Advances on Bioactive Metabolites with Potential for the Biocontrol of Plant Pathogenic Bacteria †
Abstract
1. Introduction
2. Bactericides from Bacteria
| Compounds | Source | Bacterium Target | References |
|---|---|---|---|
| Bacteriocin | Pseudomonas syringae pv. ciccaronei | Pseudomonas savastanoi pv. savastanoi | [28,29] |
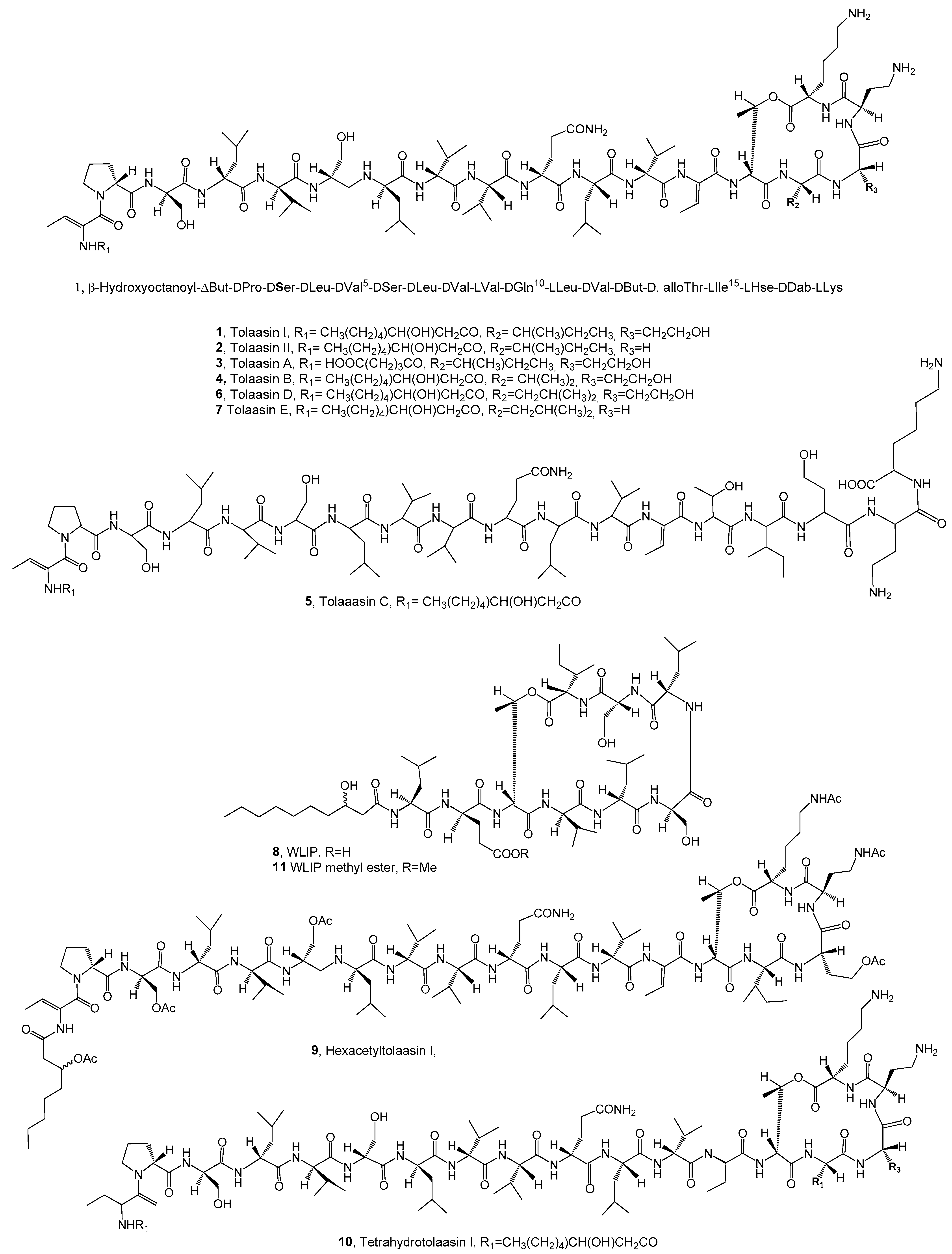
| Tolaasin I (1), Table 1 | Pseudomonas tolaasii | Bacillus megaterium and Rodococcus fascians | [31] |
| Burkholderia caryophylli, P. syringae pv. panici, Pseudomonas syringae pv. tabaci, P. syringae pv. siringae and Pseudomonas syringae pv. japonica, B. subtilis, Bacillus megaterium | [43] | ||
| Tolaasin II (2) | ″ | Bacillus megaterium and Rodococcus fascians | [31] |
| Burkholderia caryophylli, P. syringae pv. panici, Pseudomonas syringae pv. tabaci, P. syringae pv. siringae and Pseudomonas syringae pv. japonica, B. subtilis, Bacillus megaterium | [43] | ||
| Tolaasins A (3) | ″ | Bacillus megaterium and Rodococcus fascians | [31] |
| Tolaasins B (4) | ″ | ″ | ″ |
| Tolaasins C (5) | ″ | ″ | ″ |
| Tolaasins D (6) | ″ | Bacillus megaterium and Rodococcus fascians | [31] |
| Burkholderia caryophylli, P. syringae pv. panici, Pseudomonas syringae pv. tabaci, P. syringae pv. siringae and Pseudomonas syringae pv. japonica, B. subtilis, Bacillus megaterium | [43] | ||
| Tolaasins E (7) | ″ | Bacillus megaterium and Rodococcus fascian | [31] |
| Burkholderia caryophylli, P. syringae pv. panici, Pseudomonas syringae pv. tabaci, P. syringae pv. siringae and Pseudomonas syringae pv. japonica | [43] | ||
| WLIP (8) | Pseudomonas reactans | B. megaterium, Erwinia carotovora subsp. carotovora | [33] |
| B. subtilis and B. megaterium | [43] | ||
| Hexacetyltolaasin I (9) | Burkholderia caryophylli, P. syringae pv. panici, Pseudomonas syringae pv. tabaci, P. syringae pv. siringae and Pseudomonas syringae pv. japonica, | [43] | |
| Tetrahydrotolaasin I (10) | Burkholderia caryophylli, P. syringae pv. panici, Pseudomonas syringae pv. tabaci, P. syringae pv. siringae and Pseudomonas syringae pv. japonica, B. subtilis, Bacillus megateriums | [43] | |
| WLIP methyl ester (11) | B. subtilis and B. megaterium | ″ | |
| Maculosin-1, Cyclo(L-Pro-L-Tyr) (12) | Lysobacter capsici | Burkholderia caryophylli, P. syringae pv. panici, Pseudomonas syringae pv. tabaci, P. syringae pv. siringae and Pseudomonas syringae pv. japonica, B. subtilis, Bacillus megaterium | ″ |
| Cyclo(L-Pro-L-Val) (13) | ″ | Not toxic | ″ |
| Cyclo(L-pro-Leu) (14) | ″ | Burkholderia caryophylli, P. syringae pv. panici, Pseudomonas syringae pv. tabaci, P. syringae pv. siringae and Pseudomonas syringae pv. japonica, B. subtilis, Bacillus megaterium, E.coli | ″ |
| Cyclo(D-Pro-L-Tyr) (15) | ″ | ″ | ″ |
| Bicornutin A (16) | Xenorhabdus budapestensis and X. szentirmaii | Erwinia amylovora | [44] |
| L-Furanomycin (17) | Pseudomonas fluorescens SW25 | Dickeya dadantii, P. syringae, E. amylovora and b. subtilis | [45] |
| 4-Formylaminooxyvinyl glycine (18) | Pseudomonas fluorescens WH6 | Erwinia amylovora | [46,47] |
| Erucamide (19) | Bacillus megaterium | Agrobacterium tumefaciens, Erwinia carotovora and Ralstonia solanacearum | [52] |
| Behenic acid (20) | ″ | ″ | ″ |
| Palmitic acid (21) | ″ | Not toxic | ″ |
| Phenylacetic acid (22) | ″ | ″ | ″ |
| β-Sitosterol (23) | ″ | ″ | ″ |
| Guvermectin (24) | Ralstonia solanacearum, Pseudomonas syringae pv. actinidiae Xanthomonas oryzae pv. oryzae | [54] | |
| Pantocin A (25) | Pantoea sp. | Pectobacterium carotovorum subsp. carotovorum | [55] |
| Pantocin B (26) | ″ | ″ | ″ |
3. Bacteriocides from Fungi
| Compounds | Source | Bacterium Target | References |
|---|---|---|---|
| Papyracillic acid (27) | Ascochyta agropyrina var. nana | Bacillus subtilis, Xanthomonas campestris, Bacillus brevis, Microcossus luteus Enterobacter dissolvens | [56] |
| Sphaeropsidin A (28) | D. cupressi | Xanthomonas oryzae pv. oryzae | [65] |
| SMA93 (29) | Fusarium proliferatum | B. subtilis | [66] |
| 5-O-Methylated of SMA93 (30) | “ | “ | “ |
| Rhodolamprometrin (31) | “ | “ | “ |
| Radicinin (32) | “ | Not toxic | “ |
| Dehydrodroallogibberic acid (33), | “ | Not toxic | “ |
| 3-Methyl-6,8- dihydroxyisocoumarin (34) | “ | Not toxic | “ |
| (R)-Formosusin A (35) | Aspergillus candidus | “ | [67] |
| (R)-Variotin (36) | “ | “ | “ |
| Candidusin (37), | “ | “ | “ |
| Asperlin (38) | “ | Clavibacter michiganensis E. amylovora | “ |
| Chloromonilicin (39) | Alternaria sonchi | B. subtilis, E. coli, P. fluorescens and Paenibacillus polymyxa | [68] |
| Aspergillone (40) | Aspergillus niger | Not toxic | [69] |
| Aurasperone A (41) | “ | Pseudomonas aeruginosa and S. aureus | “ |
| Aurasperone D (42) | “ | Not toxic | “ |
| Asperpyrone A (43), | “ | E. coli | “ |
| Fonsecinone A (44) | “ | S. aureus, E. coli and Pseudomonas syringae pv. maculicola | “ |
| Carbonarone A (45) | “ | Dickeya solani | “ |
| Pyrophen (46) | “ | Micrococcus luteus, Aeromonas hydrophila and Listeria innocua | “ |
| Penicillic acid (47) | Penicillium sp. | Xanthomonas citri subsp. citri, Xanthomonas campestris | [72] |
| 5-Hydroxymethyl-2-furancarboxylic acid (48) | Aspergillus niger xj | Erwinia carotovora, Agrobacterium tumefaciens, Ralstonia solanacearum | [73] |
4. Bacteriocides from Plants
| Compounds | Source | Bacterium Target | References |
|---|---|---|---|
| Ungeremine (49) | Pancratium maritimum | Edwardsiella ictaluri, Flavobacterium columnare | [80] |
| 1-O-Acetyllycorine (50) | “ | “ | “ |
| 1,2-O,O’-Diacetyllycorine (51) | “ | “ | “ |
| Lycorine (55) | Sternbergia lutea | “ | [81] |
| Methyl 2,4,6-trihydroxybenzoate (59) | Cassia alata L. | Not toxic | [84] |
| Aloe-emodin (60) | “ | “ | “ |
| Kaempferol (61) | M. oryzae and Phytophthora sp. | ||
| (-)-Epiafzelechin (62) | “ | “ | “ |
| Rhein (63) | “ | Acidovorax avenae subsp. cattlvaePhytophthora sp. | “ |
| Kaempferol-3-O-glycoside (64) | “ | M. oryzae and Phytophthora sp. | “ |
| Kaempferol-3-O-gentiobiside (65) | “ | “ | “ |
| Aloe-emodin-8-O-β-D-glucoside (66) | “ | “ | “ |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strobel, G.A. Phytotoxins. Annu. Rev. Biochem. 1982, 51, 309–333. [Google Scholar] [CrossRef] [PubMed]
- Ballio, A.; Graniti, A. Phytotoxins and their involvement in plant diseases. Experientia 1991, 47, 751–826. [Google Scholar]
- Bender, C.L. Bacterial phytotoxins. Methods Microbiol. 1998, 27, 169–175. [Google Scholar]
- Durbin, R. Toxins in Plant Disease; Elsevier: Amsterdam, The Netherland, 2012. [Google Scholar]
