Antimicrobial Resistance in the Context of Animal Production and Meat Products in Poland—A Critical Review and Future Perspective
Abstract
1. Introduction
2. Antibiotics Used in Animal Production
2.1. Importance of Antibiotic Use in Livestock Production
2.2. Challenges of Antibiotic Use
2.3. Antibiotic Use in Poland
3. Influence of Food Processing Technology on the Antibiotic Content in Meat Products
4. The Problem of Antibiotic Resistance
4.1. Regulations in Antibiotic Use
4.2. Implications of Antibiotic Resistance
4.3. Strategies to Prevent Antibiotic Resistance
5. Antibiotic Resistance Among Microorganisms Isolated from Meat and Meat Products
5.1. Campylobacter spp.
5.2. Staphylococcus spp.
5.3. Enterococcus spp.
5.4. Listeria monocytogenes
5.5. Enterobacterales
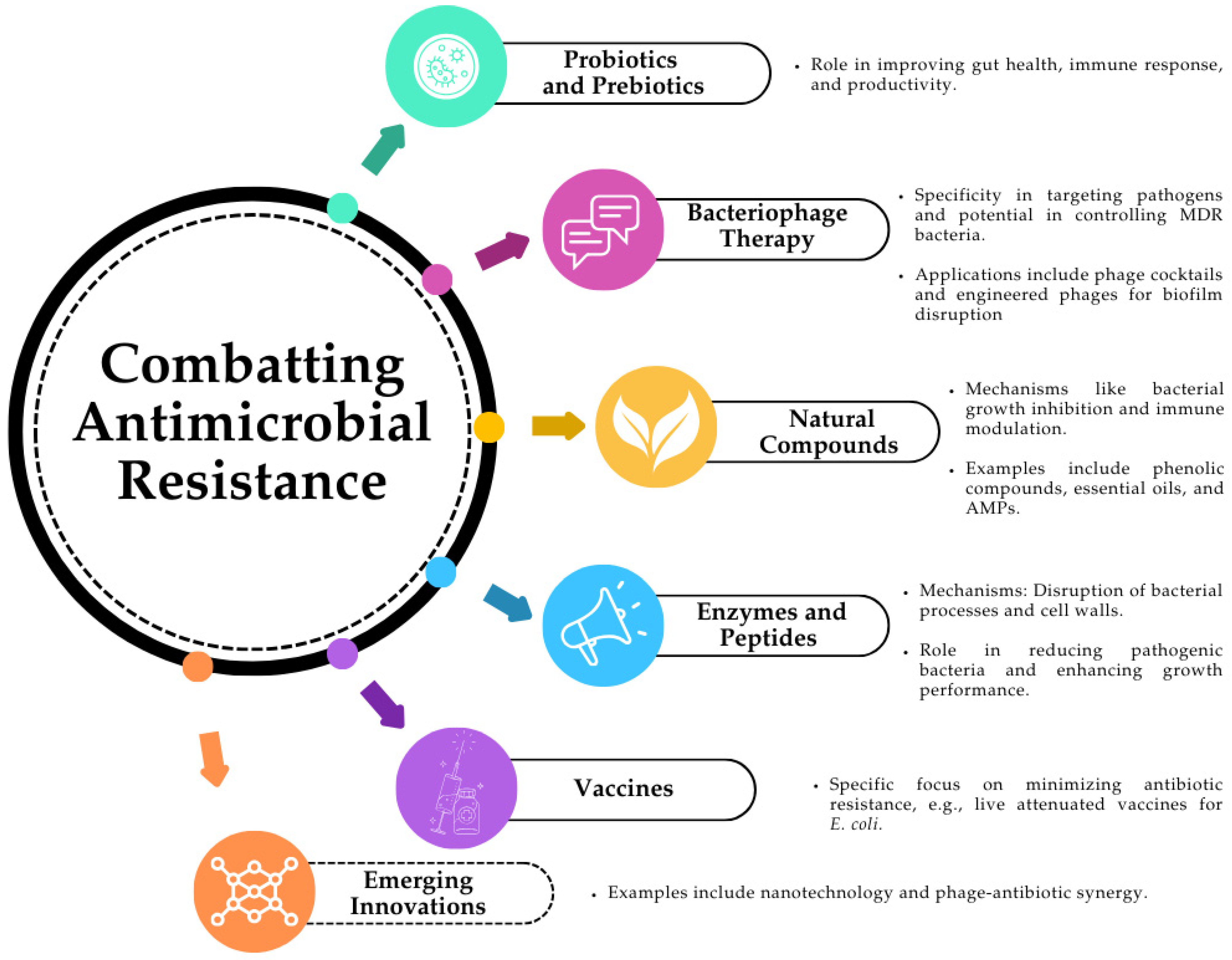
6. Alternatives to Antibiotic Therapy in Agriculture and Animal Husbandry
6.1. Probiotics and Prebiotics
6.2. Bacteriophages
6.3. Natural Compounds
6.4. Enzymes and Peptides
6.5. Vaccines
6.6. Emerging Innovations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global Trends in Antimicrobial Resistance in Animals in Low- and Middle-Income Countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef] [PubMed]
- O’neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance. AMR Review. 2016. Available online: https://apo.org.au/sites/default/files/resource-files/2016-05/apo-nid63983.pdf (accessed on 2 September 2024).
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022, 1st ed.; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-006270-2. [Google Scholar]
- van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Harbarth, S.; Balkhy, H.H.; Goossens, H.; Jarlier, V.; Kluytmans, J.; Laxminarayan, R.; Saam, M.; van Belkum, A.; Pittet, D. Antimicrobial Resistance: One World, One Fight! Antimicrob. Resist. Infect. Control 2015, 4, 49. [Google Scholar] [CrossRef]
- Bataeva, D.S.; Zaiko, E.V. Risks Associated with the Presence of Antimicrobial Drug Residues in Meat Products and Products of Animal Slaughter. Theory Pract. Meat Process. 2016, 1, 4–13. [Google Scholar] [CrossRef]
- Madhup, S.K.; Shrestha, R.; Panta, R.; Chauguthi, L.; Katuwal, N.; Shrestha, S. Prevalence of Pathogenic Bacteria in Meat Products and Their Antimicrobial Resistance Pattern. Ann. Clin. Chem. Lab. Med. 2021, 4, 13–19. [Google Scholar] [CrossRef]
- Almashhadany, D.A. Monitoring of Antibiotic Residues among Sheep Meat in Erbil City and Thermal Processing Effect on Their Remnants. Iraqi J. Vet. Sci. 2020, 34, 217–222. [Google Scholar] [CrossRef]
- Stella, O.-I.O.; Ezenduka, E.V.; Anaelom, N.J. Screening for Tylosin and Other Antimicrobial Residues in Fresh and Fermented (Nono) Cow Milk in Delta State, South-South, Nigeria. Vet. World 2020, 13, 458–464. [Google Scholar] [CrossRef]
- Almashhadany, D.A.; Mohammed, H.I.; Abdulwahid, M.; Muslat, T.; Rashid, R.F.; Hassan, R.R.; Hassan, A.O. Antimicrobial Residues in Meat and Meat Products. Health Risks of Food Additives—Recent Developments and Trends in Food Sector. IntechOpen. 2022. Available online: https://www.intechopen.com/chapters/82512 (accessed on 5 September 2024).
- Ahangaran, M.G.; Sichani, M.; Sadeghi, A.; Peimani, N.; Dastgerdi, A. The Effect of Thyme (Thymus daenensis) Supplement on Growth and Hygienic Parameters of Broilers Meat. Iraqi J. Vet. Sci. 2019, 33, 87–92. [Google Scholar] [CrossRef]
- Tian, N.; Guo, X.; Wang, M.; Chen, C.; Cui, H.; Zhang, L.; Tang, H. Bacterial Community Diversity of Shilixiang Baijiu Daqu Based on Metagenomics. J. Food Biochem. 2020, 44, e13410. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.S.; Lee, S.Y.; Kang, H.J.; Hur, S.J. Reducing Veterinary Drug Residues in Animal Products: A Review. Food Sci. Anim. Resour. 2019, 39, 687–703. [Google Scholar] [CrossRef] [PubMed]
- EMA Regulation (EC) No. 470/2009 of the European Parliament and of the Council of 6 May 2009 Laying Down Community Procedures for the Establishment of Residue Limits of Pharmacologically Active Substances in Foodstuffs of Animal Origin, Repealing Council Regulation (EEC) No. 2377/90 and Amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No. 726/2004 of the European Parliament and of the CouncilText with EEA Relevance. Available online: http://data.europa.eu/eli/reg/2009/470/oj (accessed on 15 September 2024).
