Molecular Characterisation and Antibody Response to Bovine Respiratory Syncytial Virus in Vaccinated and Infected Cattle in Turkey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sampling and Study Population
2.3. Extraction of RNA and Reverse Transcription
2.4. Determination of RNA Concentration and Purity
2.5. PCR Inhibitors of RNA Extracts
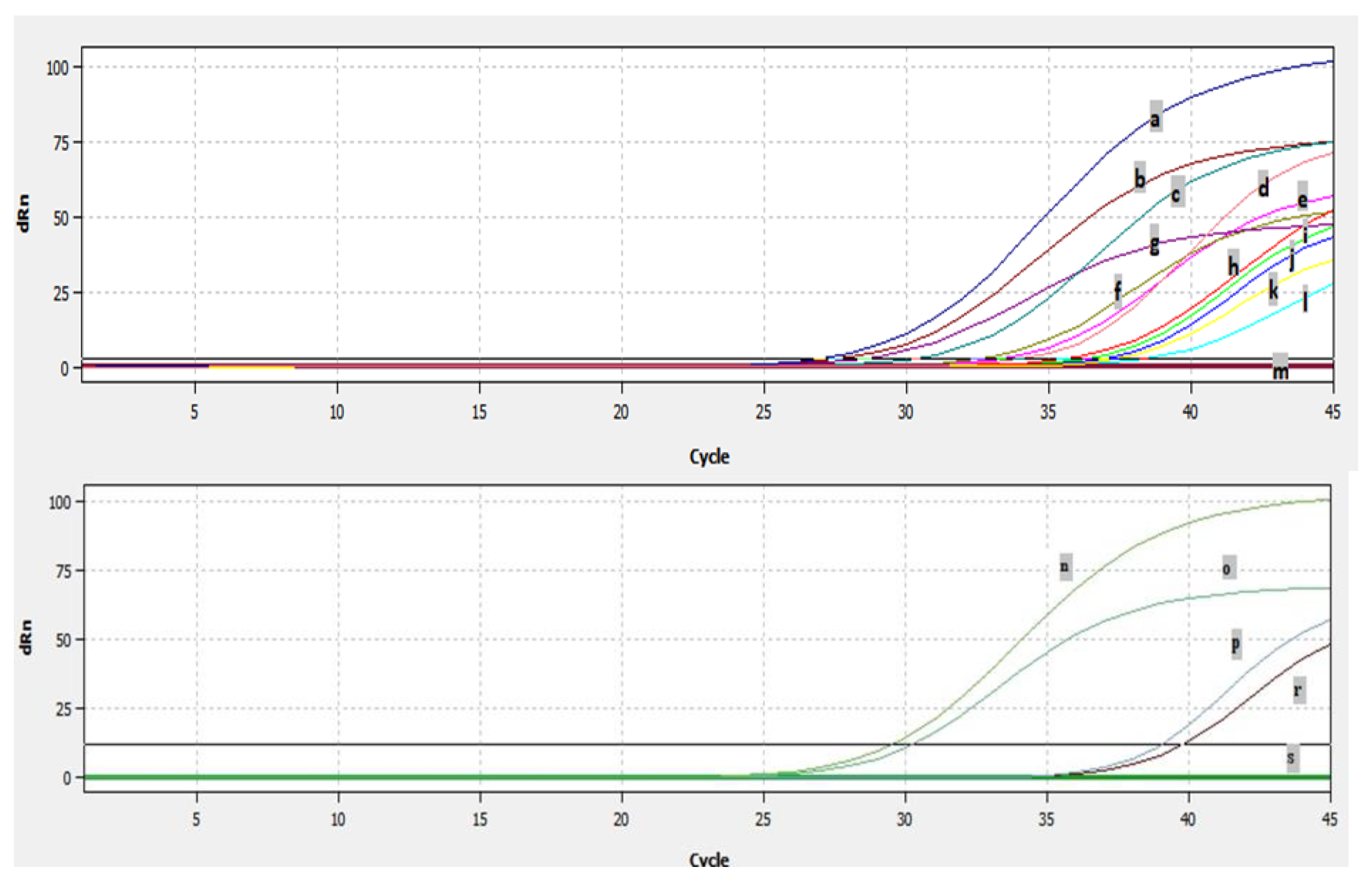
2.6. Detection of BRSV by Real Time RT-PCR
2.7. Sequencing and Phylogenetic Analysis
2.8. ELISA
2.9. Virus Isolation
2.10. Statistical Analyses
3. Results
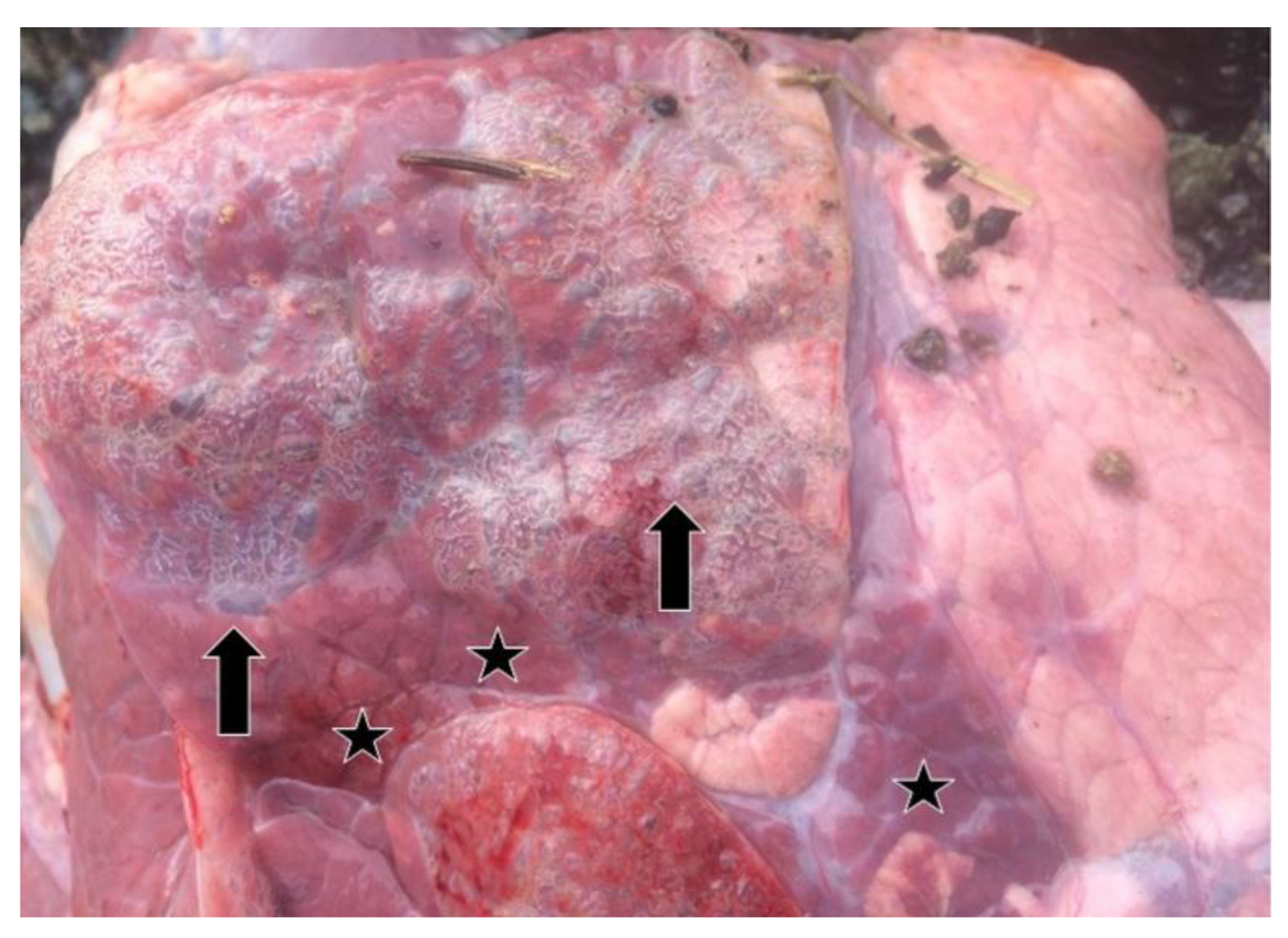
3.1. Clinical and Necropsy Findings
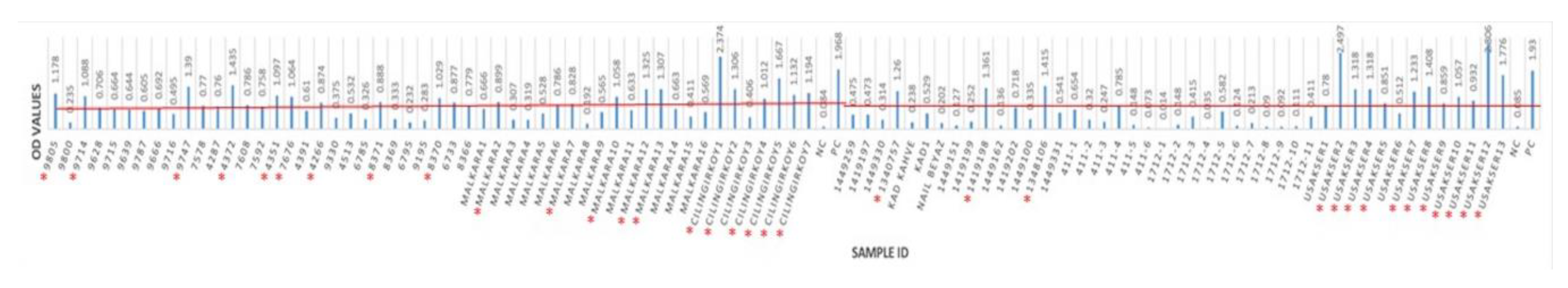
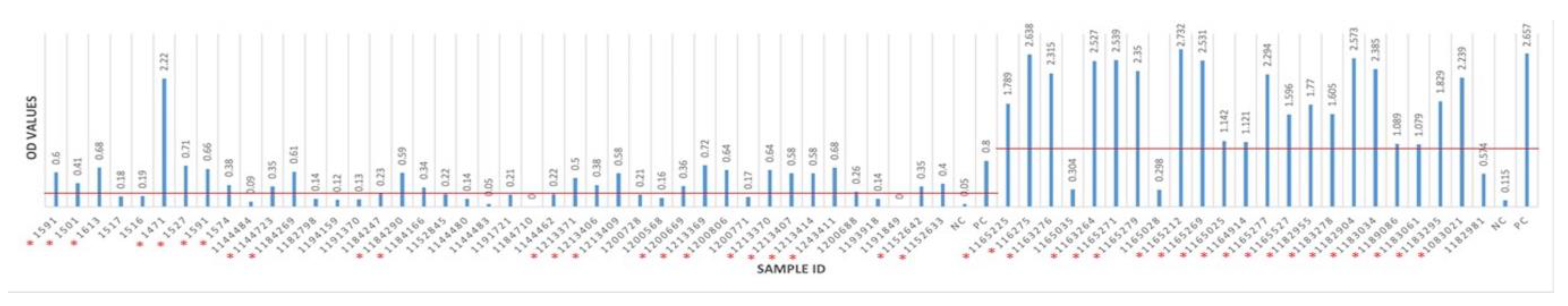
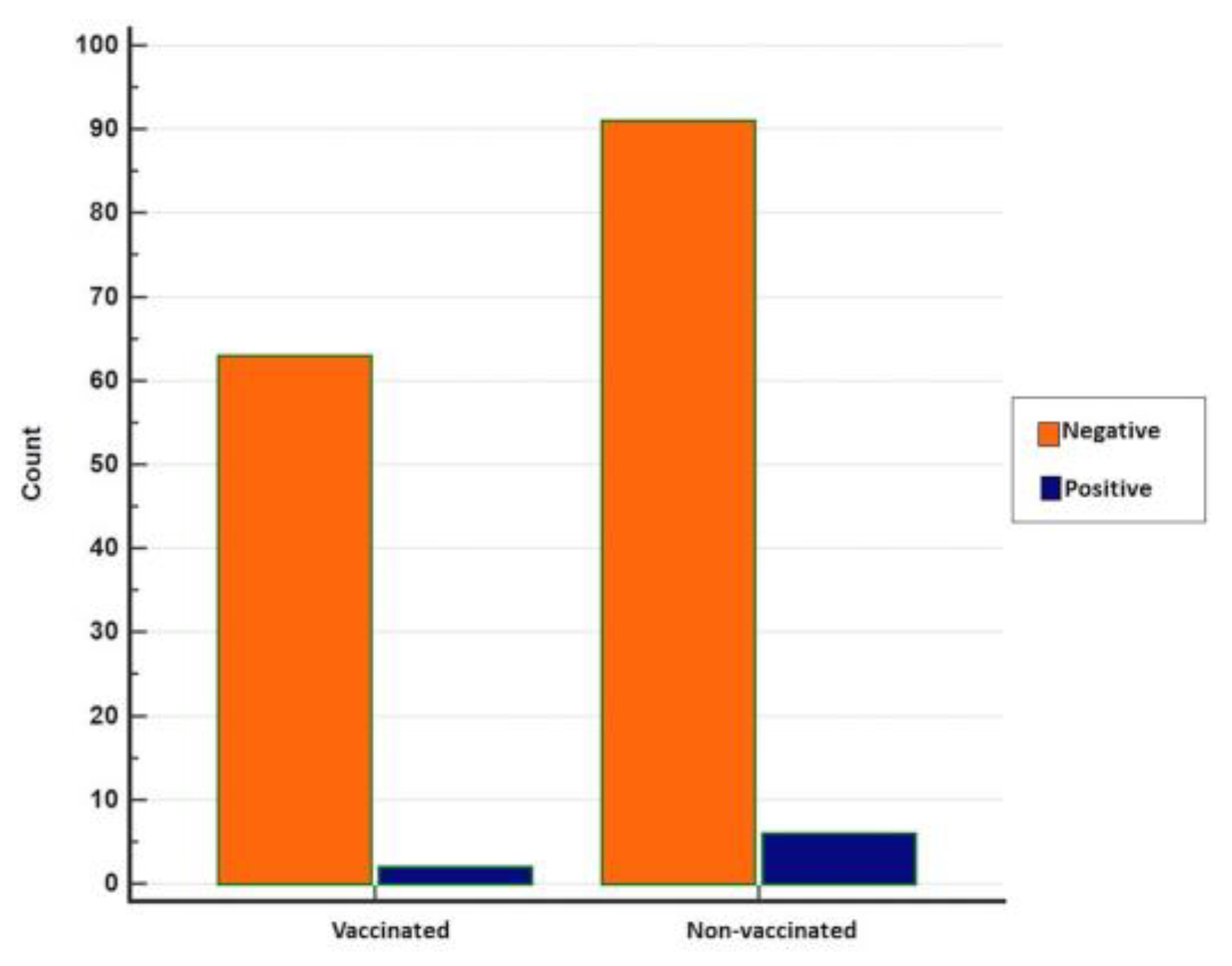
3.2. Seropositivity of BRSV Detected by ELISA
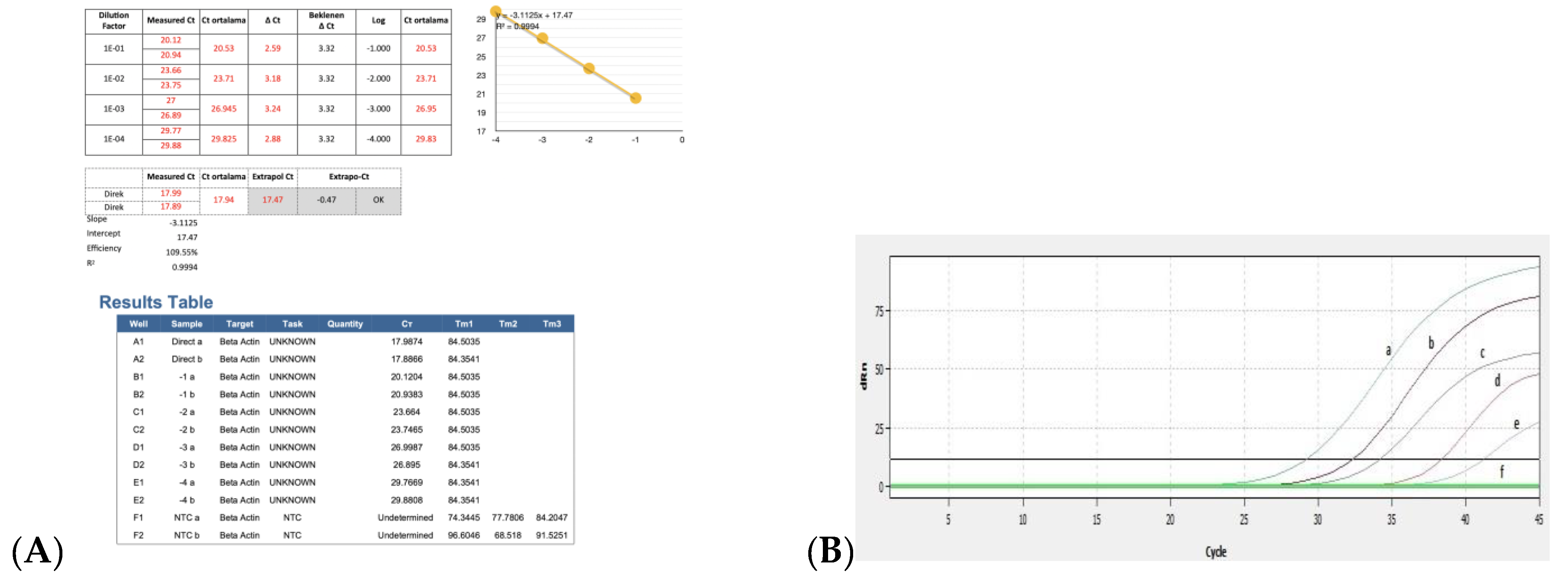
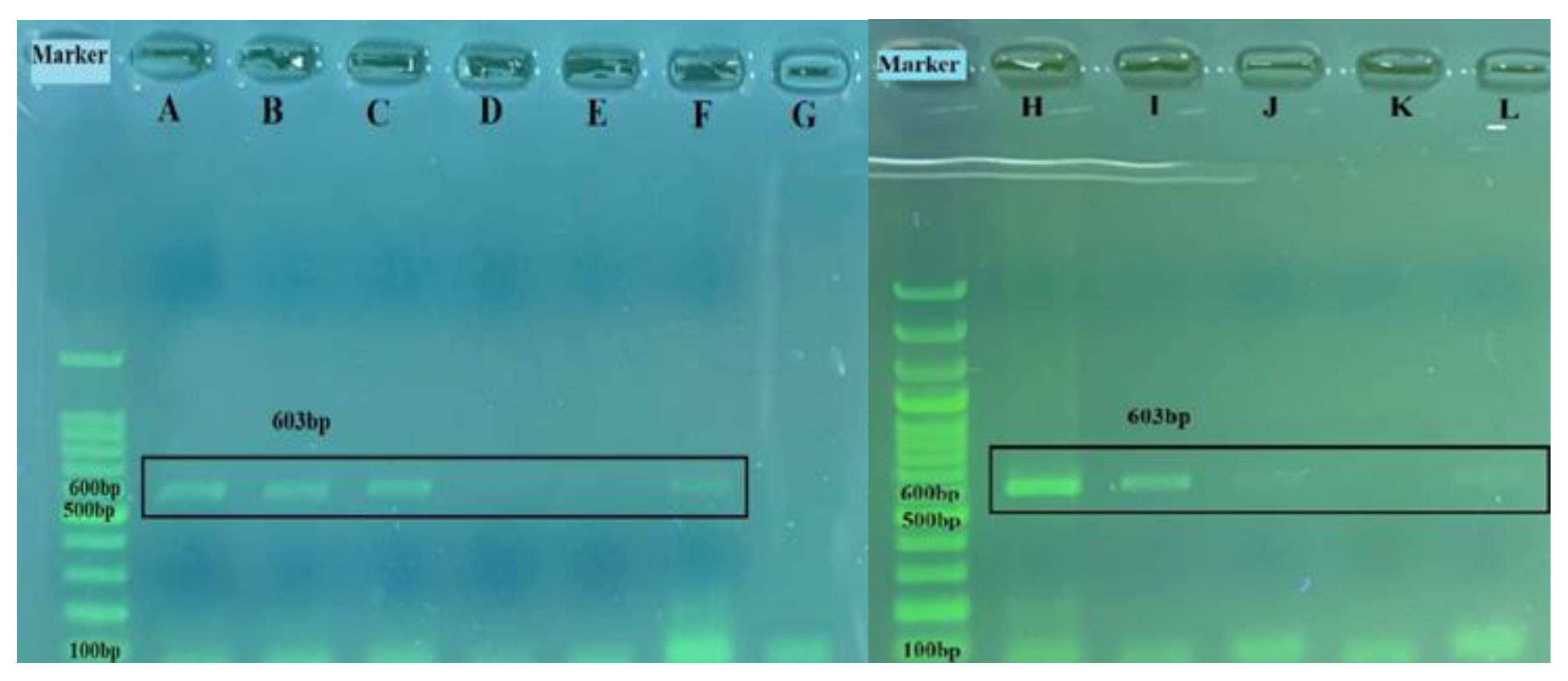
3.3. Efficiency of Real Time RT-PCR and Detection of BRSV-RNA in Clinical Samples
3.4. Sequencing and Phylogenetic Analysis
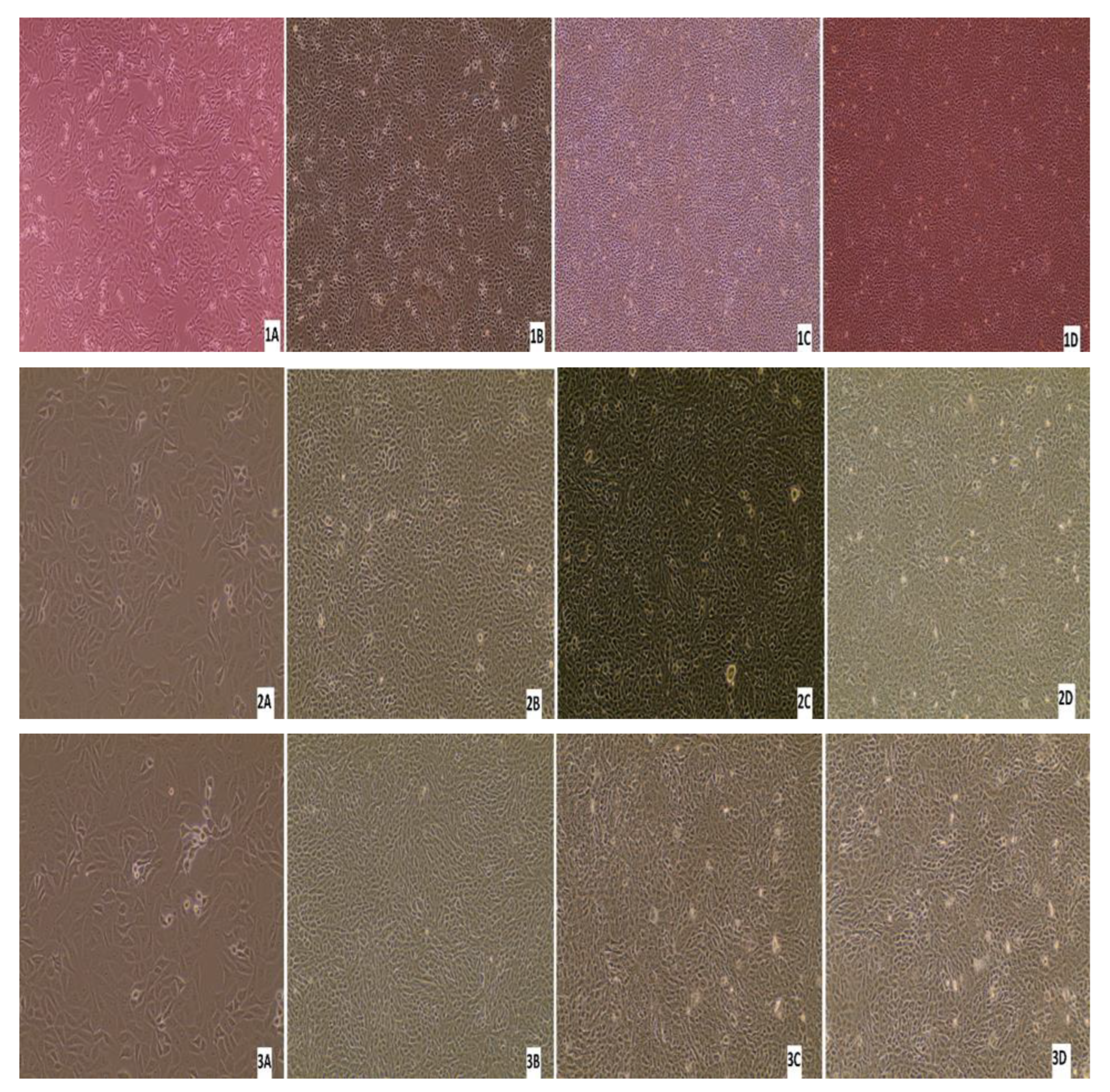
3.5. Virus Isolation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klem, T.B.; Sjurseth, S.K.; Sviland, S.; Gjerset, B.; Myrmel, M.; Stokstad, M. Bovine respiratory syncytial virus in experimentally exposed and rechallenged calves; viral shedding related to clinical signs and the potential for transmission. BMC Vet. Res. 2019, 15, 156. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Umar, S.; Turan, N.; Kayar, A.; Richt, J.A.; Yilmaz, H. Current scenario of viral diseases and vaccination strategies of cattle in Turkey. J. Infect. Dev. Ctries. 2022, 16, 1230–1242. [Google Scholar] [CrossRef] [PubMed]
- Baykan, K.Z.; Ozcan, M. Causes of Culling and Disease Incidences at First Production Year of Imported Brown Swiss and Simmental Cows from Austria. Kocatepe Vet. J. 2019, 12, 178–184. [Google Scholar]
- Valarcher, J.F.; Taylor, G. Bovine respiratory syncytial virus infection. Vet. Res. 2007, 38, 153–180. [Google Scholar] [CrossRef] [PubMed]
- Rima, B.; Collins, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.A.; Lee, B.; Maisner, A.; Rota, L.; Wang, C. ICTV Virus Taxonomy Profile: Pneumoviridae. J. Gen. Virol. 2017, 98, 2912–2913. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, L.; Giammarioli, M.; Rosati, S. Genetic characterization of bovine respiratory syncytial virus strains isolated in Italy: Evidence for the circulation of new divergent clades. J. Vet. Diagn. Investig. 2018, 30, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Krešic, N.; Bedeković, T.; Brnić, D.; Šimić, I.; Lojkić, I.; Turk, N. Genetic analysis of bovine respiratory syncytial virus in Croatia. Comp. Immunol. Microbiol. Infect. Dis. 2018, 58, 52–57. [Google Scholar] [CrossRef]
- Kumagai, A.; Kawauchi, K.; Andoh, K.; Hatama, S. Sequence and unique phylogeny of G genes of bovine respiratory syncytial viruses circulating in Japan. J. Vet. Diagn. Investig. 2021, 33, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Sausker, E.A.; Dyer, N.W. Polymerase chain reaction and DNA sequencing for detection of ovine herpesvirus 2 in American bison (Bison bison). J. Vet. Diagn. Investig. 2002, 14, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Citterio, C.; Luzzago, C.; Sala, M.; Sironi, G.; Gatti, P.; Gaffuri, A.; Lanfranchi, P. Serological study of a population of alpine chamois (Rupkapra rrupkapra) affected by an outbreak of respiratory disease. Vet. Rec. 2003, 153, 592–596. [Google Scholar] [CrossRef]
