Pentavalent Antimony Associated with G-CSF in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania (Viannia) braziliensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Endemic Area and Case Definition of CL
2.2. Patient Selection
2.3. Sample Size, Randomization and Group Assignment
2.4. Histopathology, PCR and Leishmania Skin Test
2.5. Drug Administration
2.6. Study Procedures
2.7. Clinical Endpoint Criteria
2.8. Statistical Analyses
2.9. Ethics
3. Results
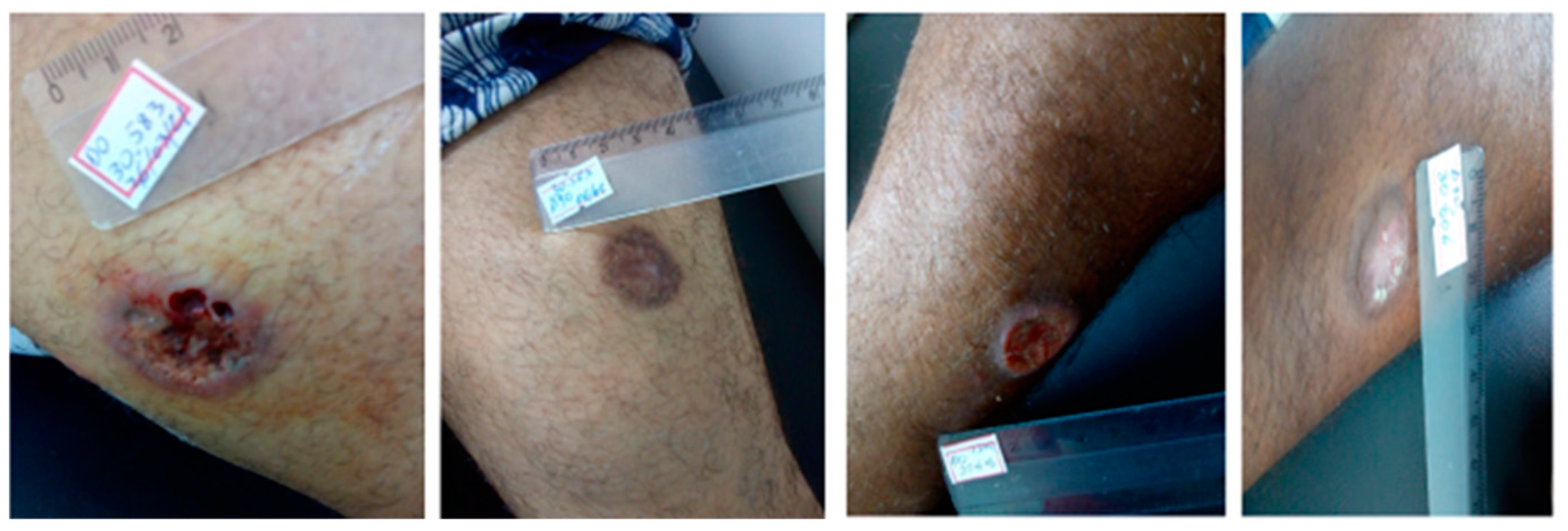
3.1. Efficacy
3.2. Cytokine Levels
3.3. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glesby, M.J.; Machado, P.R.; Carvalho, E.M.; Lago, E.; Rosa, M.E.; Guimarães, L.H.; Jirmanus, L. Epidemiological and Clinical Changes in American Tegumentary Leishmaniasis in an Area of Leishmania (Viannia) Braziliensis Transmission over a 20-Year Period. Am. J. Trop. Med. Hyg. 2012, 86, 426–433. [Google Scholar] [CrossRef]
- Saúde, M.D.S.D. Manual de Vigilância da Leishmaniose Tegumentar. 2017. 191. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_leishmaniose_tegumentar.pdf (accessed on 25 March 2024).
- Romero, G.A.; Guerra, M.V.; Paes, M.G.; Macêdo, V.O. Comparison of Cutaneous Leishmaniasis Due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: Therapeutic Response to Meglumine Antimoniate. Am. J. Trop. Med. Hyg. 2001, 65, 456–465. [Google Scholar] [CrossRef]
- Arana, B.; Rizzo, N.; Diaz, A. Chemotherapy of Cutaneous Leishmaniasis: A Review. Med. Microbiol. Immunol. 2001, 190, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, J.; Ramirez, L.; Adaui, V.; Zimic, M.; Tulliano, G.; Miranda-Verástegui, C.; Lazo, M.; Loayza-Muro, R.; Doncker, S.D.; Maurer, A.; et al. Influence of Leishmania (Viannia) Species on the Response to Antimonial Treatment in Patients with American Tegumentary Leishmaniasis. J. Infect. Dis. 2007, 195, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Df, B. Manual de Recomendações para o Controle da Tuberculose No Brasil. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/manual_recomendacoes_controle_tuberculose_brasil_2_ed.pdf (accessed on 18 April 2023).
- Guia Prático Sobre a Hanseníase—Departamento de HIV/Aids, Tuberculose, Hepatites Virais e Infecções Sexualmente Transmissíveis. Available online: https://www.gov.br/aids/pt-br/centrais-de-conteudo/publicacoes/2021/guia-pratico-sobre-a-hanseniase/view (accessed on 18 April 2023).
- Antonelli, L.R.V.; Dutra, W.O.; Almeida, R.P.; Bacellar, O.; Carvalho, E.M.; Gollob, K.J. Activated Inflammatory T Cells Correlate with Lesion Size in Human Cutaneous Leishmaniasis. Immunol. Lett. 2005, 101, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, G.; Nilforoushzadeh, M.A. Effect of Combination Therapy with Systemic Glucantime and Pentoxifylline in the Treatment of Cutaneous Leishmaniasis. Int. J. Dermatol. 2006, 45, 819–821. [Google Scholar] [CrossRef] [PubMed]
- Báfica, A.; Oliveira, F.; Freitas, L.A.R.; Nascimento, E.G.; Barral, A. American Cutaneous Leishmaniasis Unresponsive to Antimonial Drugs: Successful Treatment Using Combination of N-Methilglucamine Antimoniate plus Pentoxifylline. Int. J. Dermatol. 2003, 42, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.B.; de Jesus, A.R.; Machado, P.R.; Magalhães, A.; Salgado, K.; Carvalho, E.M.; Almeida, R.P. Antimony plus Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor Applied Topically in Low Doses Enhances Healing of Cutaneous Leishmaniasis Ulcers: A Randomized, Double-Blind, Placebo-Controlled Study. J. Infect. Dis. 2004, 190, 1793–1796. [Google Scholar] [CrossRef][Green Version]
- Almeida, R.; D’Oliveira, A., Jr.; Machado, P.; Bacellar, O.; Ko, A.I.; de Jesus, A.R.; Mobashery, N.; Brito Santos, J.; Carvalho, E.M. Randomized, Double-Blind Study of Stibogluconate Plus Human Granulocyte Macrophage Colony-Stimulating Factor versus Stibogluconate Alone in the Treatment of Cutaneous Leishmaniasis. J. Infect. Dis. 1999, 180, 1735–1737. [Google Scholar] [CrossRef]
- Deotare, U.; Al-Dawsari, G.; Couban, S.; Lipton, J.H. G-CSF-Primed Bone Marrow as a Source of Stem Cells for Allografting: Revisiting the Concept. Bone Marrow Transplant. 2015, 50, 1150–1156. [Google Scholar] [CrossRef]
- Carr, M.J.; Li, Y.; Rezakhanlou, A.M.; Ghahary, A. Keratinocyte-Releasable Factors Stimulate the Expression of Granulocyte Colony-Stimulating Factor in Human Dermal Fibroblasts. J. Cell. Biochem. 2017, 118, 308–317. [Google Scholar] [CrossRef] [PubMed]
- de Sica-Chapman, A.; Williams, G.; Soni, N.; Bunker, C.B. Granulocyte Colony-Stimulating Factor in Toxic Epidermal Necrolysis (TEN) and Chelsea & Westminster TEN Management Protocol. Br. J. Dermatol. 2010, 162, 860–865. [Google Scholar] [CrossRef]
- Fine, J.-D.; Manes, B.; Frangoul, H. Systemic Granulocyte Colony-Stimulating Factor (G-CSF) Enhances Wound Healing in Dystrophic Epidermolysis Bullosa (DEB): Results of a Pilot Trial. J. Am. Acad. Dermatol. 2015, 73, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Rutella, S.; Bonanno, G.; Pierelli, L.; Mariotti, A.; Capoluongo, E.; Contemi, A.M.; Ameglio, F.; Curti, A.; De Ritis, D.G.; Voso, M.T.; et al. Granulocyte Colony-Stimulating Factor Promotes the Generation of Regulatory DC through Induction of IL-10 and IFN-Alpha. Eur. J. Immunol. 2004, 34, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Bunse, C.E.; Tischer, S.; Lahrberg, J.; Oelke, M.; Figueiredo, C.; Blasczyk, R.; Eiz-Vesper, B. Granulocyte Colony-Stimulating Factor Impairs CD8(+) T Cell Functionality by Interfering with Central Activation Elements. Clin. Exp. Immunol. 2016, 185, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Weigle, K.A.; Labrada, L.A.; Lozano, C.; Santrich, C.; Barker, D.C. PCR-Based Diagnosis of Acute and Chronic Cutaneous Leishmaniasis Caused by Leishmania (Viannia). J. Clin. Microbiol. 2002, 40, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Weirather, J.L.; Jeronimo, S.M.B.; Gautam, S.; Sundar, S.; Kang, M.; Kurtz, M.A.; Haque, R.; Schriefer, A.; Talhari, S.; Carvalho, E.M.; et al. Serial Quantitative PCR Assay for Detection, Species Discrimination, and Quantification of Leishmania spp. in Human Samples. J. Clin. Microbiol. 2011, 49, 3892–3904. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE)|Protocol Development|CTEP. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed on 24 March 2024).
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.-C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug Resistance and Treatment Failure in Leishmaniasis: A 21st Century Challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.-C.; Tay, Y.-K. Hematological Abnormalities and the Use of Granulocyte-Colony-Stimulating Factor in Patients with Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Int. J. Dermatol. 2011, 50, 1570–1578. [Google Scholar] [CrossRef]
- Root, R.K.; Dale, D.C. Granulocyte Colony-Stimulating Factor and Granulocyte-Macrophage Colony-Stimulating Factor: Comparisons and Potential for Use in the Treatment of Infections in Nonneutropenic Patients. J. Infect. Dis. 1999, 179, S342–S352. [Google Scholar] [CrossRef]
- Brito, G.; Dourado, M.; Guimarães, L.H.; Meireles, E.; Schriefer, A.; de Carvalho, E.M.; Machado, P.R.L. Oral Pentoxifylline Associated with Pentavalent Antimony: A Randomized Trial for Cutaneous Leishmaniasis. Am. J. Trop. Med. Hyg. 2017, 96, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Lapidari, P.; Vaz-Luis, I.; Di Meglio, A. Side Effects of Using Granulocyte-Colony Stimulating Factors as Prophylaxis of Febrile Neutropenia in Cancer Patients: A Systematic Review. Crit. Rev. Oncol. Hematol. 2021, 157, 103193. [Google Scholar] [CrossRef] [PubMed]
- Khoury, H.; Adkins, D.; Brown, R.; Vij, R.; Westervelt, P.; Trinkaus, K.; Goodnough, L.T.; DiPersio, J.F. Adverse Side-Effects Associated with G-CSF in Patients with Chronic Myeloid Leukemia Undergoing Allogeneic Peripheral Blood Stem Cell Transplantation. Bone Marrow Transplant. 2000, 25, 1197–1201. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Variables | MA with G-CSF (Group A) n = 17 | MA with Placebo (Group B) n = 15 | p-Value |
|---|---|---|---|
| Gender: M (%) | 10 (59%) | 07 (46.6%) | 0.37 * |
| Age (years, mean + SD) | 31.6 ± 10.8 | 32.9 ± 12.8 | 0.94 ** |
| Duration of illness 30–60 days >60–90 days | 16 (94%) 1 (6%) | 14 (93.3%) 1 (6.7%) | 0.72 * |
| Location of the biggest lesion Cephalic segment Trunk Upper extremities Lower extremities | 0 (0%) 0 (0%) 4 (23.5%) 13 (76.5%) | 0 (0%) 2 (13.3%) 2 (13.3%) 11 (73.3%) | 0.25 * |
| Number of lesions Single lesion Two lesions Three lesions | 13 (76.5%) 3 (17.6%) 1 (6%) | 9 (60%) 5 (33.3%) 1 (7%) | 0.57 * |
| Largest diameter (mm²) | 27.18 ± 6.7 | 27.47 ± 7 | 0.90 *** |
| Lymphadenopathy (%) | 12 (70.6%) | 9 (60%) | 0.39 * |
| Positive PCR | 16 (94%) | 15 (100%) | 0.53 * |
| Positive LST (%) | 16 (94%) | 15 (100%) | 0.53 * |
| Therapeutic Result | MA with G-CSF (Group A) n = 17 | MA with Placebo (Group B) n = 15 | p-Value |
|---|---|---|---|
| Cure on D90 (%) | 9 (53%) | 7 (47%) | 0.50 * |
| Final cure rate (D180) (%) | 11 (65%) | 7 (47%) | 0.40 * |
| Rescue therapy (%) | 6 (35%) | 7 (47%) | 0.5 * |
| Healing time (days) range (M ± SD) | 90 (57.5–1 × 35) | 150 (50–2 × 10) | 0.77 ** |
| Relapse (%) | 1 (6%) | 1 (7%) | - |
| Irregular use (%) | 0 | 0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suprien, C.; Guimarães, L.H.; de Carvalho, L.P.; Machado, P.R.L. Pentavalent Antimony Associated with G-CSF in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania (Viannia) braziliensis. Pathogens 2024, 13, 301. https://doi.org/10.3390/pathogens13040301
Suprien C, Guimarães LH, de Carvalho LP, Machado PRL. Pentavalent Antimony Associated with G-CSF in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania (Viannia) braziliensis. Pathogens. 2024; 13(4):301. https://doi.org/10.3390/pathogens13040301
Chicago/Turabian StyleSuprien, Carvel, Luiz H. Guimarães, Lucas P. de Carvalho, and Paulo R. L. Machado. 2024. "Pentavalent Antimony Associated with G-CSF in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania (Viannia) braziliensis" Pathogens 13, no. 4: 301. https://doi.org/10.3390/pathogens13040301
APA StyleSuprien, C., Guimarães, L. H., de Carvalho, L. P., & Machado, P. R. L. (2024). Pentavalent Antimony Associated with G-CSF in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania (Viannia) braziliensis. Pathogens, 13(4), 301. https://doi.org/10.3390/pathogens13040301