- Kebede, G.; Zenebe, T.; Disassa, H.; Tolosa, T. Review on Detection of Antimicrobial Residues in Raw Bulk Milk in Dairy Farms. AJBAS 2014, 6, 87–97. [Google Scholar] [CrossRef]
- Almashhadany, D.A. Detecting Antibiotic Residues Among Sheep Milk Using YCT, DDA, and Acidification Method in Erbil City, Kurdistan Region, Iraq. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2020, 77, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C. Mechanisms of Antimicrobial Resistance in Bacteria. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Jha, R.; Mishra, P. Dietary Fiber in Poultry Nutrition and Their Effects on Nutrient Utilization, Performance, Gut Health, and on the Environment: A Review. J. Anim. Sci. Biotechnol. 2021, 12, 51. [Google Scholar] [CrossRef]
- Golden, C.E.; Rothrock, M.J.; Mishra, A. Mapping Foodborne Pathogen Contamination throughout the Conventional and Alternative Poultry Supply Chains. Poult. Sci. 2021, 100, 101157. [Google Scholar] [CrossRef]
- Ghimpețeanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Drăgotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food—A Public Health Threat: A Review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.; Almashhadany, D.A.; Khalid, H.S. Determination of Heavy Metals and Selenium Content in Chicken Liver at Erbil City, Iraq. Ital. J. Food Saf. 2020, 9, 189–194. [Google Scholar] [CrossRef]
- Regulation (EC) No. 1831/2003 of the European Parliament and of the Council of 22 August 2003 on Additives for Use in Animal Nutrition. Available online: http://data.europa.eu/eli/reg/2003/1831/oj (accessed on 5 September 2024).
- Albero, B.; Tadeo, J.L.; Miguel, E.; Pérez, R.A. Rapid Determination of Antibiotic Residues in Cereals by Liquid Chromatography Triple Mass Spectrometry. Anal. Bioanal. Chem. 2019, 411, 6129–6139. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC. Available online: http://data.europa.eu/eli/reg/2019/6/oj (accessed on 25 September 2024).
- Truszczyński, M.; Posyniak, A.; Pejsak, Z. Mechanizmy Powstawania Oporności Bakterii na Działanie Antybiotyków i Środków Dezynfekujących. Vet. Med. 2013, 6, 131–135. [Google Scholar]
- Jeżak, K.; Kozajda, A. Occurrence and Spread of Antibiotic-Resistant Bacteria on Animal Farms and in Their Vicinity in Poland and Ukraine—Review. Environ. Sci. Pollut. Res. 2022, 29, 9533–9559. [Google Scholar] [CrossRef] [PubMed]
- Supreme Audit Office. 2018. Available online: https://www.nik.gov.pl/aktualnosci/nik-o-stosowaniu-antybiotykow-w-nbsp-hodowli-zwierzat-w-nbsp-woj-lubuskim.html (accessed on 25 August 2024).
- Schwarz, S.; Kehrenberg, C.; Walsh, T.R. Use of Antimicrobial Agents in Veterinary Medicine and Food Animal Production. Int. J. Antimicrob. Agents 2001, 17, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Gawryjołek, K. Antibiotics in Agriculture—Application, Threats and Legal Regulations. Pol. J. Agron. 2021, 47, 10–21. [Google Scholar] [CrossRef]
- Schwarz, S.; Chaslus-Dancla, E. Use of Antimicrobials in Veterinary Medicine and Mechanisms of Resistance. Vet. Res. 2001, 32, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Anusz, K. Antibiotic Resistance of Zoonotic Pathogens Related to the Safety of Foods of Animal Origin. Życie Weter. 2018, 93, 118–121. [Google Scholar]
- Pyzik, E.; Urban-Chmiel, R.; Herman, K.; Nowaczek, A. Solutiodkns Limiting the Use of Antibiotics in Poultry Production in Poland and Other European Union Countries. Anim. Sci. Genet. 2024, 20, 41–53. [Google Scholar] [CrossRef]
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2022: Trends from 2010 to 2022: Thirteenth ESVAC Report; Publications Office of the European Union: Luxembourg, 2023; ISBN 978-92-9155-071-5. [Google Scholar]
- World Health Organization WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-155013-0.
- Florek, M.; Barłowska, J.; Litwińczuk, Z. Mleko i mięso zwierząt przeżuwających jako źródło substancji biologicznie czynnych. Prz. Hod. 2016, 84, 3. [Google Scholar]
- Mian, A.A.; Ahmad, T.; Nadeem, S.; Tanveer, Z.I.; Arshad, J. Sulfonamide Residues Determination in Commercial Poultry Meat and Eggs. J. Anim. Plant Sci. 2012, 22, 473–478. [Google Scholar]
- Heshmati, A. Impact of Cooking Procedures on Antibacterial Drug Residues in Foods: A Review. J. Food Qual. Hazards Control 2015, 2, 33–37. [Google Scholar]
- Javadi, A. Effect of Roasting, Boiling and Microwaving Cooking Method on Doxycline Residues in Edible Tissues of Poultry by Microbial Method. Afr. J. Pharm. Pharmacol. 2011, 5, 1034–1037. [Google Scholar] [CrossRef]
- Gratacós-Cubarsí, M.; Fernandez-García, A.; Picouet, P.; Valero-Pamplona, A.; García-Regueiro, J.-A.; Castellari, M. Formation of Tetracycline Degradation Products in Chicken and Pig Meat under Different Thermal Processing Conditions. J. Agric. Food Chem. 2007, 55, 4610–4616. [Google Scholar] [CrossRef]
- Vivienne, E.E.; Josephine, O.O.; Anaelom, N.J. Effect of Temperature (Cooking and Freezing) on the Concentration of Oxytetracycline Residue in Experimentally Induced Birds. Vet. World 2018, 11, 167–171. [Google Scholar] [CrossRef]
- Nguyen, V.; Li, C.; Zhou, G. The Degradation of Oxytetracycline during Thermal Treatments of Chicken and Pig Meat and the Toxic Effects of Degradation Products of Oxytetracycline on Rats. J. Food Sci. Technol. 2015, 52, 2842–2850. [Google Scholar] [CrossRef]
- Xuan, R.; Arisi, L.; Wang, Q.; Yates, S.R.; Biswas, K.C. Hydrolysis and Photolysis of Oxytetracycline in Aqueous Solution. J. Environ. Sci. Health B 2009, 45, 73–81. [Google Scholar] [CrossRef]
- Furusawa, N.; Hanabusa, R. Cooking Effects on Sulfonamide Residues in Chicken Thigh Muscle. Food Res. Int. 2002, 35, 37–42. [Google Scholar] [CrossRef]
- Cooper, K.M.; Whelan, M.; Danaher, M.; Kennedy, D.G. Stability during Cooking of Anthelmintic Veterinary Drug Residues in Beef. Food Addit. Contam. A 2011, 28, 155–165. [Google Scholar] [CrossRef]
- Khan, A.A.; Randhawa, M.A.; Butt, M.S.; Nawaz, H. Impact of Various Processing Techniques on Dissipation Behavior of Antibiotic Residues in Poultry Meat: Dissipation Behavior of Antibiotic Residues. J. Food Process. Preserv. 2016, 40, 76–82. [Google Scholar] [CrossRef]
- Darwish, W.S.; Eldaly, E.A.; El-Abbasy, M.T.; Ikenaka, Y.; Nakayama, S.; Ishizuka, M. Antibiotic Residues in Food: The African Scenario. Jpn. J. Vet. Res. 2013, 61, 13–22. [Google Scholar]
- Moyane, J.N.; Jideani, A.I.O.; Aiyegoro, O.A. Antibiotics Usage in Food-Producing Animals in South Africa and Impact on Human: Antibiotic Resistance. Afr. J. Microbiol. Res. 2013, 7, 2990–2997. [Google Scholar] [CrossRef]
- Kjeldgaard, J.; Cohn, M.T.; Casey, P.G.; Hill, C.; Ingmer, H. Residual Antibiotics Disrupt Meat Fermentation and Increase Risk of Infection. mBio 2012, 3, e00190-12. [Google Scholar] [CrossRef]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic Resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Akova, M. Epidemiology of Antimicrobial Resistance in Bloodstream Infections. Virulence 2016, 7, 252–266. [Google Scholar] [CrossRef]
- Argudín, M.; Deplano, A.; Meghraoui, A.; Dodémont, M.; Heinrichs, A.; Denis, O.; Nonhoff, C.; Roisin, S. Bacteria from Animals as a Pool of Antimicrobial Resistance Genes. Antibiotics 2017, 6, 12. [Google Scholar] [CrossRef]
- Woolhouse, M.; Ward, M.; van Bunnik, B.; Farrar, J. Antimicrobial Resistance in Humans, Livestock and the Wider Environment. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140083. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.J. Review: Livestock Production Increasingly Influences Wildlife across the Globe. Animal 2018, 12, s372–s382. [Google Scholar] [CrossRef]
- Augustyńska-Prejsnar, A.; Ormian, M.; Sokołowicz, Z.; Topczewska, J.; Lechowska, J. Oddziaływanie Ferm Trzody Chlewnej i Drobiu na Środowisko. Proc. ECOpole 2018, 12, 117–129. [Google Scholar] [CrossRef]
- Zalewska, M.; Błażejewska, A.; Czapko, A.; Popowska, M. Antibiotics and Antibiotic Resistance Genes in Animal Manure—Consequences of Its Application in Agriculture. Front. Microbiol. 2021, 12, 610656. [Google Scholar] [CrossRef]
- Chef Veterinary Officer. 2019. Available online: https://www.wetgiw.gov.pl/main/komunikaty/komunikat-w-sprawie-opublikowanego-na-portalu-onet-artykulu-pt.-polskie-mieso-jest-pelne-antybiotykow.-zawiera-ich-coraz-wiecej/idn:1164 (accessed on 29 August 2024).