- Larios Mora, A.; Detalle, L.; Gallup, J.M.; Van Geelen, A.; Stohr, T.; Duprez, L.; Ackermann, M.R. Delivery of ALX-0171 by inhalation greatly reduces respiratory syncytial virus disease in newborn lambs. MAbs 2018, 10, 778–795. [Google Scholar] [CrossRef]
- Sarmiento-Silva, R.E.; Nakamura-Lopez, Y.; Vaughan, G. Epidemiology, molecular epidemiology and evolution of bovine respiratory syncytial virus. Viruses 2012, 4, 3452–3467. [Google Scholar] [CrossRef] [PubMed]
- Urban-Chmiel, R.; Wernicki, A.; Majer-Dziedzic, B.; Gnat, S.; Puchalski, A.; Dec, M. Use of different cell lines for in vitro cultures of bovine respiratory syncytial virus. J. Virol. Methods 2014, 204, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Ohlson, A.; Heuer, C.; Lockhart, C.; Tråvén, M.; Emanuelson, U.; Alenius, S. Risk factors for seropositivity to bovine coronavirus and bovine respiratory syncytial virus in dairy herds. Vet. Rec. 2010, 167, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Saa, L.; Perea, R.A.; Jara, D.V.; Arenas, A.J.; Garcia-Bocanegra, I.; Borge, C.; Carbonero, A. Prevalence of and risk factors for bovine respiratory syncytial virus (BRSV) infection in non-vaccinated dairy and dual-purpose cattle herds in Ecuador. Trop. Anim. Health Prod. 2012, 44, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Norström, M.; Skjerve, E.; Jarp, J. Risk factors for epidemic respiratory disease in Norwegian cattle herds. Prev. Vet. Med. 2000, 44, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Zeineldin, M.; Lowe, J.; Aldridge, B. Contribution of the mucosal microbiota to bovine respiratory health. Trends Microbiol. 2019, 27, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Gow, S.; Berenik, A.; Lacoste, S.; Erickson, N. Comparative efficacy of modified-live and inactivated vaccines in boosting responses to bovine respiratory syncytial virus following neonatal mucosal priming of beef calves. Can. Vet. J. 2018, 59, 1311. [Google Scholar] [PubMed]
- Stokstad, M.; Klem, T.B.; Myrmel, M.; Oma, V.S.; Toftaker, I.; Østerås, O.; Nødtvedt, A. Using biosecurity measures to combat respiratory disease in cattle: The Norwegian control program for bovine respiratory syncytial virus and bovine coronavirus. Front. Vet. Sci. 2020, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Valarcher, J.F.; Schelcher, F.; Bourhy, H. Evolution of bovine respiratory syncytial virus. J. Virol. 2000, 74, 10714–10728. [Google Scholar] [CrossRef]
- Paccaud, M.F.; Jacquier, C.A. Respiratory syncytial virus of bovine origin. Arch. Gesamte Virusforsch. 1970, 30, 327–342. [Google Scholar] [CrossRef]
- Inaba, Y.; Tanaka, Y.; Sato, K.; Omori, T.; Matumoto, M. Bovine respiratory syncytial virus studies on an outbreak in Japan, 1968–1969. Jpn J Microbiol. 1972, 16, 373–383. [Google Scholar] [CrossRef]
- Stott, E.J.; Taylor, G. Respiratory syncytial virus. Brief review. Arch. Virol. 1985, 84, 1–52. [Google Scholar] [CrossRef]
- Prozzi, D.; Walravens, K.; Langedijk, J.P.; Daus, F.; Kramps, J.A.; Letesson, J.J. Antigenic and molecular analyses of the variability of bovine respiratory syncytial virus G glycoprotein. J. Gen. Virol. 1997, 78, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Valentova, V.; Antonis, A.; Kovarcik, K. Restriction enzyme analysis of RT-PCR amplicons as a rapid method for detection of genetic diversity among bovine respiratory syncytial virus isolates. Vet. Mic. 2005, 108, 1–12. [Google Scholar] [CrossRef]
- Mitra, N.; Cernicchiaro, N.; Torres, S.; Li, F.; Hause, B.M. Metagenomic characterization of the virome associated with bovine respiratory disease in feedlot cattle identified novel viruses and suggests an etiologic role for influenza D virus. J. Gen. Virol. 2016, 97, 1771. [Google Scholar] [CrossRef]
- Ince, Ö.B.; Şevik, M.; Özgür, E.G.; Sait, A. Risk factors and genetic characterization of bovine respiratory syncytial virus in the inner Aegean Region, Turkey. Trop. Anim. Health Prod. 2022, 54, 4. [Google Scholar] [CrossRef] [PubMed]
- Philippou, S.; Otto, P.; Reinhold, P.; Elschner, M.; Streckert, H.J. Respiratory syncytial virus-induced chronic bronchiolitis in experimentally infected calves. Virchows Arch. 2000, 436, 617–621. [Google Scholar] [CrossRef]
- Bidokhti, M.R.; Tråvén, M.; Ohlson, A.; Zarnegar, B.; Baule, C.; Belák, S.; Alenius, S.; Liu, L. Phylogenetic analysis of bovine respiratory syncytial viruses from recent outbreaks in feedlot and dairy cattle herds. Arch. Virol. 2012, 157, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Kamdi, B.; Singh, R.; Singh, V.; Singh, S.; Kumar, P.; Singh, K.P.; George, N.; Dhama, K. Immunofluorescence and molecular diagnosis of bovine respiratory syncytial virus and bovine parainfluenza virus in the naturally infected young cattle and buffaloes from India. Microb. Pathog. 2020, 145, 104165. [Google Scholar] [CrossRef]
- Thonur, L.; Maley, M.; Gilray, J.; Crook, T.; Laming, E.; Turnbull, D.; Nath, M.; Willoughby, K. One-step multiplex real time RT-PCR for the detection of bovine respiratory syncytial virus, bovine herpesvirus 1 and bovine parainfluenza virus 3. BMC Vet. Res. 2012, 8, 37. [Google Scholar] [CrossRef]
- Lubbers, B.V.; Renter, D.G.; Hesse, R.A.; Peddireddi, L.G.; Zerse, M.T.; Cox, E.A.; Meyer, B.D. Prevalence of respiratory viruses and Mycoplasma bovis in US cattle and variability among herds of origin, production systems and season of year. Bov. Pract. 2017, 159–164. [Google Scholar] [CrossRef]
- Hoppe, I.B.A.L.; de Medeiros, A.S.R.; Arns, C.W.; Samara, S.I. Bovine respiratory syncytial virus seroprevalence and risk factors in non-vaccinated dairy cattle herds in Brazil. BMC Vet. Res. 2018, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Giammarioli, M.; Mangili, P.; Nanni, A.; Pierini, I.; Petrini, S.; Pirani, S.; Gobbi, P.; De Mia, G.M. Highly pathogenic Bovine Respiratory Syncytial virus variant in a dairy herd in Italy. Vet. Med. Sci. 2020, 6, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.E.; Tjørnehøj, K.; Viuff, B.; Jensen, N.E.; Uttenthal, A. Diagnosis of enzootic pneumonia in Danish cattle: Reverse transcription-polymerase chain reaction assay for detection of bovine respiratory syncytial virus in naturally and experimentally infected cattle. J. Vet. Diagn. Investig. 1999, 11, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Timurkan, M.O.; Aydin, H.; Sait, A. Identification and Molecular Characterisation of Bovine Parainfluenza Virus-3 and Bovine Respiratory Syncytial Virus—First Report from Turkey. J. Vet. Res. 2019, 63, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Yazici, Z.; Ozan, E.; Tamer, C.; Muftuoglu, B.; Barry, G.; Kurucay, H.N.; Elhag, A.E.; Cagirgan, A.A.; Gumusova, S.; Albayrak, H. Circulation of indigenous bovine respiratory syncytial virus strains in Turkish cattle: The first isolation and molecular characterization. Animals 2020, 10, 1700. [Google Scholar] [CrossRef]
- Hougs, L.; Gatto, F.; Goerlich, O.; Grohmann, L.; Lieske, K.; Mazzara, M.; Narendja, F.; Ovesna, J.; Papazova, N.; Scholtens, I.; et al. Verification of analytical methods for GMO testing when implementing interlaboratory validated methods. In EUR 29015 EN; Publication Office of the European Union: Luxembourg, 2017. [Google Scholar]
