Respiratory Viral Coinfections: Insights into Epidemiology, Immune Response, Pathology, and Clinical Outcomes
Abstract
1. Introduction
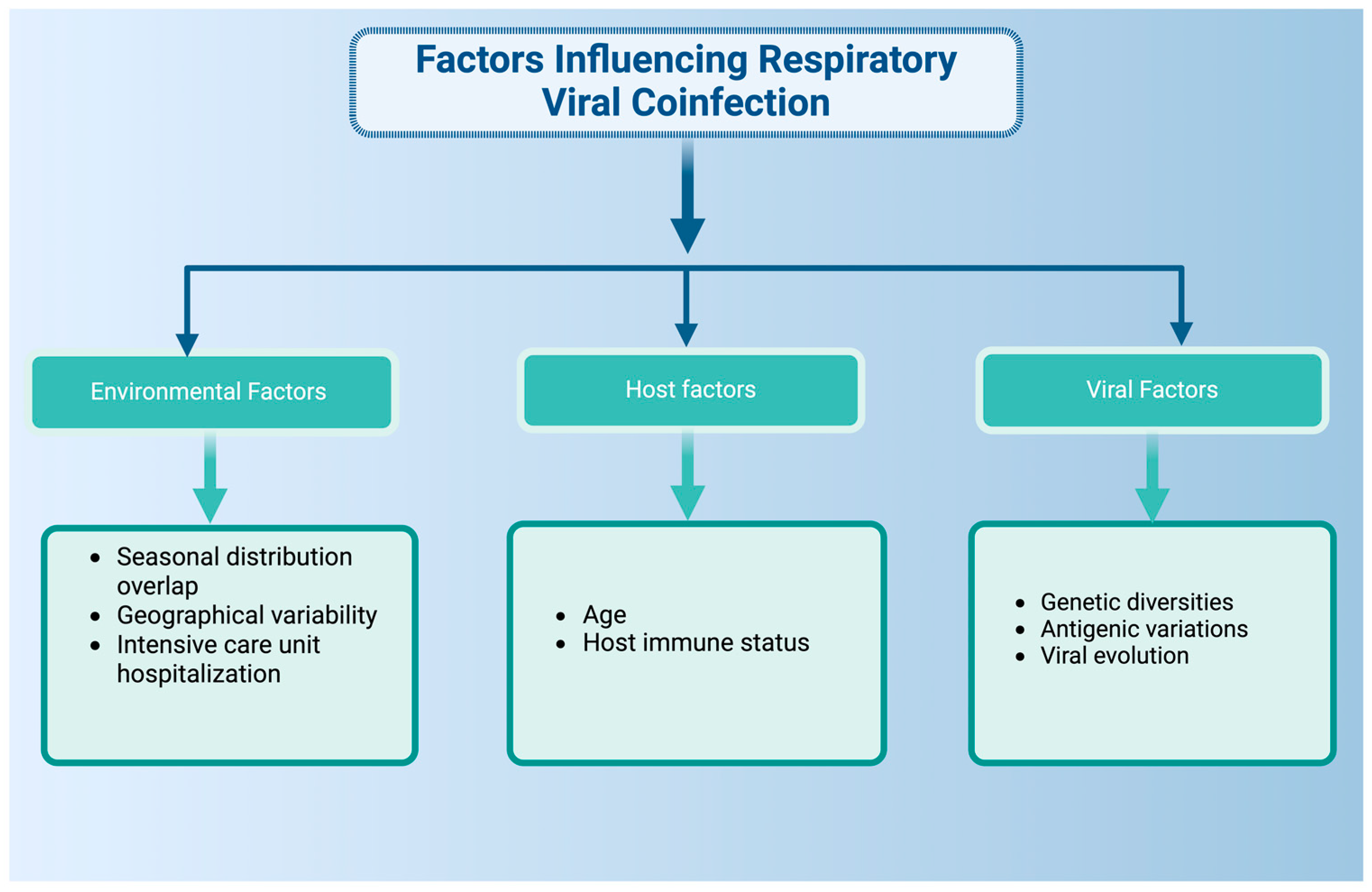
2. Epidemiology
3. Pathology, Disease Outcomes, and Severity
4. Modulation of Immune Response to Different Combinations of Respiratory Viral Infection
5. Experimental Model for Studying Respiratory Viral Coinfections
6. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Tregoning, J.S.; Schwarze, J.R. Respiratory Viral Infections in Infants: Causes, Clinical Symptoms, Virology, and Immunology. Clin. Microbiol. Rev. 2010, 23, 74–98. [Google Scholar] [CrossRef]
- Goto, H.; Ihira, H.; Morishita, K.; Tsuchiya, M.; Ohta, K.; Yumine, N.; Tsurudome, M.; Nishio, M. Enhanced growth of influenza A virus by coinfection with human parainfluenza virus type 2. Med. Microbiol. Immunol. 2016, 205, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, P.J.; Nakajima, N.; Sato, Y.; Takahashi, K.; Accola, M.; Chiba, S.; Fan, S.; Neumann, G.; Rehrauer, W.; Suzuki, T.; et al. SARS-CoV-2 Interference of Influenza Virus Replication in Syrian Hamsters. J. Infect. Dis. 2022, 225, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Nickbakhsh, S.; Thorburn, F.; Wissmann, B.V.; Mcmenamin, J.; Gunson, R.N.; Murcia, P.R. Extensive multiplex PCR diagnostics reveal new insights into the epidemiology of viral respiratory infections. Epidemiol. Infect. 2016, 144, 2064–2076. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.; Adams, O.; Willems, R.; Bonzel, L.; Neuhausen, N.; Schweizer-Krantz, S.; Ruggeberg, J.U.; Willers, R.; Henrich, B.; Schroten, H.; et al. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J. Clin. Virol. 2010, 48, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Goka, E.A.; Vallely, P.J.; Mutton, K.J.; Klapper, P.E. Single and multiple respiratory virus infections and severity of respiratory disease: A systematic review. Paediatr. Respir. Rev. 2014, 15, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Scotta, M.C.; Chakr, V.C.; de Moura, A.; Becker, R.G.; de Souza, A.P.; Jones, M.H.; Pinto, L.A.; Sarria, E.E.; Pitrez, P.M.; Stein, R.T.; et al. Respiratory viral coinfection and disease severity in children: A systematic review and meta-analysis. J. Clin. Virol. 2016, 80, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Asner, S.A.; Science, M.E.; Tran, D.; Smieja, M.; Merglen, A.; Mertz, D. Clinical Disease Severity of Respiratory Viral Co-Infection versus Single Viral Infection: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e99392. [Google Scholar] [CrossRef]
- Krumbein, H.; Kümmel, L.S.; Fragkou, P.C.; Thölken, C.; Hünerbein, B.L.; Reiter, R.; Papathanasiou, K.A.; Renz, H.; Skevaki, C. Respiratory viral co-infections in patients with COVID-19 and associated outcomes: A systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2365. [Google Scholar] [CrossRef]
- Stempel, H.E.; Martin, E.T.; Kuypers, J.; Englund, J.A.; Zerr, D.M. Multiple viral respiratory pathogens in children with bronchiolitis. Acta Paediatr. 2009, 98, 123–126. [Google Scholar] [CrossRef]