- Giedrojć-Brzana, U.; Kosek-Paszkowska, K.; Rudy, A. Problemy Inspekcji Weterynaryjnej przy nadzorowaniu stosowania antybiotyków w leczeniu zwierząt gospodarskich. Życie Weter. 2017, 92, 61–66. [Google Scholar]
- Cywińska, A.; Welz, M.; Konopka, B.; Witkowski, L. Regulacje prawne i zasady dobrej praktyki weterynaryjnej w stosowaniu leków przeciwdrobnoustrojowych u koni. Życie Weter 2020, 95, 704–710. [Google Scholar]
- Sharma, S.; Chauhan, A.; Ranjan, A.; Mathkor, D.M.; Haque, S.; Ramniwas, S.; Tuli, H.S.; Jindal, T.; Yadav, V. Emerging Challenges in Antimicrobial Resistance: Implications for Pathogenic Microorganisms, Novel Antibiotics, and Their Impact on Sustainability. Front. Microbiol. 2024, 15, 1403168. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic Resistance and Persistence—Implications for Human Health and Treatment Perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Olczak-Pieńkowska, A.; Hryniewicz, W. Impact of Social, Economic, and Healthcare Factors on the Regional Structure of Antibiotic Consumption in Primary Care in Poland (2013–2017). Front. Public Health 2021, 9, 680975. [Google Scholar] [CrossRef]
- Mąka, Ł.; Maćkiw, E.; Ścieżyńska, H.; Pawłowska, K.; Popowska, M. Antimicrobial Susceptibility of Salmonella Strains Isolated from Retail Meat Products in Poland between 2008 and 2012. Food Control 2014, 36, 199–204. [Google Scholar] [CrossRef]
- Yemeke, T.; Chen, H.-H.; Ozawa, S. Economic and Cost-Effectiveness Aspects of Vaccines in Combating Antibiotic Resistance. Hum. Vaccines Immunother. 2023, 19, 2215149. [Google Scholar] [CrossRef] [PubMed]
- Różańska, A.; Chmielarczyk, A.; Romaniszyn, D.; Bulanda, M.; Walkowicz, M.; Osuch, P.; Knych, T. Antibiotic Resistance, Ability to Form Biofilm and Susceptibility to Copper Alloys of Selected Staphylococcal Strains Isolated from Touch Surfaces in Polish Hospital Wards. Antimicrob. Resist. Infect. Control 2017, 6, 80. [Google Scholar] [CrossRef]
- Paramitadevi, Y.V.; Priadi, C.R.; Rahmatika, I.; Rukmana, A.; Moersidik, S.S. Integration of Water, Sanitation, and Hygiene Program with Biosecurity: A One Health Approach to Reduce the Prevalence and Exposure of Antibiotic-Resistant Bacteria in the Livestock Community. Int. J. One Health 2023, 9, 181–193. [Google Scholar] [CrossRef]
- Pieri, A.; Aschbacher, R.; Fasani, G.; Mariella, J.; Brusetti, L.; Pagani, E.; Sartelli, M.; Pagani, L. Country Income Is Only One of the Tiles: The Global Journey of Antimicrobial Resistance among Humans, Animals, and Environment. Antibiotics 2020, 9, 473. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.; Kang, M.; Yang, J.; Park, W. Gain and Loss of Antibiotic Resistant Genes in Multidrug Resistant Bacteria: One Health Perspective. J. Microbiol. 2021, 59, 535–545. [Google Scholar] [CrossRef]
- Andrzejewska, M.; Szczepańska, B.; Śpica, D.; Klawe, J.J. Prevalence, Virulence, and Antimicrobial Resistance of Campylobacter spp. in Raw Milk, Beef, and Pork Meat in Northern Poland. Foods 2019, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, K.; Osek, J. Characteristics and Antimicrobial Resistance of Campylobacter Isolated from Pig and Cattle Carcasses in Poland. Pol. J. Vet. Sci. 2013, 16, 501–508. [Google Scholar] [CrossRef]
- Rożynek, E.; Maćkiw, E.; Kamińska, W.; Tomczuk, K.; Antos-Bielska, M.; Dzierżanowska-Fangrat, K.; Korsak, D. Emergence of Macrolide-Resistant Campylobacter Strains in Chicken Meat in Poland and the Resistance Mechanisms Involved. Foodborne Pathog. Dis. 2013, 10, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, K.; Bocian, Ł.; Osek, J. Prevalence and Antimicrobial Resistance of Campylobacter Isolated from Carcasses of Chickens Slaughtered in Poland—A Retrospective Study. Food Control 2020, 112, 107159. [Google Scholar] [CrossRef]
- Wieczorek, K.; Osek, J. A Five-Year Study on Prevalence and Antimicrobial Resistance of Campylobacter from Poultry Carcasses in Poland. Food Microbiol. 2015, 49, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Woźniak-Biel, A.; Bugla-Płoskońska, G.; Kielsznia, A.; Korzekwa, K.; Tobiasz, A.; Korzeniowska-Kowal, A.; Wieliczko, A. High Prevalence of Resistance to Fluoroquinolones and Tetracycline Campylobacter Spp. Isolated from Poultry in Poland. Microb. Drug Resist. 2018, 24, 314–322. [Google Scholar] [CrossRef]
- Wysok, B.; Wojtacka, J.; Wiszniewska-Łaszczych, A.; Szteyn, J. Antimicrobial Resistance and Virulence Properties of Campylobacter spp. Originating from Domestic Geese in Poland. Animals 2020, 10, 742. [Google Scholar] [CrossRef]
- Maćkiw, E.; Korsak, D.; Rzewuska, K.; Tomczuk, K.; Rożynek, E. Antibiotic Resistance in Campylobacter jejuni and Campylobacter coli Isolated from Food in Poland. Food Control 2012, 23, 297–301. [Google Scholar] [CrossRef]
- Krupa, P.; Bystroń, J.; Bania, J.; Podkowik, M.; Empel, J.; Mroczkowska, A. Genotypes and Oxacillin Resistance of Staphylococcus Aureus from Chicken and Chicken Meat in Poland. Poult. Sci. 2014, 93, 3179–3186. [Google Scholar] [CrossRef] [PubMed]
- Krupa, P.; Bystroń, J.; Podkowik, M.; Empel, J.; Mroczkowska, A.; Bania, J. Population Structure and Oxacillin Resistance of Staphylococcus aureus from Pigs and Pork Meat in South-West of Poland. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; Nalepa, B.; Sierpińska, M.; Łaniewska-Trokenheim, Ł. Retail Ready-to-Eat Food as a Potential Vehicle for Staphylococcus spp. Harboring Antibiotic Resistance Genes. J. Food Prot. 2014, 77, 993–998. [Google Scholar] [CrossRef]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; Nalepa, B.; Sierpińska, M.; Łaniewska-Trokenheim, Ł. Coagulase-Negative Staphylococci (CoNS) Isolated from Ready-to-Eat Food of Animal Origin—Phenotypic and Genotypic Antibiotic Resistance. Food Microbiol. 2015, 46, 222–226. [Google Scholar] [CrossRef]
- Podkowik, M.; Bystroń, J.; Bania, J. Prevalence of Antibiotic Resistance Genes in Staphylococci Isolated from Ready-to-Eat Meat Products. Pol. J. Vet. Sci. 2012, 15, 233–237. [Google Scholar] [CrossRef]
- Pyzik, E.; Marek, A.; Stępień-Pyśniak, D.; Urban-Chmiel, R.; Jarosz, Ł.S.; Jagiełło-Podębska, I. Detection of Antibiotic Resistance and Classical Enterotoxin Genes in Coagulase -Negative Staphylococci Isolated from Poultry in Poland. J. Vet. Res. 2019, 63, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ławniczek-Wałczyk, A.; Cyprowski, M.; Górny, R.L. Distribution of Selected Drug-Resistant Enterococcus Species in Meat Plants in Poland. Rocz. Ochr. Śr. 2022, 24, 345–359. [Google Scholar] [CrossRef]
- Woźniak-Biel, A.; Bugla-Płoskońska, G.; Burdzy, J.; Korzekwa, K.; Ploch, S.; Wieliczko, A. Antimicrobial Resistance and Biofilm Formation in Enterococcus Spp. Isolated from Humans and Turkeys in Poland. Microb. Drug Resist. 2019, 25, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; Łaniewska-Trokenheim, Ł. Diversity of Antibiotic Resistance Genes in Enterococcus Strains Isolated from Ready-to-Eat Meat Products. J. Food Sci. 2016, 81, M2799–M2807. [Google Scholar] [CrossRef] [PubMed]
- Stępień-Pyśniak, D.; Marek, A.; Banach, T.; Adaszek, Ł.; Pyzik, E.; Wilczyński, J.; Winiarczyk, S. Prevalence and Antibiotic Resistance of Enterococcus Strains Isolated from Poultry. Acta Vet. Hung. 2016, 64, 148–163. [Google Scholar] [CrossRef]
- Kawacka, I.; Pietrzak, B.; Schmidt, M.; Olejnik-Schmidt, A. Listeria monocytogenes Isolates from Meat Products and Processing Environment in Poland Are Sensitive to Commonly Used Antibiotics, with Rare Cases of Reduced Sensitivity to Ciprofloxacin. Life 2023, 13, 821. [Google Scholar] [CrossRef]
- Kurpas, M.; Osek, J.; Moura, A.; Leclercq, A.; Lecuit, M.; Wieczorek, K. Genomic Characterization of Listeria monocytogenes Isolated from Ready-to-Eat Meat and Meat Processing Environments in Poland. Front. Microbiol. 2020, 11, 1412. [Google Scholar] [CrossRef]