- Duke, S.O.; Dayan, F.E. Modes of action of microbially-produced phytotoxins. Toxins 2011, 3, 1038–1064. [Google Scholar] [CrossRef]
- Dudler, R. The role of bacterial phytotoxins in inhibiting the eukaryotic proteasome. Trends Microbiol. 2012, 22, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Sharma, V.; Thakur, A. Phytotoxins—A mini review. J. Pharmacogn. Phytochem. 2018, 7, 2705–2708. [Google Scholar]
- Chen, H.; Singh, H.; Bhardwaj, N.; Bhardwaj, S.K.; Khatri, M.; Kim, K.H.; Peng, W. An exploration on the toxicity mechanisms of phytotoxins and their potential utilities. Crit. Rev. Environ. Sci. Technol. 2022, 52, 395–435. [Google Scholar] [CrossRef]
- Tringali, C. Bioactive Compounds from Natural Sources: Isolation, Characterization and Biological Properties; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- García-Pajón, C.M.; Collado, I.G. Secondary metabolites isolated from Colletotrichum species. Nat. Prod. Rep. 2003, 20, 426–431. [Google Scholar] [CrossRef]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef]
- Evidente, A.; Cimmino, A.; Masi, M. Phytotoxins produced by pathogenic fungi of agrarian plants. Phytochem. Rev. 2019, 18, 843–870. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A. Specialized metabolites produced by phytotopatogen fungi to control weeds and parasite plants. Microorganisms 2023, 11, 843. [Google Scholar] [CrossRef]
- Shettally, R.; Prasad, R.; Walia, D.S. Effect of chemical pesticides on the environment. Indian J. Environ. 1997, 17, 275–280. [Google Scholar]
- Srivastav, A.L. Chemical fertilizers and pesticides: Role in groundwater contamination. In Agrochemicals Detection, Treatment and Remediation; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 143–159. [Google Scholar]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the art and future opportunities. J. Agric. Food Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef]
- Khursheed, A.; Rather, M.A.; Jain, V.; Rasool, S.; Nazir, R.; Malik, N.A.; Majid, S.A. Plant based natural products as potential ecofriendly and safer biopesticides: A comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef] [PubMed]
- Macías-Rubalcava, M.L.; Sánchez-Fernández, R.E. Secondary metabolites of endophytic Xylaria species with potential applications in medicine and agriculture. World J. Microbiol. Biotechnol. 2017, 33, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Tumbarski, Y.; Lante, A.; Krastanov, A. Immobilization of bacteriocins from lactic acid bacteria and possibilities for application in food biopreservation. Open Biotechnol. J. 2018, 12, 25–32. [Google Scholar] [CrossRef]
- Smits, T.H.; Duffy, B.; Blom, J.; Ishimaru, C.A.; Stockwell, V.O. Pantocin A, a peptide-derived antibiotic involved in biological control by plant-associated Pantoea species. Arch. Microbiol. 2019, 201, 713–722. [Google Scholar] [CrossRef]
- Vicente, T.F.; Félix, C.; Félix, R.; Valentão, P.; Lemos, M.F. Seaweed as a natural source against phytopathogenic bacteria. Mar. Drugs 2022, 21, 23. [Google Scholar] [CrossRef]
- Akmukhanova, N.R.; Leong, Y.K.; Seiilbek, S.N.; Konysbay, A.; Zayadan, B.K.; Sadvakasova, A.K.; Sarsekeyeva, F.K.; Bauenova, M.O.; Bolatkhan, K.; Alharby, H.F.; et al. Eco-friendly biopesticides derived from CO2-Fixing cyanobacteria. Environ. Res. 2023, 239, 117419. [Google Scholar] [CrossRef] [PubMed]
- Surico, G.; Comai, L.; Kosuge, T. Pathogenicity of strains of Pseudomonas syringae pv. savastanoi and their indoleacetic acid deficient mutants on olive and oleander. Phytopathology 1984, 74, 490–493. [Google Scholar]
- Surico, G.; Evidente, A.; Iacobellis, N.S.; Randazzo, G. On the presence and level of different cytokinins in culture filtrate of Pseudomonas syringae pv. savastanoi. In Proceedings of the Plant Pathogenic Bacteria: Proceedings of the Sixth International Conference on Plant Pathogenic Bacteria, College Park, MD, USA, 2–7 June 1987; pp. 566–570. [Google Scholar]
- Sisto, A.; Cipriani, M.G.; Morea, M. Knot formation caused by Pseudomonas syringae subsp. savastanoi on olive plants is hrp-dependent. Phytopathology 2004, 94, 484–489. [Google Scholar] [PubMed]
- Lavermicocca, P.; Lonigro, S.L.; Evidente, A.; Andolfi, A. Bacteriocin production by Pseudomonas syringae pv. ciccaronei NCPPB2355. Isolation and partial characterization of the antimicrobial compound. J. Appl. Microbiol. 1999, 86, 257–265. [Google Scholar]
- Lavermicocca, P.; Lonigro, S.L.; Valerio, F.; Evidente, A.; Visconti, A. Reduction of olive knot disease by a bacteriocin from Pseudomonas syringae pv. ciccaronei. Appl. Environ. Microbiol. 2002, 68, 1403–1407. [Google Scholar] [CrossRef]
- Evidente, A. Bioactive lipodepsipeptides produced by bacteria and fungi. Int. J. Mol. Sci. 2022, 23, 12342. [Google Scholar] [CrossRef]
- Bassarello, C.; Lazzaroni, S.; Bifulco, G.; Lo Cantore, P.; Iacobellis, N.S.; Riccio, R.; Gomez-Paloma, L.; Evidente, A. Tolaasins A−E, five new lipodepsipeptides produced by Pseudomonas tolaasii. J. Nat. Prod. 2004, 67, 811–816. [Google Scholar] [CrossRef]
- Wong, W.C.; Preece, T.F. Identification of Pseudomonas tolaasii: The white line in agar and mushroom tissue block rapid pitting tests. J. Appl. Bacteriol. 1979, 47, 401–407. [Google Scholar] [CrossRef]
- Lo Cantore, P.; Lazzaroni, S.; Coraiola, M.; Serra, M.D.; Cafarchia, C.; Evidente, A.; Iacobellis, N.S. Biological characterization of white line-inducing principle (WLIP) produced by Pseudomonas reactans NCPPB1311. Mol. Plant Microbe Interact. 2006, 19, 1113–1120. [Google Scholar] [CrossRef]
- Mieczkowski, A.; Speina, E.; Trzybiński, D.; Winiewska-Szajewska, M.; Wińska, P.; Borsuk, E.M.; Podsiadła-Białoskórska, M.; Przygodzki, T.; Drabikowski, K.; Stanczyk, L.; et al. Diketopiperazine-based, flexible tadalafil analogues: Synthesis, crystal structures and biological activity profile. Molecules 2021, 26, 794. [Google Scholar] [CrossRef]
- Stierle, A.C.; Cardellina, J.H.; Strobel, G.A. Maculosin, a hostspecific phytotoxin for spotted knapweed from Alternaria alternata. Proc. Natl. Acad. Sci. USA 1988, 85, 8008–8011. [Google Scholar] [CrossRef] [PubMed]
- Puopolo, G.; Cimmino, A.; Palmieri, M.C.; Giovannini, O.; Evidente, A.; Pertot, I. Lysobacter capsici AZ78 produces cyclo (L-ProL-Tyr), a 2, 5-diketopiperazine with toxic activity against sporangia of Phytophthora infestans and Plasmopara viticola. J. Appl. Microbiol. 2014, 117, 1168–1180. [Google Scholar] [CrossRef]
- Yamaguchi, T. Horticulture in Japan. 1994. [Google Scholar]
- Young, J.M.; Fletcher, M.J. Pseudomonas syringae pv. panici (Elliott 1923) Young, Dye & Wilkie 1978 is a doubtful name. Australas. Plant Pathol. 1994, 23, 66–68. [Google Scholar]
- Liu, H.; Qiu, H.; Zhao, W.; Cui, Z.; Ibrahim, M.; Jin, G.; Li, B.; Zhu, B.; Xie, G.L. Genome sequence of the plant pathogen Pseudomonas syringae pv. panici LMG 2367. J. Bacteriol. 2012, 194, 5693–5694. [Google Scholar] [CrossRef]
- Mapuranga, N. A new race of Pseudomonas syringae pv. tabaci on tobacco in Zimbabwe. Plant Dis. 1998, 82, 1404. [Google Scholar]
- Gutiérrez-Barranquero, J.A.; Cazorla, F.M.; de Vicente, A. Pseudomonas syringae pv. syringae associated with mango trees, a particular pathogen within the “hodgepodge” of the Pseudomonas syringae complex. Front. Plant Sci. 2019, 10, 570. [Google Scholar]
- Young, J.M. Pseudomonas syringae pv. japonica (Mukoo 1955) Dye et al. 1980 is a junior synonym of Ps. syringae pv. syringae van Hall 1902. Lett. Appl. Microbiol. 1992, 15, 129–130. [Google Scholar]
- Castaldi, S.; Cimmino, A.; Masi, M.; Evidente, A. Bacterial lipodepsipeptides and some of their derivatives and cyclic dipeptides as potential agents for biocontrol of pathogenic bacteria and fungi of agrarian plants. J. Agric. Food Chem. 2022, 70, 4591–4598. [Google Scholar] [CrossRef]
- Böszörményi, E.; Érsek, T.; Fodor, A.; Fodor, A.M.; Földes, L.S.; Hevesi, M.; Hogan, J.S.; Katona, Z.; Klein, M.G.; Kormany, A.; et al. Isolation and activity of Xenorhabdus antimicrobial compounds against the plant pathogens Erwinia amylovora and Phytophthora nicotianae. J. Appl. Microbiol. 2009, 107, 746–759. [Google Scholar] [CrossRef]
- Trippe, K.; McPhail, K.; Armstrong, D.; Azevedo, M.; Banowetz, G. Pseudomonas fluorescens SBW25 produces furanomycin, a non-proteinogenic amino acid with selective antimicrobial properties. BMC Microbiol. 2013, 13, 111. [Google Scholar] [CrossRef]
- McPhail, K.L.; Armstrong, D.J.; Azevedo, M.D.; Banowetz, G.M.; Mills, D. I: 4-Formylaminooxyvinylglycine, an herbicidal germination-arrest factor from Pseudomonas rhizosphere bacteria. J. Nat. Prod. 2010, 73, 1853–1857. [Google Scholar] [CrossRef] [PubMed]
- Kimbrel, J.A.; Givan, S.A.; Halgren, A.B.; Creason, A.L.; Mills, D.I.; Banowetz, G.M.; Armstrong, D.J.; Chang, J. H: An improved, high-quality draft genome sequence of the Germination-Arrest Factor-producing Pseudomonas fluorescens WH6. BMC Genom. 2010, 11, 522. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.E.; Frey, E.J.; Benn, M.H. Rhizobitoxine and L-threo-hydroxythreonine production by the plant pathogen Pseudomonas andropogonis. Phytochemistry 1986, 25, 2711–2715. [Google Scholar]
- Sahm, U.; Knobloch, G.; Wagner, F. Isolation and characterization of the methionine antagonist L-2-amino-4-methoxy-trans-3-butenoic acid from Pseudomonas aeruginosa grown on n-paraffin. J Antibiot. 1973, 26, 389–390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Braun, S.D.; Völksch, B.; Nüske, J.; Spiteller, D. 3-Methylarginine from Pseudomonas syringae pv. syringae 22d/93 suppresses the bacterial blight caused by its close relative Pseudomonas syringae pv. glycinea. Chem. Bio Chem. 2008, 9, 1913–1920. [Google Scholar]
- Katagiri, K.; Tori, K.; Kimura, Y.; Yoshida, T.; Nagasaki, T.; Minato, H. A new antibiotic. Furanomycin, an isoleucine antagonist. J. Med. Chem. 1967, 10, 1149–1154. [Google Scholar] [CrossRef]
- Xie, Y.; Peng, Q.; Ji, Y.; Xie, A.; Yang, L.; Mu, S.; Li, Z.; He, T.; Xiao, Y.; Zhao, J.; et al. Isolation and identification of antibacterial bioactive compounds from Bacillus megaterium L2. Front. Microbiol. 2021, 12, 645484. [Google Scholar] [CrossRef]
- Liu, C.; Bai, L.; Cao, P.; Li, S.; Huang, S.X.; Wa, J.; Li, L.; Zhang, J.; Zhao, J.; Song, J.; et al. Novel plant growth regulator guvermectin from plant growthpromoting rhizobacteria boosts biomass and grain yield in rice. J. Agric. Food Chem. 2022, 70, 16229–16240. [Google Scholar] [CrossRef]
- Zhang, M.; Li, L.; Li, C.; Ma, A.; Li, J.; Yang, C.; Chen, X.; Cao, P.; Li, S.; Zhang, Y.; et al. Natural product guvermectin inhibits guanosine 5′-monophosphate synthetase and confers broad-spectrum antibacterial activity. Int. J. Biol. Macromol. 2004, 267, 131510. [Google Scholar] [CrossRef]
- Aghdam, N.M.N.; Baghaee-Ravari, S.; Shiri, A. Weeds associated bacterial endophyte producing pantocin against Pectobacterium carotovorum subsp. carotovorum. J. Agric. Sci. Tech. 2024, 25, 1443–1454. [Google Scholar]
- Evidente, A.; Berestetskiy, A.; Cimmino, A.; Tuzi, A.; Superchi, S.; Melck, D.; Andolfi, A. Papyracillic acid, a phytotoxic 1,6-dioxaspiro [4,4] nonene produced by Ascochyta agropyrina var. nana, a potential mycoherbicide for Elytrigia repens biocontrol. J. Agric. Food Chem. 2009, 57, 11168–11173. [Google Scholar]
- Hansske, F.; Sterner, O.; Satadler, M.; Anke, H.; Dorge, L.; Shan, R. Papyracillic Acid, Method for Preparation and Its Use as Synthon for Bioactive Substances. U.S. Patent 5907047, 25 May 1999. [Google Scholar]
- Shan, R.; Heidren, A.; Stadler, M.; Sterner, O. Papyracillic acid, a new penicillic acid analogue from the ascomycete Lachnum papyraceum. Tetrahedron 1996, 52, 10249–10254. [Google Scholar] [CrossRef]
- Evidente, A. The incredible story of ophiobolin A and sphaeropsidin A: Two fungal terpenes from wilt-inducing phytotoxins to promising anticancer compounds. Nat. Prod. Rep. 2024, 41, 434–468. [Google Scholar] [CrossRef] [PubMed]
- Ingels, A.; Scott, R.; Hooper, A.R.; van der Westhuyzen, A.E.; Wagh, S.B.; de Meester, J.; Maddau, L.; Marko, D.; Aichinger, G.; Berger, W.; et al. New hemisynthetic derivatives of sphaeropsidin phytotoxins triggering severe endoplasmic reticulum swelling in cancer cells. Sci. Rep. 2024, 14, 14674. [Google Scholar] [CrossRef] [PubMed]
- Akatsuka, T.; Kodama, O.; Kato, H.; Kono, Y.; Takeuchi, S. Short Communication 3-Hydroxy-7-oxo-sandaracopimaradiene (oryzalexin A), a new phytoalexin Isolated from rice blast leaves. Agric. Biol. Chem. 1983, 47, 445–447. [Google Scholar]
- Kono, Y.; Takeuchi, S.; Kodama, O.; Akatsuka, T. Absolute configuration of oryzalexin A and structures of its related phytoalexins isolated from rice blast leaves infected with Pyricularia oryzae. Agric. Biol. Chem. 1984, 48, 253–255. [Google Scholar] [CrossRef]
- Akatsuka, T.; Kodama, O.; Sekido, H.; Kono, Y.; Takeuchi, S. Novel phytoalexins (oryzalexins A, B and C) isolated from rice blast leaves infected with Pyricularia oryzae. Part I: Isolation, characterization and biological activities of oryzalexins. Agric. Biol. Chem. 1985, 49, 1689–1694. [Google Scholar] [CrossRef]
- Cartwright, D.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Chemical activation of host defence mechanisms as a basis for crop protection. Nature 1977, 267, 511–513. [Google Scholar] [CrossRef]
- Evidente, A.; Venturi, V.; Masi, M.; Degrassi, G.; Cimmino, A.; Maddau, L.; Andolfi, A. In vitro antibacterial activity of sphaeropsidins and chemical derivatives toward Xanthomonas oryzae pv. oryzae, the causal agent of rice bacterial blight. J. Nat. Prod. 2011, 74, 2520–2525. [Google Scholar]
- Li, S.; Shao, M.W.; Lu, Y.H.; Kong, L.C.; Jiang, D.H.; Zhang, Y.L. Phytotoxic and antibacterial metabolites from Fusarium proliferatum ZS07 isolated from the gut of long-horned grasshoppers. J. Agric. Food Chem. 2014, 62, 8997–9001. [Google Scholar] [CrossRef]
- Ngo, M.T.; Van Nguyen, M.; Han, J.W.; Kim, B.; Kim, Y.K.; Park, M.S.; Kim, H.; Choi, G.J. Biocontrol potential of Aspergillus species producing antimicrobial metabolites. Front. Microbiol. 2021, 12, 804333. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Pescitelli, G.; Berestetskiy, A.; Dalinova, A.; Krivorotov, D.; Tuzi, A.; Evidente, A. Biological evaluation and determination of the absolute configuration of chloromonilicin, a strong antimicrobial metabolite isolated from Alternaria sonchi. J. Antibiot. 2016, 69, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Masi, M.; Panda, S.K.; Luyten, W.; Cimmino, A.; Tayung, K.; Evidente, A. Antimicrobial secondary metabolites of an endolichenic Aspergillus niger isolated from lichen thallus of Parmotrema ravum. Nat. Prod. Res. 2020, 34, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- van Der Wolf, J.M.; Nijhuis, E.H.; Kowalewska, M.J.; Saddler, G.S.; Parkinson, N.; Elphinstone, J.G.; Pritchard, L.; Toth, I.K.; Lojkowska, E.; Potrykus, M.; et al. Dickeya solani sp. nov., a pectinolytic plant-pathogenic bacterium isolated from potato (Solanum tuberosum). Int. J. Syst. Evol. Microbiol. 2014, 64, 768–774. [Google Scholar] [CrossRef]
- Takikawa, Y.; Takahashi, F. Bacterial leaf spot and blight of crucifer plants (Brassicaceae) caused by Pseudomonas syringae pv. maculicola and P. cannabina pv. alisalensis. J. Gen. Plant. Pathol. 2014, 80, 466–474. [Google Scholar]
- Vieira, G.; Khalil, Z.G.; Capon, R.J.; Sette, L.D.; Ferreira, H.; Sass, D.C. Isolation and agricultural potential of penicillic acid against citrus canker. J. Appl. Microbiol. 2022, 132, 3081–3088. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, Q.; Xie, A.; Xiao, Y.; Guo, K.; Mu, S.; Xie, Y.; Li, Z.; He, T. Isolation of bioactive compounds, antibacterial activity, and action mechanism of spore powder from Aspergillus niger xj. Front. Microbiol. 2022, 13, 934857. [Google Scholar] [CrossRef] [PubMed]
- Avrova, A.O.; Hyman, L.J.; Toth, R.L.; Toth, I.K. Application of amplified fragment length polymorphism fingerprinting for taxonomy and identification of the soft rot bacteria Erwinia carotovora and Erwinia chrysanthemi. Appl. Environ. Microbiol. 2002, 68, 1499–1508. [Google Scholar] [CrossRef]