- Brunstein, J.D.; Cline, C.L.; McKinney, S.; Thomas, E. Evidence from multiplex molecular assays for complex multipathogen interactions in acute respiratory infections. J. Clin. Microbiol. 2008, 46, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Peci, A.; Tran, V.; Guthrie, J.L.; Li, Y.; Nelson, P.; Schwartz, K.L.; Eshaghi, A.; Buchan, S.A.; Gubbay, J.B. Prevalence of Co-Infections with Respiratory Viruses in Individuals Investigated for SARS-CoV-2 in Ontario, Canada. Viruses 2021, 13, 130. [Google Scholar] [CrossRef]
- Kim, D.; Quinn, J.; Pinsky, B.; Shah, N.H.; Brown, I. Rates of Co-infection between SARS-CoV-2 and Other Respiratory Pathogens. JAMA 2020, 323, 2085–2086. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, P.; Xiong, G.; Yang, Z.; Wang, M.; Li, Y.; Yu, X.-J. Coinfection of SARS-CoV-2 and multiple respiratory pathogens in children. Clin. Chem. Lab. Med. 2020, 58, 1160–1161. [Google Scholar] [CrossRef] [PubMed]
- Tanner, H.; Boxall, E.; Osman, H. Respiratory viral infections during the 2009–2010 winter season in Central England, UK: Incidence and patterns of multiple virus co-infections. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3001–3006. [Google Scholar] [CrossRef] [PubMed]
- Nickbakhsh, S.; Mair, C.; Matthews, L.; Reeve, R.; Johnson, P.C.; Thorburn, F.; Von Wissmann, B.; Reynolds, A.; McMenamin, J.; Gunson, R.N.; et al. Virus–virus interactions impact the population dynamics of influenza and the common cold. Proc. Natl. Acad. Sci. USA 2019, 116, 27142–27150. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.T.; Kuypers, J.; Wald, A.; Englund, J.A. Multiple versus single virus respiratory infections: Viral load and clinical disease severity in hospitalized children. Influenza Other Respir. Viruses 2012, 6, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; Irvem, A.; Guner, A.E.; Kazezoglu, C.; Kocatas, A. Investigation of respiratory tract coinfections in Coronavirus disease 2019 infected and suspected cases. North. Clin. Istanb. 2022, 9, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.J.; Pfotenhauer, B.; Weiner, J.J.; Hilleshiem, J.; Khubbar, M.; Bhattacharyya, S. Respiratory Pathogen Coinfections in SARS-CoV-2-Positive Patients in Southeastern Wisconsin: A Retrospective Analysis. Microbiol. Spectr. 2021, 9, e00831-21. [Google Scholar] [CrossRef]
- Le Glass, E.; Hoang, V.T.; Boschi, C.; Ninove, L.; Zandotti, C.; Boutin, A.; Bremond, V.; Dubourg, G.; Ranque, S.; Lagier, J.-C.; et al. Incidence and Outcome of Coinfections with SARS-CoV-2 and Rhinovirus. Viruses 2021, 13, 2528. [Google Scholar] [CrossRef]
- Pigny, F.; Wagner, N.; Rohr, M.; Mamin, A.; Cherpillod, P.; Posfay-Barbe, K.M.; Kaiser, L.; Eckerle, I.; L’Huillier, A.G. Viral co-infections among SARS-CoV-2-infected children and infected adult household contacts. Eur. J. Pediatr. 2021, 180, 1991–1995. [Google Scholar] [CrossRef]
- Swets, M.C.; Russell, C.D.; Harrison, E.M.; Docherty, A.B.; Lone, N.; Girvan, M.; Hardwick, H.E.; Visser, L.G.; Openshaw, P.J.; Groeneveld, G.H.; et al. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet 2022, 399, 1463–1464. [Google Scholar] [CrossRef]