- Maćkiw, E.; Stasiak, M.; Kowalska, J.; Kucharek, K.; Korsak, D.; Postupolski, J. Occurrence and Characteristics of Listeria monocytogenes in Ready-to-Eat Meat Products in Poland. J. Food Prot. 2020, 83, 1002–1009. [Google Scholar] [CrossRef]
- Skowron, K.; Wałecka-Zacharska, E.; Wiktorczyk-Kapischke, N.; Skowron, K.J.; Grudlewska-Buda, K.; Bauza-Kaszewska, J.; Bernaciak, Z.; Borkowski, M.; Gospodarek-Komkowska, E. Assessment of the Prevalence and Drug Susceptibility of Listeria monocytogenes Strains Isolated from Various Types of Meat. Foods 2020, 9, 1293. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Zakrzewski, A.J.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Antimicrobial Resistance and Virulence Characterization of Listeria monocytogenes Strains Isolated from Food and Food Processing Environments. Pathogens 2022, 11, 1099. [Google Scholar] [CrossRef]
- Szewczyk, M.; Czuba, Z.; Wiczkowski, A.; Hajdrowska, B. Antybiotykooporność Izolowanych z Żywności Bakterii z Rodziny Enterobacteriaceae. Vet. Med. 2019, 75, 553–557. [Google Scholar] [CrossRef]
- Sarowska, J.; Olszak, T.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Futoma-Koloch, B.; Gawel, A.; Drulis-Kawa, Z.; Choroszy-Krol, I. Comparative Characteristics and Pathogenic Potential of Escherichia Coli Isolates Originating from Poultry Farms, Retail Meat, and Human Urinary Tract Infection. Life 2022, 12, 845. [Google Scholar] [CrossRef] [PubMed]
- Zarzecka, U.; Chajęcka-Wierzchowska, W.; Zadernowska, A. Occurrence of Antibiotic Resistance among Enterobacterales Isolated from Raw and Ready-to-Eat Food—Phenotypic and Genotypic Characteristics. Int. J. Environ. Health Res. 2022, 32, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Mąka, Ł.; Maćkiw, E.; Ścieżyńska, H.; Popowska, M. Occurrence and Antimicrobial Resistance of Salmonella spp. Isolated from Food Other than Meat in Poland. Ann. Agric. Environ. Med. 2015, 22, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Pławińska-Czarnak, J.; Wódz, K.; Kizerwetter-Świda, M.; Bogdan, J.; Kwieciński, P.; Nowak, T.; Strzałkowska, Z.; Anusz, K. Multi-Drug Resistance to Salmonella Spp. When Isolated from Raw Meat Products. Antibiotics 2022, 11, 876. [Google Scholar] [CrossRef]
- Acke, E.; Carroll, C.; O’Leary, A.; McGill, K.; Kelly, L.; Lawlor, A.; Madden, R.H.; Moran, L.; Scates, P.; McNamara, E.; et al. Genotypic Characterisation and Cluster Analysis of Campylobacter jejuniisolates from Domestic Pets, Human Clinical Cases and Retail Food. Ir. Vet. J. 2011, 64, 6. [Google Scholar] [CrossRef]
- Deckert, A.E.; Reid-Smith, R.J.; Tamblyn, S.; Morrell, L.; Seliske, P.; Jamieson, F.B.; Irwin, R.; Dewey, C.E.; Boerlin, P.; McEWEN, S.A. Burden of Illness and Factors Associated with Duration of Illness in Clinical Campylobacteriosis. Epidemiol. Infect. 2013, 141, 2536–2546. [Google Scholar] [CrossRef]
- Tresse, O.; Alvarez-Ordóñez, A.; Connerton, I.F. Editorial: About the Foodborne Pathogen Campylobacter. Front. Microbiol. 2017, 8, 1908. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Sahin, O.; Kassem, I.I.; Shen, Z.; Lin, J.; Rajashekara, G.; Zhang, Q. Campylobacter in Poultry: Ecology and Potential Interventions. Avian Dis. 2015, 59, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion on Quantification of the Risk Posed by Broiler Meat to Human Campylobacteriosis in the EU. EFSA J. 2010, 8, 1437. [CrossRef]
- Damjanova, I.; Jakab, M.; Farkas, T.; Mészáros, J.; Galántai, Z.; Turcsányi, I.; Bistyák, A.; Juhász, Á.; Pászti, J.; Kiss, I.; et al. From Farm to Fork Follow-up of Thermotolerant Campylobacters throughout the Broiler Production Chain and in Human Cases in a Hungarian County during a Ten-Months Period. Int. J. Food Microbiol. 2011, 150, 95–102. [Google Scholar] [CrossRef]
- Ge, B.; Wang, F.; Sjölund-Karlsson, M.; McDermott, P.F. Antimicrobial Resistance in Campylobacter: Susceptibility Testing Methods and Resistance Trends. J. Microbiol. Methods 2013, 95, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Acheson, D.; Allos, B.M. Campylobacter jejuni Infections: Update on Emerging Issues and Trends. Clin. Infect. Dis. 2001, 32, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Migura, L.; Hendriksen, R.S.; Fraile, L.; Aarestrup, F.M. Antimicrobial Resistance of Zoonotic and Commensal Bacteria in Europe: The Missing Link between Consumption and Resistance in Veterinary Medicine. Vet. Microbiol. 2014, 170, 1–9. [Google Scholar] [CrossRef]
- Korsak, D.; Maćkiw, E.; Rożynek, E.; Żyłowska, M. Prevalence of Campylobacter spp. in Retail Chicken, Turkey, Pork, and Beef Meat in Poland between 2009 and 2013. J. Food Prot. 2015, 78, 1024–1028. [Google Scholar] [CrossRef]
- Wieczorek, K.; Osek, J. Antimicrobial Resistance and Genotypes of Campylobacter Jejuni from Pig and Cattle Carcasses Isolated in Poland During 2009–2016. Microb. Drug Resist. 2018, 24, 680–684. [Google Scholar] [CrossRef]
- Jamali, H.; Ghaderpour, A.; Radmehr, B.; Chuan Wei, K.S.; Chai, L.C.; Ismail, S. Prevalence and Antimicrobial Resistance of Campylobacter Species Isolates in Ducks and Geese. Food Control 2015, 50, 328–330. [Google Scholar] [CrossRef]
- Mencía-Gutiérrez, A.; Martín-Maldonado, B.; Pastor-Tiburón, N.; Moraleda, V.; González, F.; García-Peña, F.J.; Pérez-Cobo, I.; Revuelta, L.; Marín, M. Prevalence and Antimicrobial Resistance of Campylobacter from Wild Birds of Prey in Spain. Comp. Immunol. Microbiol. Infect. Dis. 2021, 79, 101712. [Google Scholar] [CrossRef] [PubMed]
- Fijałkowski, K.; Peitler, D.; Karakulska, J. Staphylococci Isolated from Ready-to-Eat Meat—Identification, Antibiotic Resistance and Toxin Gene Profile. Int. J. Food Microbiol. 2016, 238, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Osman, K.M.; Abd El-Razik, K.A.; Marie, H.S.H.; Arafa, A. Coagulase-Negative Staphylococci Collected from Bovine Milk: Species and Antimicrobial Gene Diversity. J. Food Saf. 2016, 36, 89–99. [Google Scholar] [CrossRef]
- Savariraj, W.R.; Ravindran, N.B.; Kannan, P.; Paramasivam, R.; Senthilkumar, T.; Kumarasamy, P.; Rao, V.A. Prevalence, Antimicrobial Susceptibility and Virulence Genes of Staphylococcus aureus Isolated from Pork Meat in Retail Outlets in India. J. Food Saf. 2019, 39, e12589. [Google Scholar] [CrossRef]
- Sudarmadi, A.A.M.; Prajitno, S.; Widodo, A.D.W. Antibiotic Resistance in Escherichia coli and Staphylococcus aureus from Retail Chicken Meat in Surabaya, Indonesia. Biomol. Health Sci. J. 2020, 3, 80. [Google Scholar] [CrossRef]
- von Eiff, C.; Peters, G.; Heilmann, C. Pathogenesis of Infections Due to Coagulasenegative Staphylococci. Lancet Infect. Dis. 2002, 2, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Witte, W. Antibiotic Resistance in Gram-Positive Bacteria: Epidemiological Aspects. J. Antimicrob. Chemother. 1999, 44, 1–9. [Google Scholar] [CrossRef]
- Zarzecka, U.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Starter Cultures as a Reservoir of Antibiotic Resistant Microorganisms. LWT 2020, 127, 109424. [Google Scholar] [CrossRef]
- Braga, J.F.V.; Leal, C.A.G.; Silva, C.C.; Fernandes, A.A.; Martins, N.R.D.S.; Ecco, R. Genetic Diversity and Antimicrobial Resistance Profile of Enterococcus Faecalis Isolated from Broilers with Vertebral Osteomyelitis in Southeast Brazil. Avian Pathol. 2018, 47, 14–22. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. External Quality Assessment of Laboratory Performance: European Antimicrobial Resistance Surveillance Network (EARSNet). 2017. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/EQA-EARS-Net.pdf (accessed on 12 September 2024).
