses to Subtype A or B Challenge. Avian Pathol. 2022, 51, 181–196. [Google Scholar] [CrossRef]
- Kaboudi, K.; Lachheb, J. Avian Metapneumovirus Infection in Turkeys: A Review on Turkey Rhinotracheitis. J. Appl. Poult. Res. 2021, 30, 100211. [Google Scholar] [CrossRef]
- Shin, H.J.; Njenga, M.K.; McComb, B.; Halvorson, D.A.; Nagaraja, K.V. Avian Pneumovirus (APV) RNA from Wild and Sentinel Birds in the United States Has Genetic Homology with RNA from APV Isolates from Domestic Turkeys. J. Clin. Microbiol. 2000, 38, 4282–4284. [Google Scholar] [CrossRef]
- Tucciarone, C.M.; Franzo, G.; Legnardi, M.; Pasotto, D.; Lupini, C.; Catelli, E.; Quaglia, G.; Graziosi, G.; Dal Molin, E.; Gobbo, F.; et al. Molecular Survey on A, B, C and New Avian Metapneumovirus (AMPV) Subtypes in Wild Birds of Northern-Central Italy. Vet. Sci. 2022, 9, 373. [Google Scholar] [CrossRef]
- Jesse, S.T.; Ribó-Molina, P.; Jo, W.K.; Rautenschlein, S.; Vuong, O.; Fouchier, R.A.M.; Ludlow, M.; Osterhaus, A.D.M.E. Molecular Characterization of Avian Metapneumovirus Subtype C Detected in Wild Mallards (Anas Platyrhynchos) in The Netherlands. Transbound. Emerg. Dis. 2022, 69, 1–11. [Google Scholar] [CrossRef]
- Rima, B.; Collins, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.A.; Lee, B.; Maisner, A.; Rota, P.; Wang, L. ICTV Virus Taxonomy Profile: Pneumoviridae. J. Virol. 2017, 98, 2912–2913. [Google Scholar] [CrossRef]
- Sugiyama, M.; Ito, H.; Hata, Y. Complete Nucleotide Sequences of Avian Metapneumovirus Subtype B Genome. Virus Genes 2010, 41, 389–395. [Google Scholar] [CrossRef]
- Brown, P.A.; Lemaitre, E.; Briand, F.X.; Courtillon, C.; Guionie, O.; Allée, C.; Toquin, D.; Bayon-Auboyer, M.H.; Jestin, V.; Eterradossi, N. Molecular Comparisons of Full Length Metapneumovirus (MPV) Genomes, Including Newly Determined French AMPV-C and -D Isolates, Further Supports Possible Subclassification within the MPV Genus. PLoS ONE 2014, 9, e102740. [Google Scholar] [CrossRef]
- Jesse, S.T.; Ludlow, M.; Osterhaus, A.D.M.E. Zoonotic Origins of Human Metapneumovirus: A Journey from Birds to Humans. Viruses 2022, 14, 677. [Google Scholar] [CrossRef]
- Rizotto, L.S.; Simão, R.M.; Scagion, G.P.; Simasaki, A.A.; Caserta, L.C.; Benassi, J.C.; Arns, C.W.; Ferreira, H.L. Detection of Avian Metapneumovirus Subtype a from Wild Birds in the State of São Paulo, Brazil. Braz. J. Vet. Res. Anim. Sci. 2019, 39, 209–213. [Google Scholar] [CrossRef]
- Rizotto, L.; Scagion, G.; Cardoso, T.; Simao, R.; Caserta, L.C.; Benassi, J.C.; Keid, L.B.; Oliveira, T.M.F.D.S.; Soares, R.M.; Arns, C.W.; et al. Complete Genome Sequence of an Avian Metapneumovirus Subtype A Strain Brazil. Genome Announc. 2017, 5, 8–9. [Google Scholar] [CrossRef]
- Chacón, J.L.; Mizuma, M.; Vejarano, M.P.; Toquín, D.; Eterradossi, N.; Patnayak, D.P.; Goyal, S.M.; Piantino Ferreira, A.J. Avian Metapneumovirus Subtypes Circulating in Brazilian Vaccinated and Nonvaccinated Chicken and Turkey Farms. Avian Dis. 2011, 55, 82–89. [Google Scholar] [CrossRef]
- Chacón, J.L.; Brandão, P.E.; Buim, M.; Villarreal, L.; Ferreira, A.J.P. Detection by Reverse Transcriptase-Polymerase Chain Reaction and Molecular Characterization of Subtype B Avian Metapneumovirus Isolated in Brazil. Avian Pathol. 2007, 36, 383–387. [Google Scholar] [CrossRef]
- Rivera-Benitez, J.F.; Martínez-Bautista, R.; Ríos-Cambre, F.; Ramírez-Mendoza, H. Molecular Detection and Isolation of Avian Metapneumovirus in Mexico. Avian Pathol. 2014, 43, 217–223. [Google Scholar] [CrossRef]
- Cavanagh, D.; Mawditt, K.; Britton, P.; Naylor, C.J. Longitudinal Field Studies of Infectious Bronchitis Virus and Avian Pneumovirus in Broilers Using Type-Specific Polymerase Chain Reactions. Avian Pathol. 1999, 28, 593–605. [Google Scholar] [CrossRef]
- Juhasz, K.; Easton, A.J. Extensive Sequence Variation in the Attachment (G) Protein Gene of Avian Pneumovirus: Evidence for Two Distinct Subgroups. J. Gen. Virol. 1994, 75, 2873–2880. [Google Scholar] [CrossRef]
- Lupini, C.; Tucciarone, C.M.; Mescolini, G.; Quaglia, G.; Graziosi, G.; Turblin, V.; Brown, P.; Cecchinato, M.; Legnardi, M.; Delquigny, T.; et al. Longitudinal Survey on AMPV Circulation in French Broiler Flocks Following Different Vaccination Strategies. Animals 2023, 13, 57. [Google Scholar] [CrossRef]
- Felippe, P.A.; da Silva, L.H.A.; dos Santos, M.B.; Sakata, S.T.; Arns, C.W. Detection of and Phylogenetic Studies with Avian Metapneumovirus Recovered from Feral Pigeons and Wild Birds in Brazil. Avian Pathol. 2011, 40, 445–452. [Google Scholar] [CrossRef]
- WOAH. High Pathogenicity Avian Influenza (Hpai)—Situation Report; World Animal Health Information System of the World Organisation for Animal Health (WAHIS): Paris, France, 2022; pp. 1–5. [Google Scholar]
- Nguyen, V.G.; Chung, H.C.; Do, H.Q.; Nguyen, T.T.; Cao, T.B.P.; Truong, H.T.; Mai, T.N.; Le, T.T.; Nguyen, T.H.; Le, T.L.; et al. Serological and Molecular Characterization of Avian Metapneumovirus in Chickens in Northern Vietnam. Vet. Sci. 2021, 8, 206. [Google Scholar] [CrossRef]
- Andreopoulou, M.; Franzo, G.; Tucciarone, C.M.; Prentza, Z.; Koutoulis, K.C.; Cecchinato, M.; Chaligianni, I. Molecular Epidemiology of Infectious Bronchitis Virus and Avian Metapneumovirus in Greece. Poult. Sci. 2019, 98, 5374–5384. [Google Scholar] [CrossRef]
- Tucciarone, C.M.; Franzo, G.; Lupini, C.; Alejo, C.T.; Listorti, V.; Mescolini, G.; Brandão, P.E.; Martini, M.; Catelli, E.; Cecchinato, M. Avian Metapneumovirus Circulation in Italian Broiler Farms. Poult. Sci. 2018, 97, 503–509. [Google Scholar] [CrossRef]
- Tegegne, D.; Deneke, Y.; Sori, T.; Abdurahaman, M.; Kebede, N.; Cecchinato, M.; Franzo, G. Molecular Epidemiology and Genotyping of Infectious Bronchitis Virus and Avian Metapneumovirus in Backyard and Commercial Chickens in Jimma Zone, Southwestern Ethiopia. Vet. Sci. 2020, 7, 187. [Google Scholar] [CrossRef]
- Dani, M.A.C.; Arns, C.W.; Durigon, E.L. Molecular Characterization of Brazilian Avian Pneumovirus Isolates Using Reverse Transcription-Polymerase Chain Reaction, Restriction Endonuclease Analysis and Sequencing of a G Gene Fragment. Avian Pathol. 1999, 28, 473–476. [Google Scholar] [CrossRef]
- Gobbo, F.; Moronato, M.; Franzo, G.; Cecchinato, M.; Martini, M. Real-Time PCR Data Express the Different Distribution of Avian Metapneumovirus and Mycoplasma Synoviae in Broiler Chickens Experimentally Infected with One or Both Pathogens. Preliminary Results. In 20th World Veterinary Poultry Association Congress—Abstracts Book; Padua Research Archive: Padova, Italy, 2017; p. 345. [Google Scholar]
- Sharifi, A.; Allymehr, M.; Talebi, A. Concurrent Occurrence of Infectious Bursal Disease and Multicausal Respiratory Infections Caused by Newcastle Disease and Avian Metapneumovirus in Broilers. Arch. Razi Inst. 2022, 77, 1007–1016. [Google Scholar] [CrossRef]
- Cecchinato, M.; Lupini, C.; Ricchizzi, E.; Falchieri, M.; Meini, A.; Jones, R.C.; Catelli, E. Italian Field Survey Reveals a High Diffusion of Avian Metapneumovirus Subtype B in Layers and Weaknesses in the Vaccination Strategy Applied. Source Avian Dis. 2012, 56, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Hananeh, W.M.; Al-Natour, M.Q.; Alaboudi, A.R.; Abo-Shehada, M.N.; Bani Ismail, Z.A. Congenital Abnormalities in Dead-in-Shell Chicks Associated with Mixed Bacterial Infections. Heliyon 2021, 7, e06272. [Google Scholar] [CrossRef]
- Shin, H.-J.; Njenga, M.; Halvorson, D.; Shaw, D.; Nagaraja, K. Susceptibility of Ducks to Avian Pneumovirus of Turkey Origin. Am. J. Vet. Res. 2001, 62, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Catelli, E.; Lupini, C.; Listorti, V.; Marziali, A.; Matteo, P.D.; Clive, J.N.; Cecchinato, M. The Pigeon (Columba Livia) Is Not Sensitive to Avian Metapneumovirus Subtype B and Does Not Play Any Role in Virus Spread in Experimental Conditions. In VII. International Symposium on Avian Corona-and Pneumoviruses and Complicating Pathogens; Druckerei, S., Ed.; Druckerei Schroder: Rauischholzhausen, Germany, 2012; pp. 285–291. [Google Scholar]
- Gharaibeh, S.; Shamoun, M. Avian Metapneumovirus Subtype B Experimental Infection and Tissue Distribution in Chickens, Sparrows, and Pigeons. Vet. Pathol. 2012, 49, 704–709. [Google Scholar] [CrossRef]
- Restrepo-Cardona, J.S.; Ocampo-Velásquez, J.D.; Delgado, A.; Mikkola, H.; Rodríguez-Villamil, D.R. Feeding Habits of the Stygian Owl (Asio stygius) and the Short-Eared Owl (A. fLammeus) in the Southwest of Bogotá Savanna, Cundinamarca, Colombia. Ornitol. Neotrop. 2021, 32, 92–96. [Google Scholar] [CrossRef]
- Lachheb, J.; Bouslama, Z.; Nsiri, J.; Badr, C.; al Gallas, N.; Souissi, N.; Khazri, I.; Larbi, I.; Kaboudi, K.; Ghram, A. Phylogenetic and Phylodynamic Analyses of Subtype-B Metapneumovirus from Chickens in Tunisia. Poult. Sci. 2023, 102, 102253. [Google Scholar] [CrossRef]

| Sampled Organic Systems | Breeders | Laying Hens | Broilers | Wild Birds | Total |
|---|---|---|---|---|---|
| Upper respiratory tract | 99 | 49 | 27 | 69 | 244 |
| Reproductive tract | 6 | 23 | 0 | 0 | 29 |
| Total | 105 | 72 | 27 | 69 | 273 |
| ID | Year | Age | Sample Type | Initial Diagnostic Objective | Reported Symptoms |
|---|---|---|---|---|---|
| Breeders (n = 105, 7.62%) | |||||
| LBMv18-1 | 2018 | Dead-in-shell embryo | Swabs | MG-MS | NR |
| LBMv19-1 | 2019 | 7 weeks | Tracheal swabs | MG-MS | NR |
| LBMv19-2 | 2019 | 36 weeks | Tracheal and air sac swabs | MG-MS-NDV-LTA-IBV-aMPV | Unspecified respiratory symptoms |
| LBMv19-3 | 2019 | 25 weeks | Tracheal swabs | MG-MS | Routine diagnostics |
| LBMv19-4 a | 2019 | 128 weeks | Tracheal swabs | MG-MS | Routine diagnostics |
| LBMv19-5 | 2019 | 15 weeks | Tracheal swabs | MG-MS | Routine diagnostics |
| LBMv20-2 a | 2020 | 10 weeks | Tracheal swabs | MG-MS | Routine diagnostics |
| LBMv22-7 | 2022 | 48 weeks | Tracheal swabs | NDV-IBV-aMPV | Routine diagnostics |
| Layer hens (n = 72, 15.28%) | |||||
| LBMv20-1 | 2020 | 33 weeks | Tracheal swabs | NDV-IBV | Increased mortality |
| LBMv21-1 | 2021 | 50 weeks | URT organ pool | IBV | Unspecified respiratory symptoms |
| LBMv22-1 | 2022 | 83 weeks | Uterine swabs | aMPV | Respiratory rales and loss of eggshell pigmentation |
| LBMv22-2 | 2022 | 85 weeks | Uterine swabs | aMPV | Respiratory rales and loss of eggshell pigmentation |
| LBMv22-3 | 2022 | 15 weeks | Tracheal swabs | aMPV | Respiratory rales |
| LBMv22-4 | 2022 | 21 weeks | Tracheal swabs | IBV | Routine diagnostics |
| LBMv22-5 | 2022 | 7 weeks | Infraorbital sinus swabs | aMPV | Respiratory rales, mucus, and facial edema |
| LBMv22-6 | 2022 | 23 weeks | Uterine imprint | aMPV | Respiratory rales and decrease in egg production |
| LBMv23-1 | 2023 | 42 weeks | Tracheal and choanal swabs | IBV | Decrease in egg production |
| LBMv23-2 | 2023 | 25 weeks | Tracheal and choanal swabs | aMPV | Unspecified respiratory symptoms |
| LBMv23-3 | 2023 | 10 weeks | Tracheal swabs | aMPV | Unspecified respiratory symptoms |
| Broilers (n = 27, 7.41%) | |||||
| LBMv23-4 | 2023 | 40 days | Choanal swabs | aMPV | Unspecified respiratory symptoms |
| LBMv23-5 b | 2023 | 35 days | Choanal swabs | aMPV | Respiratory rales and increased mortality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar-Alfonso, S.; Alvarez-Mira, D.M.; Beltran-Leon, M.; Ramirez-Nieto, G.; Gomez, A.P. Avian Metapneumovirus Subtype B Circulation in Poultry and Wild Birds of Colombia. Pathogens 2024, 13, 882. https://doi.org/10.3390/pathogens13100882
Escobar-Alfonso S, Alvarez-Mira DM, Beltran-Leon M, Ramirez-Nieto G, Gomez AP. Avian Metapneumovirus Subtype B Circulation in Poultry and Wild Birds of Colombia. Pathogens. 2024; 13(10):882. https://doi.org/10.3390/pathogens13100882
Chicago/Turabian StyleEscobar-Alfonso, Santiago, Diana M. Alvarez-Mira, Magda Beltran-Leon, Gloria Ramirez-Nieto, and Arlen P. Gomez. 2024. "Avian Metapneumovirus Subtype B Circulation in Poultry and Wild Birds of Colombia" Pathogens 13, no. 10: 882. https://doi.org/10.3390/pathogens13100882
APA StyleEscobar-Alfonso, S., Alvarez-Mira, D. M., Beltran-Leon, M., Ramirez-Nieto, G., & Gomez, A. P. (2024). Avian Metapneumovirus Subtype B Circulation in Poultry and Wild Birds of Colombia. Pathogens, 13(10), 882. https://doi.org/10.3390/pathogens13100882