- Zhu, X.; Ge, Y.; Wu, T.; Zhao, K.; Chen, Y.; Wu, B.; Zhu, F.; Zhu, B.; Cui, L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020, 285, 198005. [Google Scholar] [CrossRef] [PubMed]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef] [PubMed]
- Dikranian, L.; Barry, S.; Ata, A.; Chiotos, K.; Gist, K.; Bhalala, U.; Danesh, V.; Heavner, S.; Gharpure, V.; Bjornstad, E.C.; et al. SARS-CoV-2 with Concurrent Respiratory Viral Infection as a Risk Factor for a Higher Level of Care in Hospitalized Pediatric Patients. Pediatr. Emerg. Care 2022, 38, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Shannon, K.L.; Osula, V.O.; Shaw-Saliba, K.; Hardick, J.; McBryde, B.; Dugas, A.; Hsieh, Y.H.; Hansoti, B.; Rothman, R.E. Viral co-infections are associated with increased rates of hospitalization in those with influenza. Influenza Other Respir. Viruses 2022, 16, 780–788. [Google Scholar] [CrossRef]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Do, A.H.; van Doorn, H.R.; Nghiem, M.N.; Bryant, J.E.; Hoang, T.H.; Do, Q.H.; Van, T.L.; Tran, T.T.; Wills, B.; Nguyen, V.C.; et al. Viral etiologies of acute respiratory infections among hospitalized Vietnamese children in Ho Chi Minh City, 2004–2008. PLoS ONE 2011, 6, e18176. [Google Scholar] [CrossRef]
- Mandelia, Y.; Procop, G.W.; Richter, S.S.; Worley, S.; Liu, W.; Esper, F. Dynamics and predisposition of respiratory viral co-infections in children and adults. Clin. Microbiol. Infect. 2021, 27, e1–e631. [Google Scholar] [CrossRef]
- Hazra, A.; Collison, M.; Pisano, J.; Kumar, M.; Oehler, C.; Ridgway, J.P. Coinfections with SARS-CoV-2 and other respiratory pathogens. Infect. Control Hosp. Epidemiol. 2020, 41, 1228–1229. [Google Scholar] [CrossRef]
- Singh, V.; Upadhyay, P.; Reddy, J.; Granger, J. SARS-CoV-2 respiratory co-infections: Incidence of viral and bacterial co-pathogens. Int. J. Infect. Dis. 2021, 105, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Horemheb-Rubio, G.; Eggeling, R.; Schmeiβer, N.; Pfeifer, N.; Lengauer, T.; Gärtner, B.C.; Prifert, C.; Kochanek, M.; Scheid, C.; Adams, O.; et al. Respiratory viruses dynamics and interactions: Ten years of surveillance in central Europe. BMC Public Health 2022, 22, 1167. [Google Scholar] [CrossRef] [PubMed]
- Huguenin, A.; Moutte, L.; Renois, F.; Leveque, N.; Talmud, D.; Abely, M.; Nguyen, Y.; Carrat, F.; Andreoletti, L. Broad respiratory virus detection in infants hospitalized for bronchiolitis by use of a multiplex RT-PCR DNA microarray system. J. Med. Virol. 2012, 84, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Cilla, G.; Oñate, E.; Perez-Yarza, E.G.; Montes, M.; Vicente, D.; Perez-Trallero, E. Viruses in community-acquired pneumonia in children aged less than 3 years old: High rate of viral coinfection. J. Med. Virol. 2008, 80, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Boivin, G.; De Serres, G.; Côté, S.; Gilca, R.; Abed, Y.; Rochette, L.; Bergeron, M.G.; Déry, P. Human Metapneumovirus Infections in Hospitalized Children1. Emerg. Infect. Dis. 2003, 9, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Kouni, S.; Karakitsos, P.; Chranioti, A.; Theodoridou, M.; Chrousos, G.; Michos, A. Evaluation of viral co-infections in hospitalized and non-hospitalized children with respiratory infections using microarrays. Clin. Microbiol. Infect. 