- Fuller, S.L.; Savory, E.A.; Weisberg, A.J.; Buser, J.Z.; Gordon, M.I.; Putnam, M.L.; Ghang, J.H. Isothermal amplification and lateral-flow assay for detecting crown-gall-causing Agrobacterium spp. Phytopathology 2017, 107, 1062–1068. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machados, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Schrader, K.K.; Dayan, F.E.; Allen, S.N.; de Regt, M.Q.; Tucker, C.S.; Paul, R.N., Jr. 9,10-Anthraquinone reduces the photosynthetic efficiency of Oscillatoria perornata and modifies cellular inclusions. Int. J. Plant Sci. 2000, 161, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Klesius, P.H.; Evans, J.; Shoemaker, C. Advancements in fish vaccine development. Aquac Int. 2006, 4, 20–21. [Google Scholar]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Kluwer Academic Publishers: Boston, MA, USA, 1998; p. 700. [Google Scholar]
- Schrader, K.K.; Andolfi, A.; Cantrell, C.L.; Cimmino, A.; Duke, S.O.; Osbrink, W.; Wedge, E.W.; Evidente, A. A survey of phytotoxic microbial and plant metabolites as potential natural products for pest management. Chem. Biodivers. 2010, 7, 2261–2280. [Google Scholar] [CrossRef]
- Kornienko, A.; Evidente, A. Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef] [PubMed]
- Schrader, K.K.; Avolio, F.; Andolfi, A.; Cimmino, A.ì.; Evidente, A. Ungeremine and its hemisynthesized analogs as bactericides against Flavobacterium columnare. J. Agric. Food Chem. 2013, 61, 1179–1183. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and antibacterial capacities of Origanum vulgare L. essential oil from the arid Andean Region of Chile and its chemical characterization by GC-MS. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef]
- Pham, D.Q.; Pham, H.T.; Han, J.W.; Nguyen, T.H.; Nguyen, H.T.; Nguyen, T.D.; Nguyen, T.T.T.; Cuong, T.H.; Pham, H.M.; Vu, H.D.; et al. Extracts and metabolites derived from the leaves of Cassia alata L. exhibit in vitro and in vivo antimicrobial activities against fungal and bacterial plant pathogens. Ind. Crops. Prod. 2021, 166, 113465. [Google Scholar] [CrossRef]
- Dongmo, A.N.; Nguefack, J.; Dongmo, J.B.L.; Fouelefack, F.R.; Azah, R.U.; Nkengfack, E.A.; Stefani, E. Chemical characterization of an aqueous extract and the essential oil of Tithonia diversifolia and their biocontrol activity against seed-borne pathogens of rice. J. Plant Dis. Prot. 2021, 128, 703–713. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reveglia, P.; Corso, G.; Evidente, A. Advances on Bioactive Metabolites with Potential for the Biocontrol of Plant Pathogenic Bacteria. Pathogens 2024, 13, 1000. https://doi.org/10.3390/pathogens13111000
Reveglia P, Corso G, Evidente A. Advances on Bioactive Metabolites with Potential for the Biocontrol of Plant Pathogenic Bacteria. Pathogens. 2024; 13(11):1000. https://doi.org/10.3390/pathogens13111000
Chicago/Turabian StyleReveglia, Pierluigi, Gaetano Corso, and Antonio Evidente. 2024. "Advances on Bioactive Metabolites with Potential for the Biocontrol of Plant Pathogenic Bacteria" Pathogens 13, no. 11: 1000. https://doi.org/10.3390/pathogens13111000
APA StyleReveglia, P., Corso, G., & Evidente, A. (2024). Advances on Bioactive Metabolites with Potential for the Biocontrol of Plant Pathogenic Bacteria. Pathogens, 13(11), 1000. https://doi.org/10.3390/pathogens13111000