- Heuer, O.E.; Pedersen, K.; Jensen, L.B.; Madsen, M.; Olsen, J.E. Persistence of Vancomycin-Resistant Enterococci (VRE) in Broiler Houses after the Avoparcin Ban. Microb. Drug Resist. 2002, 8, 355–361. [Google Scholar] [CrossRef]
- de Niederhäusern, S.; Sabia, C.; Messi, P.; Guerrieri, E.; Manicardi, G.; Bondi, M. VanA-Type Vancomycin-Resistant Enterococci in Equine and Swine Rectal Swabs and in Human Clinical Samples. Curr. Microbiol. 2007, 55, 240–246. [Google Scholar] [CrossRef]
- Aksono, E.B.; Riwu, K.H.P.; Estoepangestie, A.T.S.; Pertiwi, H. Phylogenetic Analysis and Antibiotics Resistance of Listeria monocytogenes Contaminating Chicken Meat in Surabaya, Indonesia. Vet. Med. Int. 2020, 2020, 9761812. [Google Scholar] [CrossRef] [PubMed]
- Gómez, D.; Azón, E.; Marco, N.; Carramiñana, J.J.; Rota, C.; Ariño, A.; Yangüela, J. Antimicrobial Resistance of Listeria monocytogenes and Listeria innocua from Meat Products and Meat-Processing Environment. Food Microbiol. 2014, 42, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Laorden, A.; Arraiz-Fernandez, C.; Cantalejo, M.J.; Gonzalez-Fandos, E. Prevalence, Identification and Antimicrobial Resistance of Listeria monocytogenes and Listeria spp. Isolated from Poultry and Pork Meat. Int. J. Food Sci. Technol. 2024, 59, 2667–2675. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Chajęcka-Wierzchowska, W.; Zadernowska, A. High-Pressure Processing—Impacts on the Virulence and Antibiotic Resistance of Listeria monocytogenes Isolated from Food and Food Processing Environments. Foods 2023, 12, 3899. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Monden, S.; Suzuki, H.; Nakama, A.; Ida, M.; Igimi, S. Antimicrobial Susceptibilities of Listeria monocytogenes Isolated from the Imported and the Domestic Foods in Japan. J. Food Nutr. Sci. 2015, 3, 70. [Google Scholar] [CrossRef][Green Version]
- Wiśniewski, P.; Chajęcka-Wierzchowska, W.; Zadernowska, A. Impact of High-Pressure Processing (HPP) on Listeria monocytogenes—An Overview of Challenges and Responses. Foods 2023, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority, European Centre for Disease Prevention and Control. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, e06971. [Google Scholar] [CrossRef]
- Gutema, F.D.; Agga, G.E.; Abdi, R.D.; De Zutter, L.; Duchateau, L.; Gabriël, S. Prevalence and Serotype Diversity of Salmonella in Apparently Healthy Cattle: Systematic Review and Meta-Analysis of Published Studies, 2000–2017. Front. Vet. Sci. 2019, 6, 102. [Google Scholar] [CrossRef]
- European Food Safety Authority, European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2019–2020. EFSA J. 2022, 20, e07209. [Google Scholar] [CrossRef]
- Lai, J.; Wu, C.; Wu, C.; Qi, J.; Wang, Y.; Wang, H.; Liu, Y.; Shen, J. Serotype Distribution and Antibiotic Resistance of Salmonella in Food-Producing Animals in Shandong Province of China, 2009 and 2012. Int. J. Food Microbiol. 2014, 180, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Barilli, E.; Bacci, C.; Villa, Z.S.; Merialdi, G.; D’Incau, M.; Brindani, F.; Vismarra, A. Antimicrobial Resistance, Biofilm Synthesis and Virulence Genes in Salmonella Isolated from Pigs Bred on Intensive Farms. Ital. J. Food Saf. 2018, 7, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.; Mourão, J.; Peixe, L.; Antunes, P. Non-Typhoidal Salmonella in the Pig Production Chain: A Comprehensive Analysis of Its Impact on Human Health. Pathogens 2019, 8, 19. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Q.; Zhang, J.; Huang, J.; Chen, L.; Wu, S.; Zeng, H.; Wang, J.; Chen, M.; Wu, H.; et al. Prevalence, Bacterial Load, and Antimicrobial Resistance of Salmonella Serovars Isolated from Retail Meat and Meat Products in China. Front. Microbiol. 2019, 10, 2121. [Google Scholar] [CrossRef] [PubMed]
- Commission Implementing Decision of 12 November 2013 on the Monitoring and Reporting of Antimicrobial Resistance in Zoonotic and Commensal Bacteria (Notified Under Document C(2013) 7145) Text with EEA Relevance. Available online: http://data.europa.eu/eli/dec_impl/2013/652/oj (accessed on 12 October 2024).