2013, 19, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Boivin, G. Viral Interference between Respiratory Viruses. Emerg. Infect. Dis. 2022, 28, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.J.; Lee, A.C.; Chan, J.F.; Liu, F.; Li, C.; Chen, Y.; Chu, H.; Lau, S.Y.; Wang, P.; Chan, C.C.; et al. Coinfection by Severe Acute Respiratory Syndrome Coronavirus 2 and Influenza A(H1N1)pdm09 Virus Enhances the Severity of Pneumonia in Golden Syrian Hamsters. Clin. Infect. Dis. 2021, 72, e978–e992. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.F.; Carolan, L.A.; Korenkov, D.; Druce, J.; McCaw, J.; Reading, P.C.; Barr, I.G.; Laurie, K.L. Investigating Viral Interference between Influenza A Virus and Human Respiratory Syncytial Virus in a Ferret Model of Infection. J. Infect. Dis. 2018, 218, 406–417. [Google Scholar] [CrossRef]
- Laurie, K.L.; Horman, W.; Carolan, L.A.; Chan, K.F.; Layton, D.; Bean, A.; Vijaykrishna, D.; Reading, P.C.; McCaw, J.M.; Barr, I.G. Evidence for Viral Interference and Cross-reactive Protective Immunity between Influenza B Virus Lineages. J. Infect. Dis. 2018, 217, 548–559. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Nair, H. Global Seasonality of Human Seasonal Coronaviruses: A Clue for Postpandemic Circulating Season of Severe Acute Respiratory Syndrome Coronavirus 2? J. Infect. Dis. 2020, 222, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Sullender, W.M. Respiratory Syncytial Virus Genetic and Antigenic Diversity. Clin. Microbiol. Rev. 2000, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lau SK, P.; Li, K.S.; Huang, Y.; Shek, C.-T.; Tse, H.; Wang, M.; Choi, G.K.; Xu, H.; Lam, C.S.; Guo, R.; et al. Ecoepidemiology and Complete Genome Comparison of Different Strains of Severe Acute Respiratory Syndrome-Related Rhinolophus Bat Coronavirus in China Reveal Bats as a Reservoir for Acute, Self-Limiting Infection That Allows. J. Virol. 2010, 84, 2808–2819. [Google Scholar] [PubMed]
- Haney, J.; Vijayakrishnan, S.; Streetley, J.; Dee, K.; Goldfarb, D.M.; Clarke, M.; Mullin, M.; Carter, S.D.; Bhella, D.; Murcia, P.R. Coinfection by influenza A virus and respiratory syncytial virus produces hybrid virus particles. Nat. Microbiol. 2022, 7, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gong, Y.; Zhang, C.; Sun, J.; Wong, G.; Shi, W.; Liu, W.; Gao, G.F.; Bi, Y. Co-existence and co-infection of influenza A viruses and coronaviruses: Public health challenges. Innovation 2022, 3, 100306. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cui, X.; Cai, X.; An, T. Recombination in Positive-Strand RNA Viruses. Front. Microbiol. 2022, 13, 870759. [Google Scholar] [CrossRef] [PubMed]
- Pisano, M.B.; Sicilia, P.; Zeballos, M.; Lucca, A.; Fernandez, F.; Castro, G.M.; Goya, S.; Viegas, M.; López, L.; Barbás, M.G.; et al. SARS-CoV-2 Genomic Surveillance Enables the Identification of Delta/Omicron Co-Infections in Argentina. Front. Virol. 2022, 2, 910839. [Google Scholar] [CrossRef]