- Boxus, M.; Letellier, C.; Kerkhofs, P. Real Time RT-PCR for the detection and quantitation of bovine respiratory syncytial virus. J. Virol. Methods. 2005, 125, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Yao, X.; Yang, Y.; Niu, C.; Zhao, Y.; Zhang, X.; Pan, R.; Jiang, X.; Xiaobo, S.; Qiao, X.; et al. Isolation, identification, and phylogenetic analysis of subgroup III strain of bovine respiratory syncytial virus contributed to outbreak of acute respiratory disease among cattle in Northeast China. Virulence 2021, 12, 404–414. [Google Scholar] [CrossRef]
- Jiménez-Ruiz, S.; García-Bocanegra, I.; Acevedo, P.; Espunyes, J.; Triguero-Ocaña, R.; Cano-Terriza, D.; Torres-Sánchez, M.J.; Vicente, J.; Risalde, M.Á. A survey of shared pathogens at the domestic–wild ruminants’ interface in Doñana National Park (Spain). Transbound. Emerg. Dis. 2022, 69, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.C.; O’Grady, L.; McAloon, C.G.; Mee, J. Longitudinal Prevalence of Antibodies to Endemic Pathogens in Bulk Tank Milk Samples from Dairy Herds Engaged or Not in Contract Heifer Rearing. Front. Vet. Sci. 2021, 25, 785128. [Google Scholar] [CrossRef] [PubMed]
- Bugarski, D.; Petrović, T.; Milanov, D.; Lazić, S. Seroprevalence of bovine respiratory syncytial virus (BRSV) in Vojvodina [Serbia]. Arch. Vet. Med. 2011, 4, 23–29. [Google Scholar] [CrossRef]
- Grubbs, S.T.; Kania, S.A.; Potgieter, L. Prevalence of ovine and bovine respiratory syncytial virus infections in cattle determined with a synthetic peptide-based immunoassay. J. Vet. Diagn. Investig. 2001, 13, 128–132. [Google Scholar] [CrossRef]
- Ferella, A.; Aguirreburualde, M.S.P.; Margineda, C.; Aznar, N.; Sammarruco, A.; Santos, M.J.D.; Mozgovoj, M. Bovine respiratory syncytial virus seroprevalence and risk factors in feedlot cattle from Córdoba and Santa Fe, Argentina. Rev. Argent. Microbiol. 2018, 50, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Burgu, I.; Toker, A.; Akca, Y.; Alkan, F. A seroepidemiologic study of bovine respiratory syncytial virus (BRSV) in Turkey. Dtsch. Tierarztl. Wochenschr. 1990, 97, 88–89. [Google Scholar] [PubMed]
- Alkan, F. Sığırlarda viral nedenli solunum sistemi enfeksiyonlarının seroepidemiyolojisi. Ankara Üniv Vet. Fak. Derg. 1997, 44, 1–8. [Google Scholar]
- Çabalar, M.; Can-Şahna, K. Doğu ve güneydoğu anadolu bölgesinde süt sığırlarında parainfluenza virus-3, bovine herpes virus-1 ve respiratory syncytial virus enfeksiyonlarının seroepidemiyolojisi. YYÜ Vet. Fak. Derg. 2000, 11, 101–105. [Google Scholar]
- Yeşilbağ, K.; Güngör, B. Seroprevalence of bovine respiratory viruses in North-Western Turkey. Trop. Anim. Health Prod. 2008, 40, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Karaotcu, A.; Yildirim, Y. Denizli ve Burdur yörelerindeki sığırlarda bovine respiratory syncytial virus enfeksiyonunun serolojik olarak araştırılması. MAKU J. Health Sci. Inst. 2019, 7, 114–123. [Google Scholar] [CrossRef]
- Öner, E.B.; Yesilbag, K. Besi sığırlarında solunum sistemi virüslerinin seroprevalansı ve persiste BVD virüs enfeksiyonu tespiti. Ankara Üniv Vet. Fak. Derg. 2018, 65, 1–7. [Google Scholar]
- Hussain, K.J.; Al-Farwachi, M.I.; Hassan, S.D. Seroprevalence and risk factors of bovine respiratory syncytial virus in cattle in the Nineveh Governorate, Iraq. Vet. World 2019, 12, 1862. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, E.; Lotfi, M.; Kamalzadeh, M.; Noaman, V.; Bahriari, M.; Morovati, H.; Hatami, A. Seroepidemiological study of bovine respiratory viruses (BRSV, BoHV-1, PI-3V, BVDV, and BAV-3) in dairy cattle in central region of Iran (Esfahan province). Trop. Anim. Health Prod. 2012, 44, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Farzinpour, M.; Badiei, K.; Pourjafar, M.; Ghane, M.; Karim, Z. Antibody Tracing, Seroepidemiology and Risk Factors of Bovine Respiratory Syncytial Virus and Bovine Adenovirus-3 in Dairy Holstein Farms. J. Fac. Vet. Med. Istanbul Univ. 2016, 42, 5–10. [Google Scholar]
- Willoughby, K.; Thomson, K.; Maley, M.; Gilray, J.; Scholes, S.; Howie, F.; Caldow, G.; Nettleton, P.F. Development of a real time reverse transcriptase polymerase chain reaction for the detection of bovine respiratory syncytial virus in clinical samples and its comparison with immunohistochemistry and immunofluorescence antibody testing. Vet. Microbiol. 2008, 126, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Klem, T.B.; Rimstad, E.; Stokstad, M. Occurrence and phylogenetic analysis of bovine respiratory syncytial virus in outbreaks of respiratory disease in Norway. BMC Vet. Res. 2014, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Nefedchenko, A.; Glotov, A.; Koteneva, S.; Glotova, T. Developing and testing a real-time polymerase chain reaction to identify and quantify bovine respiratory syncytial viruses. Mol. Gen. Microbiol. Virol. 2020, 35, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Timsit, E.; Maingourd, C.; Dréan, E.L.; Belloc, C.; Seegers, H.; Douart, A.; Assié, S. Evaluation of a commercial real-time reverse transcription polymerase chain reaction kit for the diagnosis of bovine respiratory syncytial virus infection. J. Vet. Diagn. Investig. 2010, 22, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Hakhverdyan, M.; Hägglund, S.; Larsen, L.E.; Belák, S. Evaluation of a single-tube fluorogenic RT-PCR assay for detection of bovine respiratory syncytial virus in clinical samples. J. Virol. Methods 2005, 123, 195–202. [Google Scholar] [CrossRef]
- Selim, A.; Gaede, W. Evaluation of reverse transcription-PCR protocols based on the fusion gene for diagnosis of bovine respiratory syncytial virus infections. Biotech. Anim. Hus. 2013, 29, 53–64. [Google Scholar] [CrossRef]
- Studer, E.; Schönecker, L.; Meylan, M.; Stucki, D.; Dijkman, R.; Holwerda, M.; Glaus, A.; Becker, J. Prevalence of BRD-related viral pathogens in the upper respiratory tract of swiss veal calves. Animals. 2021, 11, 1940. [Google Scholar] [CrossRef] [PubMed]
- Pratelli, A.; Cirone, F.; Capozza, P.; Trotta, A.; Corrente, M.; Balestrieri, A.; Buonavoglia, C. Bovine respiratory disease in beef calves supported long transport stress: An epidemiological study and strategies for control and prevention. Res. Vet. Sci. 2021, 135, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Pardon, B.; Callens, J.; Maris, J.; Allais, L.; Van Praet, W.; Deprez, P.; Ribbens, S. Pathogen-specific risk factors in acute outbreaks of respiratory disease in calves. J. Dairy. Sci. 2020, 103, 2556–2566. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.; D’Offay, J.; Landis, C.; Miles, D.; Smith, R.; Saliki, J.; Ridpath, J.; Confer, A.; Neill, J.; Eberle, R. Detection and characterization of viruses as field and vaccine strains in feedlot cattle with bovine respiratory disease. Vaccine. 2016, 34, 3478–3492. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, E.; Khamesipor, F.; Momeni, M. Evaluate the frequency of respiratory syncytial virus (RSV) in dairy herds in the Chaharmahal va Bakhtyari province-IRAN. Iran. J. Med. Microbiol. 2015, 9, 32–38. [Google Scholar]
- Hacioglu, I.K.; Coşkun, N.; Yelken, S.D.; Sevinc, S.; Alkan, F. Phylogenetic analysis of bovine respiratory syncytial virus from calves with respiratory disorders. Kafkas Univ. Vet. Fak. Derg. 2019, 25, 251–256. [Google Scholar]
- Stine, L.C.; Hoppe, D.K.; Kelling, C.L. Sequence conservation in the attachement glycoprotein and antigenic diversity among bovine respiratory syncytial virus isolates. Vet. Microbiol. 1997, 54, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Leme, R.A.; Agnol, A.M.D.; Balbo Pereira, F.L.; Possatti, F.; Alfieri, A.F.; Alfieri, A.A. Molecular characterization of Brazilian wild-type strains of bovine respiratory syncytial virus reveals genetic diversity and a putative new subgroup of the virus. Vet. Q. 2020, 40, 83–96. [Google Scholar] [CrossRef]
- Socha, W.; Rola, J. Comparison of four RT-PCR assays for detection of bovine respiratory syncytial virus. Pol. J. Vet. Sci. 2011, 14, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.F.; Weiblen, R.; Medeiros, M.; Botton, S.A.; Irigoyen, L.F.; Driemeier, D.; Schuch, L.F.; Moraes, M. A retrospective search for bovine respiratory syncytial virus (BRSV) antigens in histological specimens by immunofluorescence and immunohistochemistry. Pesq. Vet. Bras. 2000, 20, 139–143. [Google Scholar] [CrossRef]
- Arns, C.; Campalans, J.; Costa, S.; Domingues, H.; D’Arce, R.; Almeida, R.; Coswig, L. Characterization of bovine respiratory syncytial virus isolated in Brazil. Br. J. Med. Biol. Res. 2003, 36, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.; Salem, G. Isolation and molecular identification of BHV-1 from cattle suffering from respiratory signs. Kafrelsheikh Vet. Med. J. 2012, 10, 119–140. [Google Scholar] [CrossRef]
- Toker, E.B.; Yesilbag, K. Molecular characterization and comparison of diagnostic methods for bovine respiratory viruses (BPIV-3, BRSV, BVDV, and BoHV-1) in field samples in northwestern Turkey. Trop. Anim. Health Prod. 2021, 53, 79. [Google Scholar] [CrossRef] [PubMed]




| Sample Collection Date | Sample Region | Age (Months) | Number of Samples | Clinical Signs/Pathology | ||
|---|---|---|---|---|---|---|
| Blood | Swabs | Lungs | ||||
| 21 January 2019 | Luleburgaz | 0–6 | 10 | 10 | Dyspnea, cough, serous nasal secretions, fatigue | |
| 20 February 2019 | Luleburgaz | 0–6 | 2 | 2 | Dyspnea, cough, serous nasal secretions, fatigue | |
| 20 February 2019 | Catalca-Istanbul | 0–6 | 18 | Dyspnea, cough, serous nasal secretions, fatigue | ||
| 21 February 2019 | Luleburgaz | 0–6 | 11 | 11 | 2 | Dead calf |
| 26 February 2019 | Usak | 0–6 | 20 | Dyspnea, cough, serous nasal secretions, fatigue | ||
| 13 January 2019 | Malkara | 0–6 | 18 | 18 | Dyspnea, cough, serous nasal secretions, fatigue | |
| 14 January 2019 | Kirklareli | 0–3 | 9 | 9 | Cough, serous nasal secretions, fatigue | |
| 26 January 2019 | Kirklareli | 0–6 | 25 | Dyspnea, cough, serous nasal secretions, fatigue | ||
| 10 February 2019 | Edirne | 0–3 | 15 | 15 | Cough, serous nasal secretions, fatigue | |
| 13 February 2019 | Cilingirkoy-Istanbul | 0–12 | 11 | 10 | Fever and cough | |
| 22 February 2019 | Luleburgaz | 0–6 | 19 | Dyspnea, cough, serous nasal secretions, fatigue | ||
| 23 February 2019 | Kirklareli | 0–12 | 3 | 1 | Dyspnea, cough, serous nasal secretions, fatigue | |
| 24 February 2019 | Kirklareli | 0–6 | 5 | Dyspnea, cough, serous nasal secretions, fatigue | ||
| 4 March 2021 | Luleburgaz | 0–6 | 25 | 25 | 1 | Dead calf |
| 9 March 2021 | Kirklareli | 0–6 | 4 | Dyspnea, cough, serous nasal secretions, fatigue | ||
| 18 March 2021 | Kirklareli | 0–6 | 9 | 9 | Dyspnea, cough, serous nasal secretions, fatigue | |
| 2 April 2021 | Kirklareli | 0–6 | 5 | Dyspnea, cough, serous nasal secretions, fatigue | ||
| 2 April 2021 | Buyukcekmece-Istanbul | 6–18 | 5 | Abattoir sample: Consolidation, hyperemia | ||
| 7 April 2021 | Cilingirkoy-Istanbul | 0–12 | 7 | 7 | Fever and cough | |
| 19 April 2021 | Edirne | 0–12 | 12 | 12 | Cough, serous nasal secretions, fatigue | |
| 7 April 2021 | Balikesir | 12–24 | 19 | Abattoir sample: Consolidation, hyperemia | ||
| 4 November 2021 | Edirne | 0–6 | 6 | 6 | Cough, serous nasal secretions, fatigue | |
| 17 December 2021 | Edirne | 0–6 | 11 | 11 | Cough, serous nasal secretions, fatigue | |
| 5 January 2022 | Esenyurt-Istanbul | 12–24 | 9 | Abattoir sample: Consolidation, hyperemia | ||
| 20 January 2022 | Usak | 0–6 | 3 | 3 | 1 | Dead calf Cough, serous nasal secretions, fatigue |
| 1 March 2022 | Edirne | 0–3 | 2 | Cough, serous nasal secretions, fatigue | ||
| 4 March 2022 | Usak | 0–6 | 20 | Cough, serous nasal secretions, fatigue | ||
| 7 March 2022 | Kirklareli | 0–3 | 10 | 10 | Fever, serous nasal secretions, fatigue | |
| Total | 162 | 277 | 37 | |||
| Primers and Probe | Target Genes | Primers and Probe Sequences | Size (bp) | Positions | References |
|---|---|---|---|---|---|
| B5A-B6A RT-PCR | G gene | F: 5′-CCACCCTAGCAATGATAACCTTGAC-3′ R: 5′-AAGAGAGGATGC(T/C)TTGCTGTGG-3′ | 603 | 110–134 691–712 | [29] |
| BRSV Real time RT-PCR | N gene | F: 5′-GCAATGCTGCAGGACTAGGTATAAT-3′ R: 5′-ACACTGTAATTGATGACCCCATTCT-3′ | 123 | 977–1001 1076–1100 | [39] |
| Probe | FAM-5-ACCAAGACTTGTATGATGCTGCCAAAGCA-3-TAMRA | 1028–1056 | [39] |
| Distance/ Distance | OQ713830_9716 2019_TÜRKİYE | OQ689745_7578 2019_TÜRKİYE | OQ736254_USOS1 2022_TÜRKİYE | OQ743526_USOS3 2022_TÜRKİYE | OQ789520_USAKC 2022_TÜRKİYE | Distance/ Distance | OQ713830_9716 2019_TÜRKİYE | OQ689745_7578 2019_TÜRKİYE | OQ736254_USOS1 2022_TÜRKİYE | OQ743526_USOS3 2022_TÜRKİYE | OQ789520_USAKC 2022_TÜRKİYE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OQ713830_9716_2019_TÜRKİYE | 0.00 | 96.36 | 89.88 | 89.88 | 88.26 | KY753468.1_2017_ITALY | 87.85 | 87.04 | 87.04 | 87.04 | 85.43 |
| OQ689745_7578_2019_TÜRKİYE | 96.36 | 0.00 | 89.07 | 89.07 | 87.45 | M58307.1_1990_USA | 87.45 | 87.45 | 86.64 | 86.64 | 85.02 |
| AY910755.1_2005_CZECHIA | 92.71 | 93.12 | 91.50 | 91.50 | 89.88 | Y08719.1_1997_ENGLAND | 87.45 | 88.26 | 87.45 | 87.45 | 85.83 |
| L08414.1_1997_USA | 92.71 | 93.12 | 90.69 | 90.69 | 89.47 | AY910765.1_2005_CZECHIA | 87.04 | 85.83 | 87.04 | 87.04 | 85.43 |
| AY910756.1_2005_CZECHIA | 92.31 | 92.71 | 91.09 | 91.09 | 89.47 | KF501149.1_2014_NORWAY | 87.04 | 87.85 | 86.23 | 86.23 | 84.62 |
| L08416.1_1997_USA | 91.90 | 92.31 | 90.69 | 90.69 | 89.07 | FJ555202.1_2008_BRASIL | 87.04 | 86.23 | 86.23 | 86.23 | 84.62 |
| L08415.1_1997_USA | 91.90 | 92.31 | 90.69 | 90.69 | 89.07 | AF188583.1_2000_FRANCE | 87.04 | 87.04 | 87.04 | 87.04 | 85.43 |
| MW881233.1_2021_TÜRKİYE | 91.09 | 91.90 | 93.93 | 93.93 | 92.31 | Y08717.1_1997_ENGLAND | 86.64 | 86.23 | 86.64 | 86.64 | 85.02 |
| U24716.1_1997_BELGIUM | 91.09 | 91.50 | 90.69 | 90.69 | 89.07 | U92100.1_1997_DENMARK | 86.23 | 86.23 | 86.23 | 86.23 | 84.62 |
| LC499991.1_2019_JAPAN | 91.09 | 92.31 | 89.88 | 89.88 | 88.26 | AF188588.1_2000_FRANCE | 85.83 | 85.43 | 85.83 | 85.83 | 84.21 |
| L08411.1_1997_USA | 90.69 | 91.09 | 89.47 | 89.47 | 87.85 | KY660261.1_2017_CROATIA | 85.43 | 85.43 | 83.81 | 83.81 | 82.19 |
| MH133326.1_2018_TÜRKIYE | 90.28 | 89.47 | 98.79 | 98.79 | 97.17 | KY753469.1_2017_ITALY | 85.43 | 83.81 | 84.62 | 84.62 | 83.00 |
| MH133327.1_2018_TÜRKİYE | 90.28 | 89.47 | 98.79 | 98.79 | 97.17 | AF188604.1_2000_FRANCE | 85.43 | 85.02 | 86.23 | 86.23 | 84.62 |
| OQ736254_USOS1_2022_TÜRKİYE | 89.88 | 89.07 | 0.00 | 100.00 | 98.38 | AF188603.1_2000_FRANCE | 84.62 | 84.21 | 85.43 | 85.43 | 83.81 |
| OQ743526_USOS3_2022_TÜRKİYE | 89.88 | 89.07 | 100.00 | 0.00 | 98.38 | KY753463.1_2017_ITALY | 84.62 | 85.02 | 83.81 | 83.81 | 82.19 |
| MW881234.1_2021_TÜRKiYE | 89.88 | 90.69 | 95.14 | 95.14 | 93.52 | KF501172.1_2014_NORWAY | 84.21 | 85.02 | 82.59 | 82.59 | 80.97 |
| KY680331.1_2018_CROATIA | 89.88 | 88.66 | 88.66 | 88.66 | 87.04 | KY753456.1_2017_ITALY | 84.21 | 83.81 | 85.02 | 85.02 | 83.40 |
| LC499984.1_2019_JAPAN | 89.88 | 90.28 | 89.47 | 89.47 | 87.85 | KY753464.1_2017_ITALY | 84.21 | 83.81 | 83.00 | 83.00 | 81.38 |
| KY680337.1_2018_CROATIA | 89.47 | 88.26 | 88.26 | 88.26 | 86.64 | KY680318.1_2018_CROATIA | 84.21 | 84.62 | 84.21 | 84.21 | 82.59 |
| KY680336.1_2018_CROATIA | 89.47 | 88.26 | 88.26 | 88.26 | 86.64 | KF501153.1_2014_NORWAY | 83.81 | 85.43 | 82.19 | 82.19 | 80.57 |
| KY680333.1_2018_CROATIA | 89.47 | 88.26 | 88.26 | 88.26 | 86.64 | AF188597.1_2000_FRANCE | 83.81 | 83.40 | 83.81 | 83.81 | 82.19 |
| KY753451.1_2017_ITALY | 89.47 | 88.26 | 88.26 | 88.26 | 86.64 | MK599397.1_2019_BRASIL | 83.81 | 84.21 | 82.59 | 82.59 | 80.97 |
| MN129181.1_2019_IRAQ | 89.07 | 87.85 | 87.85 | 87.85 | 86.23 | KY680327.1_2018_CROATIA | 83.40 | 83.81 | 84.21 | 84.21 | 82.59 |
| AF092942.1_1998_GERMANY | 89.07 | 89.07 | 87.45 | 87.45 | 85.83 | KY680328.1_2018_CROATIA | 83.00 | 83.40 | 83.81 | 83.81 | 82.19 |
| KY753452.1_2017_ITALY | 89.07 | 87.85 | 88.66 | 88.66 | 87.04 | Y11205.1_1997_ENGLAND | 82.59 | 81.38 | 81.38 | 81.38 | 79.76 |
| LC499985.1_2019_JAPAN | 89.07 | 88.66 | 87.85 | 87.85 | 86.23 | U57823.1_1997_BELGIUM | 82.59 | 81.38 | 81.38 | 81.38 | 79.76 |
| OQ789520_USAKC_2022_TÜRKİYE | 88.26 | 87.45 | 98.38 | 98.38 | 0.00 | AF295544.1_2001_USA | 82.59 | 81.38 | 81.38 | 81.38 | 79.76 |
| AF248593.1_2000_DENMARK | 88.26 | 88.26 | 87.45 | 87.45 | 85.83 | MK599389.1_2019_BRASIL | 82.19 | 83.40 | 80.57 | 80.57 | 78.95 |
| AF188584.1_2000_FRANCE | 88.26 | 88.26 | 87.45 | 87.45 | 85.83 | AF188585.1_2000_FRANCE | 81.38 | 81.78 | 82.19 | 82.19 | 80.57 |
| AF188582.1_2000_FRANCE | 88.26 | 88.26 | 86.64 | 86.64 | 85.02 | AF188586.1_2000_FRANCE | 81.38 | 81.78 | 82.19 | 82.19 | 80.57 |
| LC499994.1_2019_JAPAN | 88.26 | 88.66 | 88.66 | 88.66 | 87.04 | AF188587.1_2000_FRANCE | 81.38 | 81.78 | 82.19 | 82.19 | 80.57 |
| U24714.1_1997_BELGIUM | 87.85 | 87.85 | 87.04 | 87.04 | 85.43 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydin, O.; Yilmaz, A.; Turan, N.; Richt, J.A.; Yilmaz, H. Molecular Characterisation and Antibody Response to Bovine Respiratory Syncytial Virus in Vaccinated and Infected Cattle in Turkey. Pathogens 2024, 13, 304. https://doi.org/10.3390/pathogens13040304
Aydin O, Yilmaz A, Turan N, Richt JA, Yilmaz H. Molecular Characterisation and Antibody Response to Bovine Respiratory Syncytial Virus in Vaccinated and Infected Cattle in Turkey. Pathogens. 2024; 13(4):304. https://doi.org/10.3390/pathogens13040304
Chicago/Turabian StyleAydin, Ozge, Aysun Yilmaz, Nuri Turan, Juergen A. Richt, and Huseyin Yilmaz. 2024. "Molecular Characterisation and Antibody Response to Bovine Respiratory Syncytial Virus in Vaccinated and Infected Cattle in Turkey" Pathogens 13, no. 4: 304. https://doi.org/10.3390/pathogens13040304