- Svircev, A.; Roach, D.; Castle, A. Framing the Future with Bacteriophages in Agriculture. Viruses 2018, 10, 218. [Google Scholar] [CrossRef]
- Rehman, A.; Arif, M.; Sajjad, N.; Al-Ghadi, M.Q.; Alagawany, M.; Abd El-Hack, M.E.; Alhimaidi, A.R.; Elnesr, S.S.; Almutairi, B.O.; Amran, R.A.; et al. Dietary Effect of Probiotics and Prebiotics on Broiler Performance, Carcass, and Immunity. Poult. Sci. 2020, 99, 6946–6953. [Google Scholar] [CrossRef] [PubMed]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, A.S.M.R.U.; Jahid, I.K. Characterization and Evaluation of Lactic Acid Bacteria from Indigenous Raw Milk for Potential Probiotic Properties. J. Dairy Sci. 2020, 103, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Niemiałtowski, M.; Schollenberger, A.; Kluciński, W. Chapter 13 Mucosal Immunity and the Bovine Entero-Mammary Link: Evolutionary Established Dialogue between Antigen and Arms of Immune System. In Biology of Growing Animals; Elsevier: Amsterdam, The Netherlands, 2005; Volume 2, pp. 293–313. ISBN 978-0-444-50926-0. [Google Scholar]
- Arowolo, M.A.; He, J. Use of Probiotics and Botanical Extracts to Improve Ruminant Production in the Tropics: A Review. Anim. Nutr. 2018, 4, 241–249. [Google Scholar] [CrossRef]
- Al-Sagheer, A.A.; El-Hack, M.E.A.; Alagawany, M.; Naiel, M.A.; Mahgoub, S.A.; Badr, M.M.; Hussein, E.O.S.; Alowaimer, A.N.; Swelum, A.A. Paulownia Leaves as A New Feed Resource: Chemical Composition and Effects on Growth, Carcasses, Digestibility, Blood Biochemistry, and Intestinal Bacterial Populations of Growing Rabbits. Animals 2019, 9, 95. [Google Scholar] [CrossRef]
- Xiang, Q.; Wang, C.; Zhang, H.; Lai, W.; Wei, H.; Peng, J. Effects of Different Probiotics on Laying Performance, Egg Quality, Oxidative Status, and Gut Health in Laying Hens. Animals 2019, 9, 1110. [Google Scholar] [CrossRef]
- Soomro, R.N.; El-Hack, M.E.A.; Shah, S.S.; Taha, A.E.; Alagawany, M.; Swelum, A.A.; Hussein, E.O.S.; Ba-Aawdh, H.A.; Saadeldin, I.; El-Edel, M.A.; et al. Impact of Restricting Feed and Probiotic Supplementation on Growth Performance, Mortality and Carcass Traits of Meat-type Quails. Anim. Sci. J. 2019, 90, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Reuben, R.C.; Sarkar, S.L.; Ibnat, H.; Roy, P.C.; Jahid, I.K. Novel Mono- and Multi-strain Probiotics Supplementation Modulates Growth, Intestinal Microflora Composition and Haemato-biochemical Parameters in Broiler Chickens. Vet. Med. Sci. 2022, 8, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Reuben, R.C.; Sarkar, S.L.; Ibnat, H.; Setu, A.A.; Roy, P.C.; Jahid, I.K. Novel Multi-Strain Probiotics Reduces Pasteurella Multocida Induced Fowl Cholera Mortality in Broilers. Sci. Rep. 2021, 11, 8885. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Hao, H.; Xie, S.; Wang, X.; Dai, M.; Huang, L.; Yuan, Z. Antibiotic Alternatives: The Substitution of Antibiotics in Animal Husbandry? Front. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef]
- Low, C.X.; Tan, L.T.-H.; Mutalib, N.-S.A.; Pusparajah, P.; Goh, B.-H.; Chan, K.-G.; Letchumanan, V.; Lee, L.-H. Unveiling the Impact of Antibiotics and Alternative Methods for Animal Husbandry: A Review. Antibiotics 2021, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Srivastava, A.; Lall, R. Nutraceuticals in Veterinary Medicine; Springer: Cham, Switzerland, 2019; ISBN 978-3-030-04623-1. [Google Scholar]
- Śmiałek, M.; Burchardt, S.; Koncicki, A. The Influence of Probiotic Supplementation in Broiler Chickens on Population and Carcass Contamination with Campylobacter spp.—Field Study. Res. Vet. Sci. 2018, 118, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Alomari, M.M.M.; Dec, M.; Urban-Chmiel, R. Bacteriophages as an Alternative Method for Control of Zoonotic and Foodborne Pathogens. Viruses 2021, 13, 2348. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.Y.K.; Morales, S.; Okamoto, Y.; Chan, H.-K. Topical Application of Bacteriophages for Treatment of Wound Infections. Transl. Res. 2020, 220, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hong, Q.; Chang, R.Y.K.; Kwok, P.C.L.; Chan, H.-K. Phage–Antibiotic Therapy as a Promising Strategy to Combat Multidrug-Resistant Infections and to Enhance Antimicrobial Efficiency. Antibiotics 2022, 11, 570. [Google Scholar] [CrossRef] [PubMed]
- Ragupathi, N.K.D.; Sethuvel, D.P.M.; Gopikrishnan, M.; Dwarakanathan, H.T.; Murugan, D.; Biswas, I.; Bakthavachalam, Y.D.; Murugesan, M.; Doss, C.G.P.; Monk, P.N.; et al. Phage-Based Therapy against Biofilm Producers in Gram-Negative ESKAPE Pathogens. Microb. Pathog. 2023, 178, 106064. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Loessner, M.J. Beyond Antibacterials—Exploring Bacteriophages as Antivirulence Agents. Curr. Opin. Biotechnol. 2021, 68, 166–173. [Google Scholar] [CrossRef]
- Chung, K.M.; Nang, S.C.; Tang, S.S. The Safety of Bacteriophages in Treatment of Diseases Caused by Multidrug-Resistant Bacteria. Pharmaceuticals 2023, 16, 1347. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Fekrazad, R.; Zhang, L.; Jiang, X.; He, G.; Wen, X. Polyphenolic Natural Products as Photosensitizers for Antimicrobial Photodynamic Therapy: Recent Advances and Future Prospects. Front. Immunol. 2023, 14, 1275859. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; El-Hossary, E.M.; Oelschlaeger, T.A.; Donia, M.S.; Quinn, R.J.; Abdelmohsen, U.R. Potential of Marine Natural Products against Drug-Resistant Bacterial Infections. Lancet Infect. Dis. 2019, 19, e237–e245. [Google Scholar] [CrossRef]
- Gao, J.; Yang, Z.; Zhao, C.; Tang, X.; Jiang, Q.; Yin, Y. A Comprehensive Review on Natural Phenolic Compounds as Alternatives to In-Feed Antibiotics. Sci. China Life Sci. 2023, 66, 1518–1534. [Google Scholar] [CrossRef]
- Tiwari, P.; Mishra, R.; Mazumder, A.; Mazumder, R.; Singh, A. An Insight into Diverse Activities and Targets of Flavonoids. Curr. Drug Targets 2023, 24, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Setzer, M.; Byler, K.; Ogungbe, I.; Setzer, W. Natural Products as New Treatment Options for Trichomoniasis: A Molecular Docking Investigation. Sci. Pharm. 2017, 85, 5. [Google Scholar] [CrossRef] [PubMed]
- Teneva, D.; Denev, P. Biologically Active Compounds from Probiotic Microorganisms and Plant Extracts Used as Biopreservatives. Microorganisms 2023, 11, 1896. [Google Scholar] [CrossRef] [PubMed]
- Luong, H.X.; Ngan, H.D.; Thi Phuong, H.B.; Quoc, T.N.; Tung, T.T. Multiple Roles of Ribosomal Antimicrobial Peptides in Tackling Global Antimicrobial Resistance. R. Soc. Open Sci. 2022, 9, 211583. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, Z.; Li, S.; Liu, T.; Song, J.; Li, J.; Zhang, X. Two Antimicrobial Peptides Derived from Bacillus and Their Properties. Molecules 2023, 28, 7899. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Shady, N.H.; Abdeljawad, K.M.; Elzamkan, M.B.; Helmy, H.H.; Tarshan, E.A.; Adly, A.N.; Hussien, Y.H.; Sayed, N.G.; Zayed, A.; et al. Antimicrobial Potentials of Natural Products against Multidrug Resistance Pathogens: A Comprehensive Review. RSC Adv. 2022, 12, 29078–29102. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Matuła, K.; Karoń, S.; Paczesny, J. Resistance and Adaptation of Bacteria to Non-Antibiotic Antibacterial Agents: Physical Stressors, Nanoparticles, and Bacteriophages. Antibiotics 2021, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Śmiałek, M.; Kowalczyk, J.; Koncicki, A. Influence of Vaccination of Broiler Chickens against Escherichia coli with Live Attenuated Vaccine on General Properties of E. coli Population, IBV Vaccination Efficiency, and Production Parameters—A Field Experiment. Poult. Sci. 2020, 99, 5452–5460. [Google Scholar] [CrossRef] [PubMed]
- Hemeg, H. Nanomaterials for Alternative Antibacterial Therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef]
- Joost, U.; Juganson, K.; Visnapuu, M.; Mortimer, M.; Kahru, A.; Nõmmiste, E.; Joost, U.; Kisand, V.; Ivask, A. Photocatalytic Antibacterial Activity of Nano-TiO2 (Anatase)-Based Thin Films: Effects on Escherichia Coli Cells and Fatty Acids. J. Photochem. Photobiol. B 2015, 142, 178–185. [Google Scholar] [CrossRef]
- Reddy, L.S.; Nisha, M.M.; Joice, M.; Shilpa, P.N. Antimicrobial Activity of Zinc Oxide (ZnO) Nanoparticle against Klebsiella pneumoniae. Pharm. Biol. 2014, 52, 1388–1397. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic Disinfection Using Titanium Dioxide: Spectrum and Mechanism of Antimicrobial Activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Umamaheswari, K.; Baskar, R.; Chandru, K.; Rajendiran, N.; Chandirasekar, S. Antibacterial Activity of Gold Nanoparticles and Their Toxicity Assessment. BMC Infect. Dis. 2014, 14, P64. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A New Paradigm for Treating Infectious Diseases Using Nanomaterials in the Antibiotics Resistant Era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Saha, B.; Bhattacharya, J.; Mukherjee, A.; Ghosh, A.; Santra, C.; Dasgupta, A.K.; Karmakar, P. In Vitro Structural and Functional Evaluation of Gold Nanoparticles Conjugated Antibiotics. Nanoscale Res. Lett. 2007, 2, 614. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Girilal, M.; Mahdy, S.A.; Somsundar, S.S.; Venkatesan, R.; Kalaichelvan, P.T. Vancomycin Bound Biogenic Gold Nanoparticles: A Different Perspective for Development of Anti VRSA Agents. Process Biochem. 2011, 46, 636–641. [Google Scholar] [CrossRef]
- Baker, S.; Pasha, A.; Satish, S. Biogenic Nanoparticles Bearing Antibacterial Activity and Their Synergistic Effect with Broad Spectrum Antibiotics: Emerging Strategy to Combat Drug Resistant Pathogens. Saudi Pharm. J. 2017, 25, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, A.; Tahami, S.; Naji, M. Synthesis and Characterization of Core-Shell Bimetallic Nanoparticles for Synergistic Antimicrobial Effect Studies in Combination with Doxycycline on Burn Specific Pathogens. J. Photochem. Photobiol. B 2017, 169, 21–26. [Google Scholar] [CrossRef]
- Chopade, B.A.; Singh, R.; Nawale, L.; Arkile, M.; Wadhwani, S.; Shedbalkar, U.; Chopade, S.; Sarkar, D. Phytogenic Silver, Gold, and Bimetallic Nanoparticles as Novel Antitubercular Agents. Int. J. Nanomed. 2016, 11, 1889–1897. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Processing | Product Type | Parameters | Antibiotic | Initial Value [μg/kg] | Value After Heat Treatment [μg/kg] | Reduction [%] | Reference |
|---|---|---|---|---|---|---|---|
| Boiling | Chicken | 100 °C/5 min | ENO | 746.34 ± 5.62 | 237.53 ± 2.13 | 68.17 | [46] |
| OTC | 824.16 ± 7.20 | 383.33 ± 3.70 | 53.49 | ||||
| DOX | 680.84 ± 8.84 | 425.53 ± 5.65 | 37.50 | ||||
| CIP | 643.14 ± 6.97 | 205.46 ± 9.72 | 68.05 | ||||
| 100 °C/2 min | TET | 100,000 | 43,898 ± 2362 | 56.10 | [40] | ||
| Pork | 42,332 ± 2881 | 57.67 | |||||
| Chicken | 100 °C/3 min | OTC | 500 | 365.95 ± 6.84 | 26.81 | [42] | |
| 100 °C/6 min | 325.71 ± 4.92 | 34.86 | |||||
| 100 °C/15 min | 249.13 ± 4.89 | 50.17 | |||||
| Pork | 100 °C/3 min | 360.09 ± 3.65 | 27.98 | ||||
| 100 °C/6 min | 317.08 ± 4.17 | 36.58 | |||||
| 100 °C/15 min | 236.56 ± 7.96 | 52.69 | |||||
| Chicken | 100 °C/3 min | SDZ | 84 | 50 | 40.48 | [44] | |
| SMX | 252 | 172 | 31.75 | ||||
| SMM | 436 | 315 | 27.75 | ||||
| SQ | 970 | 669 | 31.03 | ||||
| 100 °C/6 min | SDZ | 84 | 41 | 51.19 | |||
| SMX | 252 | 145 | 42.46 | ||||
| SMM | 436 | 274 | 37.16 | ||||
| SQ | 970 | 584 | 39.79 | ||||
| 100 °C/9 min | SDZ | 84 | 34 | 59.52 | |||
| SMX | 252 | 129 | 48.81 | ||||
| SMM | 436 | 248 | 43.12 | ||||
| SQ | 970 | 539 | 44.43 | ||||
| 100 °C/12 min | SDZ | 84 | 33 | 60.71 | |||
| SMX | 252 | 117 | 53.57 | ||||
| SMM | 436 | 239 | 45.18 | ||||
| SQ | 970 | 518 | 46.60 | ||||
| Roasting | Chicken | 200 °C/30 min | ENO | 746.34 ± 5.62 | 233.23 ± 10.19 | 68.75 | [46] |
| OTC | 824.16 ± 7.20 | 274.72 ± 3.40 | 66.67 | ||||
| DOX | 680.84 ± 8.84 | 340.42 ± 4.92 | 50.00 | ||||
| CIP | 643.14 ± 6.97 | 200.98 ± 10.02 | 68.75 | ||||
| 170 °C/3 min | SDZ | 82 | 77 | 6.10 | [44] | ||
| SMX | 322 | 301 | 6.52 | ||||
| SMM | 560 | 462 | 17.50 | ||||
| SQ | 1145 | 1005 | 12.23 | ||||
| 170 °C/6 min | SDZ | 82 | 80 | 2.44 | |||
| SMX | 322 | 271 | 15.84 | ||||
| SMM | 560 | 420 | 25.00 | ||||
| SQ | 1145 | 897 | 21.66 | ||||
| 170 °C/9 min | SDZ | 82 | 80 | 2.44 | |||
| SMX | 322 | 255 | 20.81 | ||||
| SMM | 560 | 400 | 28.57 | ||||
| SQ | 1145 | 851 | 25.68 | ||||
| 170 °C/12 min | SDZ | 82 | 79 | 3.66 | |||
| SMX | 322 | 198 | 38.51 | ||||
| SMM | 560 | 337 | 39.82 | ||||
| SQ | 1145 | 713 | 37.73 | ||||
| Microwave cooking | Chicken | 900 W/3 min | ENO | 746.34 ± 5.62 | 334.68 ± 3.63 | 55.16 | [46] |
| OTC | 824.16 ± 7.20 | 227.67 ± 2.10 | 72.38 | ||||
| DOX | 680.84 ± 8.84 | 544.67 ± 6.67 | 20.00 | ||||
| CIP | 643.14 ± 6.97 | 288.40 ± 3.23 | 55.16 | ||||
| 440 W/0.75 min | TET | 100,000 | 40,111 ± 13,979 | 59.89 | [40] | ||
| Pork | 19,463 ± 2652 | 80.54 | |||||
| Chicken | 800 W/0.5 min | OTC | 500 | 342.18 ± 5.32 | 31.56 | [42] | |
| 800 W/1 min | 275.69 ± 3.21 | 44.86 | |||||
| 800 W/2 min | 223.56 ± 4.45 | 55.29 | |||||
| Pork | 800 W/0.5 min | 355.82 ± 1.71 | 28.84 | ||||
| 800 W/1 min | 309.07 ± 0.72 | 38.19 | |||||
| 800 W/2 min | 204.75 ± 1.17 | 59.05 | |||||
| Grilling | Chicken | 8 kW/2.5 min | ENO | 746.34 ± 5.62 | 497.56 ± 4.75 | 33.33 | [46] |
| OTC | 824.16 ± 7.20 | 686.80 ± 6.50 | 16.67 | ||||
| DOX | 680.84 ± 8.84 | 567.37 ± 6.20 | 16.66 | ||||
| CIP | 643.14 ± 6.97 | 535.95 ± 5.31 | 16.67 |
| No. | Microorganisms | Product Type | Antimicrobial Resistance | References | ||
|---|---|---|---|---|---|---|
| Methods for Detecting Antimicrobial Resistance | Antimicrobial Resistance Tested | Resistance Genes Tested | ||||
| 1 | C. jejuni, C. coli | Beef and pork (raw meat) | Disk diffusion | CIP, E, CN, TET, AZM | NA | [69] |
| 2 | Bovine and pork carcasses | Microbroth dilution | CN, C, NAL, CIP, STR, E, TET | [70] | ||
| 3 | Raw chicken meat (wings, legs, carcass frames, filets, and ground meat) and offal (livers, hearts, and gizzards) | Disk diffusion | TET, CIP, E | [71] | ||
| 4 | Chicken broiler carcasses | E, CIP, CN, NAL, STR, TET | [72] | |||
| 5 | Poultry broiler carcasses | Microbroth dilution | CIP, TET, E | [73] | ||
| 6 | Turkey and broiler carcasses | Microbroth dilution and PCR assay | AZM, CIP, E, CN, TET, FLR, NAL, TEL, DA | gyrA, tetO, cmeB | [74] | |
| 7 | Domestic geese | Disk diffusion | E, CN, CIP, AMP, TET, C, NAL | NA | [75] | |
| 8 | C. jejuni, C. coli, Campylobacter spp. | Raw chicken meat from wings, legs, corpuses, filets, ground meat, and offal samples (livers, hearts, and gizzards) | Disk diffusion and PCR assay | CIP, TET, E, CN | gyrA, tetO | [76] |