- Canducci, F.; Debiaggi, M.; Sampaolo, M.; Marinozzi, M.C.; Berrè, S.; Terulla, C.; Gargantini, G.; Cambieri, P.; Romero, E.; Clementi, M. Two-year prospective study of single infections and co-infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease. J. Med. Virol. 2008, 80, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, L.E.; Nasser, A.M.; Dove, W.; Gurgel, R.Q.; Greensill, J.; Hart, C.A. Human metapneumovirus and respiratory syncytial virus, Brazil. Emerg. Infect. Dis. 2003, 9, 1626–1628. [Google Scholar] [CrossRef]
- Escobar, K.; Godinez, A.; Funes Merida, E.; Moscoso, F. Metapneumovirus infection vs. metapneumovirus and RSV coinfection. Is there a difference? Eur. Respir. J. 2015, 46, PA3622. [Google Scholar]
- Semple, M.G.; Cowell, A.; Dove, W.; Greensill, J.; McNamara, P.S.; Halfhide, C.; Shears, P.; Smyth, R.L.; Hart, C.A. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J. Infect. Dis. 2005, 191, 382–386. [Google Scholar] [CrossRef]
- McNamara, P.S.; Flanagan, B.F.; Smyth, R.L.; Hart, C.A. Impact of human metapneumovirus and respiratory syncytial virus co-infection in severe bronchiolitis. Pediatr. Pulmonol. 2007, 42, 740–743. [Google Scholar] [CrossRef]
- Li, Y.; Pillai, P.; Miyake, F.; Nair, H. The role of viral co-infections in the severity of acute respiratory infections among children infected with respiratory syncytial virus (RSV): A systematic review and meta-analysis. J. Glob. Health 2020, 10, 010426. [Google Scholar] [CrossRef]
- Greensill, J.; McNamara, P.S.; Dove, W.; Flanagan, B.; Smyth, R.L.; Hart, C.A. Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg. Infect. Dis. 2003, 9, 372–375. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, Y.; Dong, J.; Liang, S.; Guo, M.; Liu, X.; Wang, X.; Huang, Z.; Sun, X.; Zhang, Z.; et al. Coinfection with influenza A virus enhances SARS-CoV-2 infectivity. Cell Res. 2021, 31, 395–403. [Google Scholar] [CrossRef]
- Geiser, J.; Boivin, G.; Huang, S.; Constant, S.; Kaiser, L.; Tapparel, C.; Essaidi-Laziosi, M. RSV and HMPV Infections in 3D Tissue Cultures: Mechanisms Involved in Virus-Host and Virus-Virus Interactions. Viruses 2021, 13, 139. [Google Scholar] [CrossRef]
- Wu, A.; Mihaylova, V.T.; Landry, M.L.; Foxman, E.F. Interference between rhinovirus and influenza A virus: A clinical data analysis and experimental infection study. Lancet Microbe 2020, 1, e254–e262. [Google Scholar] [CrossRef]
- Kim, H.K.; Kang, J.A.; Lyoo, K.S.; Le, T.B.; Yeo, Y.H.; Wong, S.S.; Na, W.; Song, D.; Webby, R.J.; Zanin, M.; et al. Severe acute respiratory syndrome coronavirus 2 and influenza A virus co-infection alters viral tropism and haematological composition in Syrian hamsters. Transbound. Emerg. Dis. 2022, 69, e3297–e3304. [Google Scholar] [CrossRef]
- Kim, E.H.; Nguyen, T.Q.; Casel MA, B.; Rollon, R.; Kim, S.M.; Kim, Y.I.; Yu, K.M.; Jang, S.G.; Yang, J.; Poo, H.; et al. Coinfection with SARS-CoV-2 and Influenza A Virus Increases Disease Severity and Impairs Neutralizing Antibody and CD4+ T Cell Responses. J. Virol. 2022, 96, e0187321. [Google Scholar] [CrossRef]
- Drews, A.L.; Atmar, R.L.; Glezen, W.P.; Baxter, B.D.; Piedra, P.A.; Greenberg, S.B. Dual Respiratory Virus Infections. Clin. Infect. Dis. 1997, 25, 1421–1429. [Google Scholar] [CrossRef]
- Le Hingrat, Q.; Bouzid, D.; Choquet, C.; Laurent, O.; Lescure, F.X.; Timsit, J.F.; Houhou-Fidouh, N.; