Pharmacokinetics and Pharmacodynamics Evaluation of Amoxicillin Against Staphylococcus pseudintermedius in Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Bacterial Strains and Culture Conditions
2.3. Minimum Inhibitory Concentration (MIC)
2.4. Minimum Bactericidal Concentration (MBC)
2.5. Mutant Prevention Concentration (MPC)
2.6. Post-Antibiotic Effect (PAE)
2.7. Animals and Experimental Design
2.8. Plasma Sample Extraction Procedure
2.9. Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry (UPLC-MS/MS) Analysis
2.10. Pharmacokinetic Data Analysis
2.11. Ex Vivo Time-Killing Curve Assay
2.12. PK/PD Relationship of AMX Against S. pseudintermedius
- E0, log10 change in bacterial count in drug-free control;
- EC50, AUC24h/MIC ratio required to achieve 50% of the Emax;
- n, Hill coefficient representing the slope of dose–response curve.
2.13. Protein Binding of AMX in Dog Plasma
- ƒu, free fraction;
- CTP, total plasma concentration; CFP, filtered plasma concentration;
- CTPBS, total PBS concentration; CFPBS, filtered PBS concentration.
2.14. Dose Estimations
- AUC, area under the curve;
- AUC/MIC, targeted endpoint for optimal efficacy;
- MIC90, MICs required to inhibit the growth of 90% of bacteria;
- Cl, clearance; ƒu, free fraction; F, bioavailability.
3. Results
3.1. In Vitro Susceptibility Test
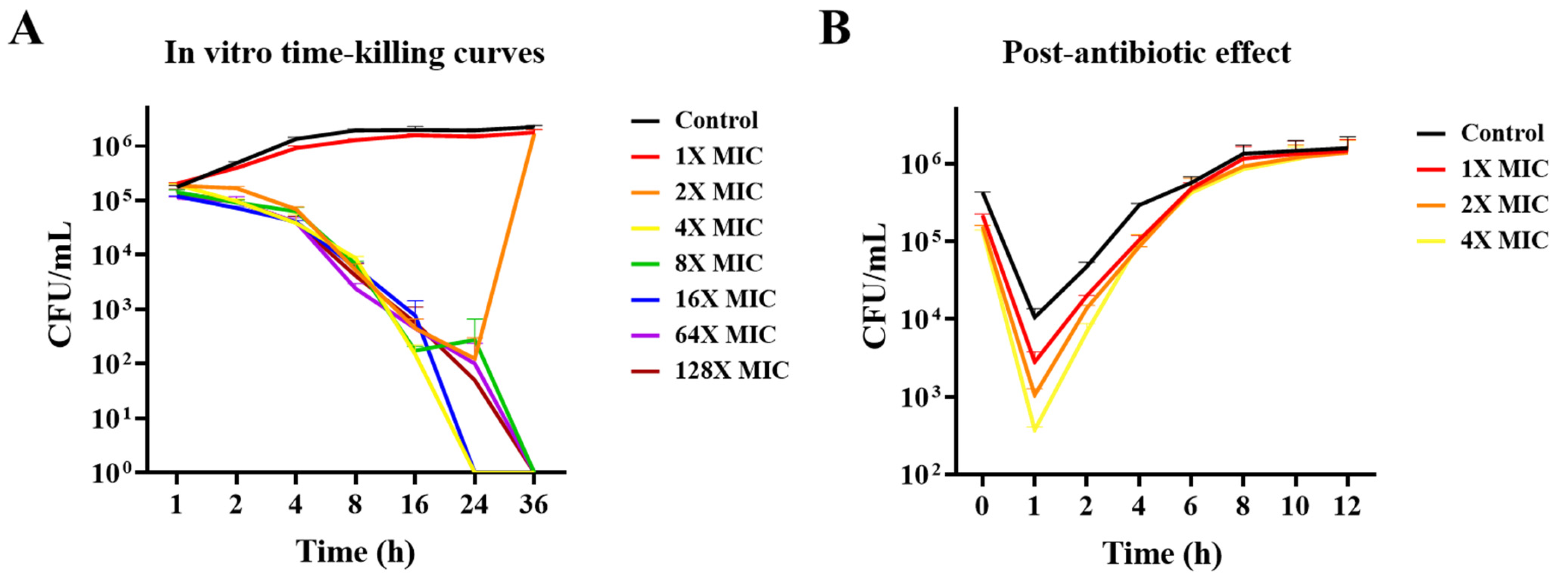
3.2. In Vitro Time-Killing Curves and PAE Determination
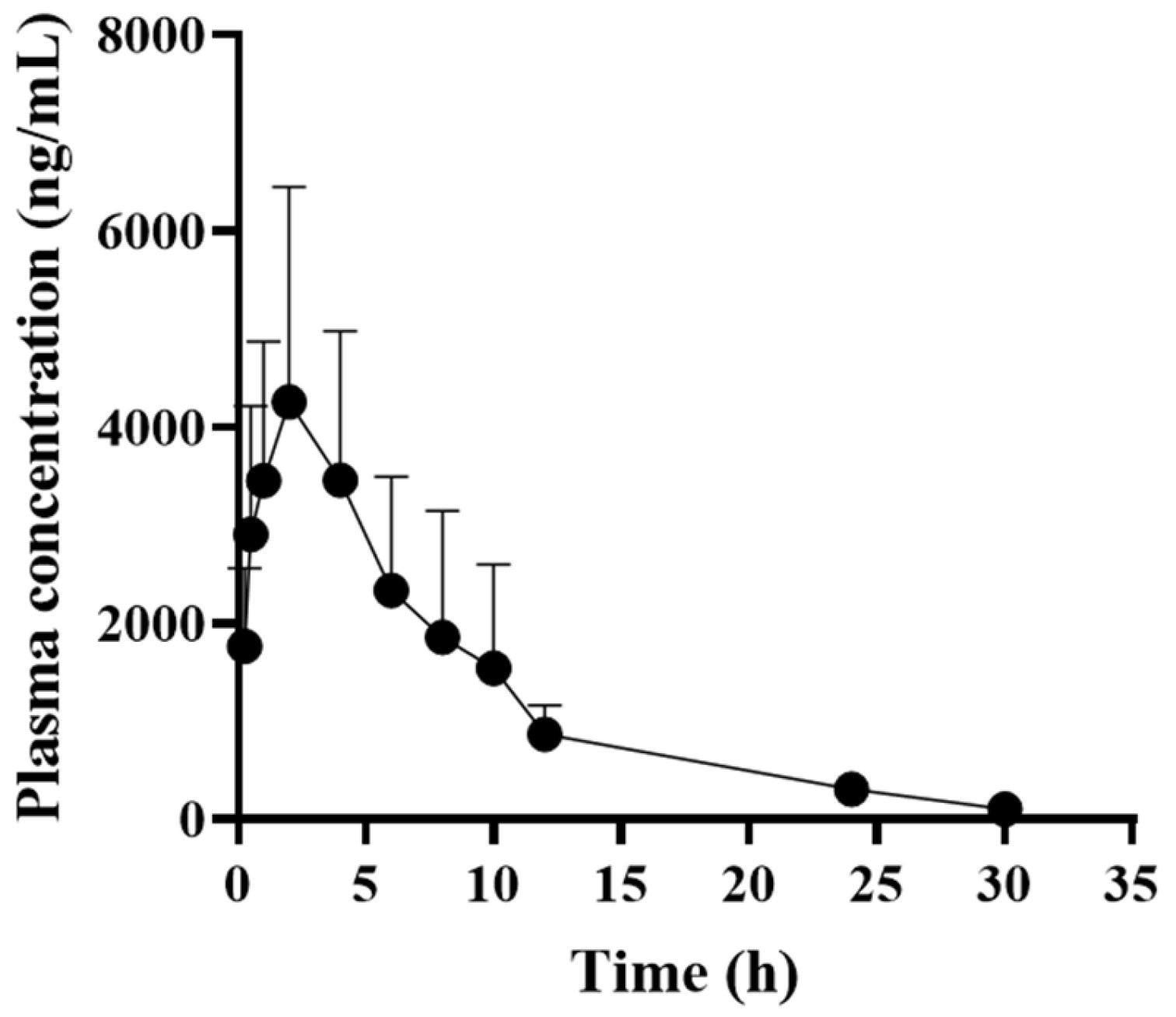
3.3. Pharmacokinetic Profiles of AMX in Dogs
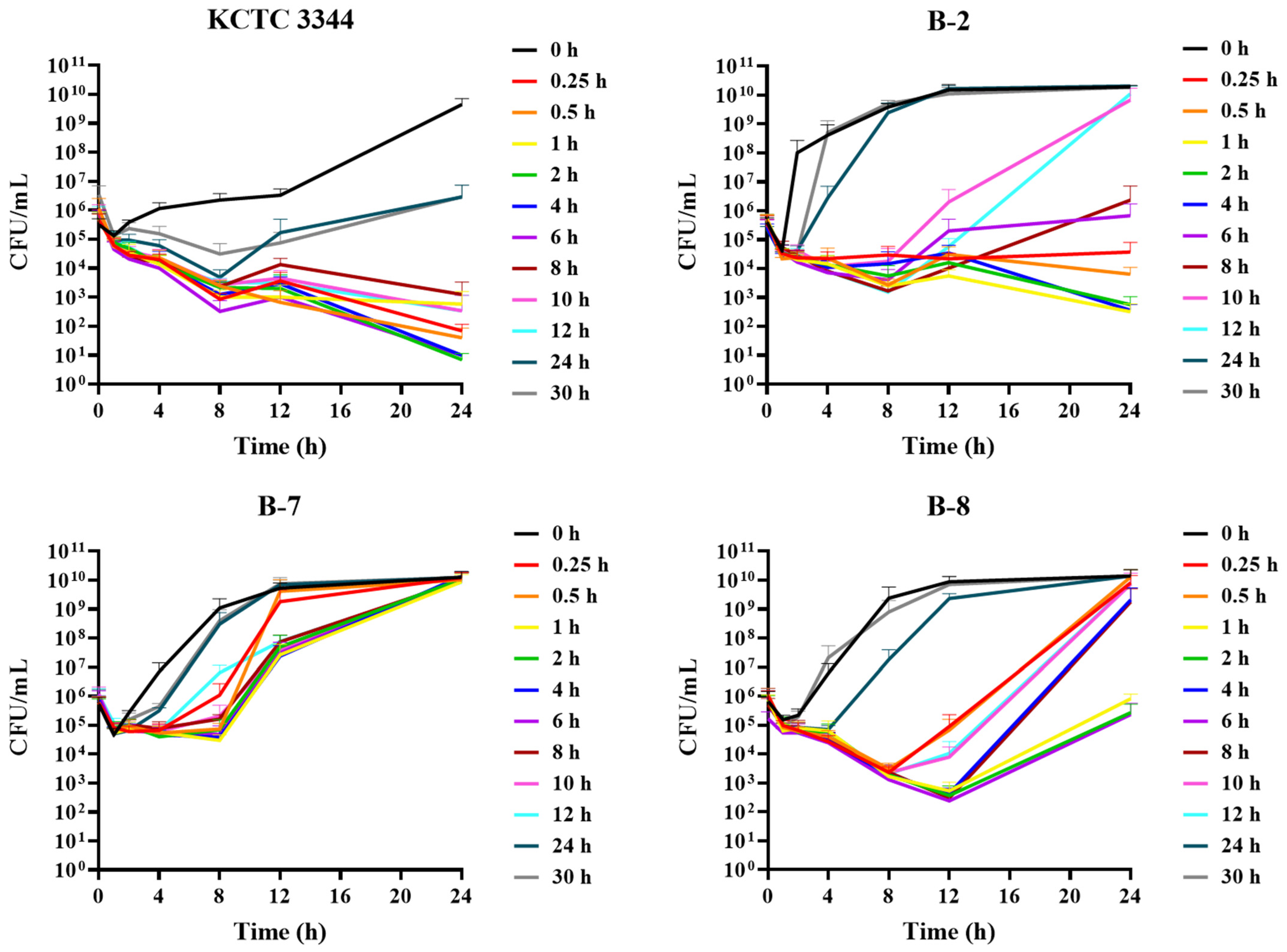
3.4. Ex Vivo Antimicrobial Activity
3.5. PK/PD Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial resistance in veterinary medicine: An overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef] [PubMed]
- Lenart-Boroń, A.; Stankiewicz, K.; Czernecka, N.; Ratajewicz, A.; Bulanda, K.; Heliasz, M.; Sosińska, D.; Dworak, K.; Ciesielska, D.; Siemińska, I.; et al. Wounds of companion animals as a habitat of antibiotic-resistant bacteria that are potentially harmful to humans-phenotypic, proteomic and molecular detection. Int. J. Mol. Sci. 2024, 25, 3121. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gascón, A.; Solinís, M.Á.; Isla, A. The role of PK/PD analysis in the development and evaluation of antimicrobials. Pharmaceutics 2021, 13, 833. [Google Scholar] [CrossRef] [PubMed]
- Ferradas, C.; Cotter, C.; Shahbazian, J.H.; Iverson, S.A.; Baron, P.; Misic, A.M.; Brazil, A.M.; Rankin, S.C.; Nachamkin, I.; Ferguson, J.M.; et al. Risk factors for antimicrobial resistance among Staphylococcus isolated from pets living with a patient diagnosed with methicillin-resistant Staphylococcus aureus infection. Zoonoses Public Health 2022, 69, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.A.; Helbig, K.J. The complex diseases of Staphylococcus pseudintermedius in canines: Where to next? Vet. Sci. 2021, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Hillier, A.; Lloyd, D.H.; Weese, J.S.; Blondeau, J.M.; Boothe, D.; Breitschwerdt, E.; Guardabassi, L.; Papich, M.G.; Rankin, S.; Turnidge, J.D.; et al. Guidelines for the diagnosis and antimicrobial therapy of canine superficial bacterial folliculitis (Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases). Vet. Dermatol. 2014, 25, 163-e43. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.M.; Cohen, N.D.; Lawhon, S.D. Amikacin resistance in Staphylococcus pseudintermedius isolated from dogs. J. Clin. Microbiol. 2014, 52, 3641–3646. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.K.; Kumar, M.; Singh, D.K. Insight into the amoxicillin resistance, ecotoxicity, and remediation strategies. J. Water Process Eng. 2021, 39, 101858. [Google Scholar] [CrossRef]
- Marier, J.F.; Beaudry, F.; Ducharme, M.P.; Fortin, D.; Moreau, J.P.; Massé, R.; Vachon, P. A pharmacokinetic study of amoxycillin in febrile beagle dogs following repeated administrations of endotoxin. J. Vet. Pharmacol. Ther. 2001, 24, 379–383. [Google Scholar] [CrossRef]
- Vegas Cómitre, M.D.; Cortellini, S.; Cherlet, M.; Devreese, M.; Roques, B.B.; Bousquet-Melou, A.; Toutain, P.L.; Pelligand, L. Population pharmacokinetics of intravenous amoxicillin combined with clavulanic acid in healthy and critically Ill dogs. Front. Vet. Sci. 2021, 8, 770202. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th ed.; CLSI Standard M02; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Minimum Bactericidal Concentration Testing. In Clinical Microbiology Procedures Handbook; ASM Press: Washington, DC, USA, 2016; pp. 5.14.1.1–5.14.3.6. [CrossRef]
- Gianvecchio, C.; Lozano, N.A.; Henderson, C.; Kalhori, P.; Bullivant, A.; Valencia, A.; Su, L.; Bello, G.; Wong, M.; Cook, E.; et al. Variation in mutant prevention concentrations. Front. Microbiol. 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A.; Gudmundsson, S. Post-antibiotic effect. In Antibiotics in Laboratory Medicine; Lorian, V., Ed.; Williams & Wilkins: Baltimore, MD, USA, 1996; pp. 296–329. [Google Scholar]
- National Office of Animal Health. Compendium of Data Sheets for Animal Medicines; NOAH Enfield: London, UK, 2021. [Google Scholar]
- Guideline on Bioanalytical Method Validation. Available online: https://www.mfds.go.kr/eng/brd/m_1127/view.do?seq=2&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1 (accessed on 11 August 2023).
- Abbas, M.A.; Lee, E.B.; Boby, N.; Biruhanu, B.T.; Park, S.C. A pharmacodynamic investigation to assess the synergism of orbifloxacin and propyl gallate against Escherichia coli. Front. Pharmacol. 2022, 13, 989395. [Google Scholar] [CrossRef] [PubMed]
- MacGowan, A.; Bowker, K. Developments in PK/PD: Optimising efficacy and prevention of resistance. A critical review of PK/PD in in vitro models. Int. J. Antimicrob. Agents 2002, 19, 291–298. [Google Scholar] [CrossRef]
- Zeng, Q.L.; Mei, X.; Su, J.; Li, X.H.; Xiong, W.G.; Lu, Y.; Zeng, Z.L. Integrated pharmacokinetic-Pharmacodynamic (PK/PD) model to evaluate the in vivo antimicrobial activity of Marbofloxacin against Pasteurella multocida in piglets. BMC Vet. Res. 2017, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, A.; Liebchen, U.; Schleibinger, M.; Kees, M.G.; Kees, F. Determination of free vancomycin, ceftriaxone, cefazolin and ertapenem in plasma by ultrafiltration: Impact of experimental conditions. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 961, 97–102. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, Q.; Qi, Y.; Yang, B.; Khaliq, H.; Xiong, J.; Moku, G.K.; Ahmed, S.; Li, K.; Zhang, H.; et al. Optimal regimens and cutoff evaluation of Tildipirosin against Pasteurella multocida. Front. Pharmacol. 2018, 9, 765. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Somayaji, R.; Priyantha, M.A.; Rubin, J.E.; Church, D. Human infections due to Staphylococcus pseudintermedius, an emerging zoonosis of canine origin: Report of 24 cases. Diagn. Microbiol. Infect. Dis. 2016, 85, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.; Rezusta, A.; Ferrer, I.; Pérez-Laguna, V.; Zarazaga, M.; Ruiz-Ripa, L.; Revillo, M.J.; Torres, C. Staphylococcus pseudintermedius human infection cases in Spain: Dog-to-human transmission. Vector-Borne Zoonotic Dis. 2017, 17, 268–270. [Google Scholar] [CrossRef]
- Robb, A.R.; Wright, E.D.; Foster, A.M.E.; Walker, R.; Malone, C. Skin infection caused by a novel strain of Staphylococcus pseudintermedius in a Siberian husky dog owner. JMM Case Rep. 2017, 4, jmmcr005087. [Google Scholar] [CrossRef] [PubMed]
- Joosten, P.; Ceccarelli, D.; Odent, E.; Sarrazin, S.; Graveland, H.; Van Gompel, L.; Battisti, A.; Caprioli, A.; Franco, A.; Wagenaar, J.A.; et al. Antimicrobial usage and resistance in companion animals: A cross-sectional study in three European countries. Antibiotics 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Asín-Prieto, E.; Rodríguez-Gascón, A.; Isla, A. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. J. Infect. Chemother. 2015, 21, 319–329. [Google Scholar] [CrossRef]
- Jacobs, M.R. Optimisation of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin. Microbiol. Infect. 2001, 7, 589–596. [Google Scholar] [CrossRef]
- DeRyke, C.A.; Lee, S.Y.; Kuti, J.L.; Nicolau, D.P. Optimising dosing strategies of antibacterials utilising pharmacodynamic principles: Impact on the development of resistance. Drugs 2006, 66, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P.; Cottrell, M.L.; Kashuba, A.D.M.; Bertino, J.S. Pharmacokinetics and pharmacodynamics of anti-infective agents. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; W.B. Saunders: Philadelphia, PA, USA, 2015; pp. 252–262.e2. [Google Scholar] [CrossRef]
- Thorburn, C.E.; Molesworth, S.J.; Sutherland, R.; Rittenhouse, S. Postantibiotic and post-beta-lactamase inhibitor effects of amoxicillin plus clavulanate. Antimicrob. Agents Chemother. 1996, 40, 2796–2801. [Google Scholar] [CrossRef] [PubMed]
- Proma, F.H.; Shourav, M.K.; Choi, J. Post-antibiotic effect of ampicillin and levofloxacin to Escherichia coli and Staphylococcus aureus based on microscopic imaging analysis. Antibiotics 2020, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Pelligand, L.; Baker, D.; Sivagurunathan, A.; Kovačević, Z.; Suemanotham, N.; Stair, J.L.; Scott, M.; Liu, F.; Page, S.W.; Guardabassi, L.; et al. Therapeutic guidelines group of the world small animal veterinary association. Quality of amoxicillin/clavulanic acid oral formulations for intended veterinary use in the UK, Malaysia, Serbia and Thailand. J. Small Anim. Pract. 2023, 64, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Moczarnik, J.; Berger, D.J.; Noxon, J.O.; LeVine, D.N.; Lin, Z.; Coetzee, J.F.; Mochel, J.P. Relative oral bioavailability of two amoxicillin-clavulanic acid formulations in healthy dogs: A pilot study. J. Am. Anim. Hosp. Assoc. 2019, 55, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, F.; Wang, G.; Xi, W.; Zhang, C.; Wang, H. Pharmacokinetics of the amoxicillin-clavulanic acid combination after intravenous and oral administration in cats. J. Vet. Pharmacol. Ther. 2019, 42, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Wen, X.; Xu, W.; Xu, Y.; Deng, X.; Yan, G.; Wu, L.; Liang, Q.; Liang, Z.; Peng, J.; et al. Development of an amoxicillin-radix scutellaria extract formulation and evaluation of its pharmacokinetics in pigs. BMC Vet. Res. 2023, 19, 164. [Google Scholar] [CrossRef] [PubMed]
- Kimble, B.; Vogelnest, L.; Gharibi, S.; Izes, A.M.; Govendir, M. Pharmacokinetic profile of amoxicillin and its glucuronide-like metabolite when administered subcutaneously to koalas (Phascolarctos cinereus). J. Vet. Pharmacol. Ther. 2020, 43, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Veiga, R.P.; Paiva, J.A. Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit. Care 2018, 22, 233. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.I.; Cars, O.; Friberg, L.E. Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: A step toward model-based dose optimization. Antimicrob. Agents Chemother. 2011, 55, 4619–4630. [Google Scholar] [CrossRef] [PubMed]
- Makade, C.S.; Shenoi, P.R.; Bhongade, B.A.; Shingane, S.A.; Ambulkar, P.C.; Shewale, A.M. Estimation of MBC: MIC ratio of herbal extracts against common endodontic pathogens. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. S2), S1414–S1416. [Google Scholar] [CrossRef] [PubMed]

| KCTC 3344 | B-2 | B-7 | B-8 | |
|---|---|---|---|---|
| MICs (μg/mL) | 0.25 | 0.5 | 64 | 16 |
| MBCs (μg/mL) | 0.25 | 4 | 128 | 64 |
| MPCs (μg/mL) | 4 | - | - | - |
| Pharmacokinetic Parameters | |
|---|---|
| T1/2 (h) | 7.1 2.1 |
| Tmax (h) a | 2.0 (0.5 − 2.0) |
| Cmax (ng/mL) | 4309.3 2161.1 |
| AUClast (h × ng/mL) | 37,802.1 14,719.8 |
| AUCINF (h × ng/mL) | 39,782.0 13,645.2 |
| AUC_%Extrap (%) | 6.1 5.8 |
| MRTlast (h) | 7.6 1.3 |
| AUMClast (h × h × ng/mL) | 274,927.2 74,853.4 |
| Vd/F (mL/kg) | 4558.7 2726.6 |
| Cl/F (mL/h/kg) | 414.8 144.0 |
| Kel (1/h) | 0.1 0.0 |
| PK/PD Parameters | KCTC 3344 | B-2 | B-8 |
|---|---|---|---|
| Emax (log10 CFU/mL) | 5.2 0.3 | 4.6 0.2 | 1.3 1.1 |
| EC50 (log10 CFU/mL) | 10.3 7.1 | 85.3 9.6 | 4.6 2.6 |
| N | 1.0 0.5 | 2.1 0.4 | 6.6 2.5 |
| AUC24h/MIC for bacteriostatic (h) | 8.5 4.6 | 85.6 11.9 | 6.6 1.3 |
| AUC24h/MIC for bactericidal (h) | 41.1 23.2 | 180.5 8.8 | - |
| AUC24h/MIC for eradication (h) | 104.0 75.6 | 307.9 20.5 | - |
| Predicted doses (mg/kg·bw) | KCTC 3344 | B-2 | B-8 |
| Bacteriostatic (E = 0) | 0.97 0.54 | 16.31 2.60 | 41.04 9.00 |
| Bactericidal (E = −3) | 4.40 2.21 | 34.77 4.98 | - |
| Eradication (E = −4) | 10.22 5.82 | 59.64 9.38 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-S.; Kim, J.-W.; Kim, J.-H.; Kim, C.-Y.; Chung, E.-H.; Boo, S.-Y.; Lee, S.-H.; Ko, J.-W.; Kim, T.-W. Pharmacokinetics and Pharmacodynamics Evaluation of Amoxicillin Against Staphylococcus pseudintermedius in Dogs. Pathogens 2024, 13, 1121. https://doi.org/10.3390/pathogens13121121
Jeong J-S, Kim J-W, Kim J-H, Kim C-Y, Chung E-H, Boo S-Y, Lee S-H, Ko J-W, Kim T-W. Pharmacokinetics and Pharmacodynamics Evaluation of Amoxicillin Against Staphylococcus pseudintermedius in Dogs. Pathogens. 2024; 13(12):1121. https://doi.org/10.3390/pathogens13121121
Chicago/Turabian StyleJeong, Ji-Soo, Jeong-Won Kim, Jin-Hwa Kim, Chang-Yeop Kim, Eun-Hye Chung, So-Young Boo, Su-Ha Lee, Je-Won Ko, and Tae-Won Kim. 2024. "Pharmacokinetics and Pharmacodynamics Evaluation of Amoxicillin Against Staphylococcus pseudintermedius in Dogs" Pathogens 13, no. 12: 1121. https://doi.org/10.3390/pathogens13121121
APA StyleJeong, J.-S., Kim, J.-W., Kim, J.-H., Kim, C.-Y., Chung, E.-H., Boo, S.-Y., Lee, S.-H., Ko, J.-W., & Kim, T.-W. (2024). Pharmacokinetics and Pharmacodynamics Evaluation of Amoxicillin Against Staphylococcus pseudintermedius in Dogs. Pathogens, 13(12), 1121. https://doi.org/10.3390/pathogens13121121