| 9 | S. aureus | Chicken meat samples (legs and wings) | P, CE, TET, DA, CN, E, OXA | mecA, mecC, blaZ | [77] | |
| 10 | Samples of pork meat from company shops | P, TET, DA, CN, E, CIP, NOR, VA | blaZ | [78] | ||
| 11 | S. aureus, S. xylosus | Cured meat | CE, TGC, QD, DA, TET, CN, RD, CIP, W, SXT | mec(A), tet(L), tet(M), tet(K) | [79,80] | |
| 12 | S. aureus, CNS (S. xylosus, S. epidermidis, S. xylosus), Staphylococcus spp. | Sausage | DA, CE, F, TGC, SXT, C, RD, CN, LZD, E, NOR, W, CIP, QD, TET | |||
| 13 | S. epidermidis | Poultry | DA, CE, LZD, QD | mec(A), tet(M), tet(K) | ||
| 14 | S. aureus, CNS (S. epidermidis, S. pasteuri, S. haemolyticus, S. carnosus, S. saprophyticus, S. sciuri, S. chromogenes, S. capitis, S. xylosus, S. equorum, S. lugdunensis) | Ready-to-eat meat products | OXA, P, TET, E, CN, VA | mecA, blaZ, tetO/K/M, ermA/B/C, aph, vanA/B/C/D | [81] | |
| 15 | CNS (S. cohnii, S. epidermidis, S. haemolyticus, S. hominis, S. simulans, S. saprophyticus, S. lentus, S. xylosus, S. sciuri, S. chromogenes) | Broiler chickens and turkeys | AMX/CL, AMX, AMP, P, CE, DA, C, E, CN, TET, SXT | blaZ, mecA, aac(6′)-aph(2″), ermA, ermB, msrA/B, tetM, tetK, tetL, tetO, cfr | [82] | |
| 16 | E. faecalis, E. faecium | Raw pork meat | E, TET, VA | ermB, vanA, vanB | [83] | |
| 17 | E. faecalis, E. faceium, E. gallinarum | Turkeys | Disk diffusion and Multiplex PCR assay | AMP, AMX/CL, VA, CIP, TET, E, CN | blaZ, vanA, vanB, vanC-1, tetK, tetM, tetO, ermA, ermB, ermC, aac(6′)Ie-aph(2″)Ia | [84] |
| 18 | E. faecalis, E. faecium, E. casseliflavus, E. durans, E. hirae, Enterococcus spp. | Ready-to-eat meat products: smoked meat (ham, shoulder, bacon, and tenderloin); sausages (simmered and boiled); offal products (liver sausage, blood sausage, and brawn); formed meat products; tinned products (meat, offal products, and terrine) | Disk diffusion and PCR assay | AMP, PM, CN, STR, TEC, NOR, LEV, CIP, TET, TGC, RD, F, LZD, FOS, C, QD, E | aac(6′)-le-aph (2‴)-la, aph (2″)-Ib, aph(2‴)-1c, aph (2″) -Id, aph(3″)-Illa, ant (4′)-la, ant (6′)-la, tetM, tetL, tetK, tetO, ermA, ermB, ermC, msrC, mefA/E, vanC2/C3 | [85] |
| 19 | E. cecorum, E. faecalis, E. faecium, E. hirae, E. gallinarum, E. casseliflavus, E. avium, E. columbae | Hearts, livers, brains, bone marrow, and oviduct swabs from poultry | Disk diffusion | VA, AMX, AMX/CL, DOX, E, FLR, LIN/SP, TY, SXT | NA | [86] |
| 20 | L. monocytogenes | Meat food samples (raw and processed) and meat processing environment (both contacting and non-contacting with food) | AMP, C, E, CN, P, STR, SXT, TET, VA, CIP | [87] | ||
| 21 | Different kinds of ready-to-eat (RTE) foods of animal origin (e.g., ham, sausages, or meat) | Genotypic data–BIGSdb-Lm platform (Institut Pasteur, Paris, France) | NA | fosB, tetM, ermB, aacA, blaZ, sulI | [88] | |
| 22 | RTE meat and meat product samples (Dumplings with meat, chicken cutlet, chicken gyros, chicken in jelly, pork in jelly, chicken salad, rice with meat, roll with chicken gyros and vegetables, steak tartare (raw beef), pork stew, chicken shish kebab, poultry meat, beef meat, smoked poultry sausage, headcheese, luncheon meat, chicken paste, mett (raw sausage), roasted pork loin, cooked pork ham, polish type sausage, and pâté with boletus | Disk diffusion | CN, MEM, AMP, SXT, AMX/CL, C, CIP, E, TET | NA | [89] | |
| 23 | Various types of meat (pork, beef, and poultry) | P, AMP, MEM, E, SXT | [90] | |||
| 24 | Chicken breast filet | Disk diffusion and MTSTM (MIC Test Strips) (Liofilchem®, Roseto degli Abruzzi, TE, Italy) | DA | [91] | ||
| 25 | Enterobacterales: Escherichia sp., Klebsiella sp., Serratia sp., Enterobacter sp., Proteus sp., Hafnia sp., Citrobacter sp., Salmonella sp., Shigella sp. | Samples of fresh raw meat (poultry, pork, beef, and mechanically minced meat) and processed meat (cured meats) intended for sale, obtained from meat processing plants | ETEST® (BioMérieux, Craponne, France) | CFU, PIP, NAL, CIP, CAZ, CTX, SXT, IMP, TOB, PIP/TAZ | [92] | |
| 26 | E. coli | Raw meat (chicken, turkey, pork, and beef) | Disk diffusion | AMX, TET, SXT, CIP, PIP, NIT, C, AMX/CL, CFT, CN, PIP/TAZ, CE, CAZ, MEM, IMP, AMK | [93] | |
| 27 | E. cloacae, S. enterica, P. penneri, C. braakii, P. penneri P. mirabilis, K. oxytoca, E. coli, C. braakii, C. freundii, K. pneumoniae | Ready-to-eat foods of animal origin (sausages, bacon, pate, gammon, brawn, salami, and roasted meat) and raw meat (beef, poultry, pork, and veal) | ESBL + AmpC screen disk kit (Liofilchem®, Roseto degli Abruzzi, TE, Italy) and PCR assay | AMP, PIP, AMX/CL, CTX, CAZ, IMP, CN, TOB, LEV, TET, SXT | BlaCTX-M, blaTEM,blaoxa, blaSHV, ACC, mox, dha, cit, ebc, fox, tet(M), tet(L), tet(K), aac(6′)-Ii, ant(6)-Ia, aac(6ʹ)-Ie-aph(2″)-Ia, aph(3ʹ)-IIIa | [94] |
| 28 | S. enteritidis, S. infantis, S. typhimurium | Retail meat product samples: poultry, meat, pork, beef, and mixed meat | Disk diffusion | NAL, TET, AMP, STR, SUL | NA | [95] |
| 29 | S. enteritidis, S. infantis, S. typhimurium, S. hadar, S. newport, S. virchow, S. chester, S. agonal, S. saintpaul, S. derby, S. duisburg, S. sandiego, S. anatum, S. brandenburg, S. eko, S. glostrup, S. heidelberg, S. indiana, S. kottbus, S. mbandaka, S. wippra | Poultry meat, pork, beef, and mixed meat | ATM, AMX/CL, AMP, CE, C, CN, NAL, SUL, STR, TET, W, SXT | [63] | ||
| 30 | S. enterica spp. Enterica, S. enteritidis S. infantis, S. newport, S. derby, S. indiana, S. mbandaka, S. kentucky | Pork and poultry sample of meat | VITEK® 2 System and AST-GN96 cards for Gramnegative bacteria (BioMérieux, Craponne, France), microdilution, PCR assay | AMP, AMX, AMX/CL, CFX, CFT, CPH, CFX, CFTI, CFQ, IMP, CN, NEO, STR, ENR, UB, MRB, NOR, DOX, OXY, TET, FLR, LIN/SP, SXT | blaCMY-2, blaPSE-1, blaTEM, aadA, aadB, strA/strB, floR, dfrA1, dfrA12, sul1, sul2, sul3, blaSHV, aphA1, aphA2, tetA, tetB, blaPSE-1 | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniewski, P.; Trymers, M.; Chajęcka-Wierzchowska, W.; Tkacz, K.; Zadernowska, A.; Modzelewska-Kapituła, M. Antimicrobial Resistance in the Context of Animal Production and Meat Products in Poland—A Critical Review and Future Perspective. Pathogens 2024, 13, 1123. https://doi.org/10.3390/pathogens13121123
Wiśniewski P, Trymers M, Chajęcka-Wierzchowska W, Tkacz K, Zadernowska A, Modzelewska-Kapituła M. Antimicrobial Resistance in the Context of Animal Production and Meat Products in Poland—A Critical Review and Future Perspective. Pathogens. 2024; 13(12):1123. https://doi.org/10.3390/pathogens13121123
Chicago/Turabian StyleWiśniewski, Patryk, Miłosz Trymers, Wioleta Chajęcka-Wierzchowska, Katarzyna Tkacz, Anna Zadernowska, and Monika Modzelewska-Kapituła. 2024. "Antimicrobial Resistance in the Context of Animal Production and Meat Products in Poland—A Critical Review and Future Perspective" Pathogens 13, no. 12: 1123. https://doi.org/10.3390/pathogens13121123
APA StyleWiśniewski, P., Trymers, M., Chajęcka-Wierzchowska, W., Tkacz, K., Zadernowska, A., & Modzelewska-Kapituła, M. (2024). Antimicrobial Resistance in the Context of Animal Production and Meat Products in Poland—A Critical Review and Future Perspective. Pathogens, 13(12), 1123. https://doi.org/10.3390/pathogens13121123












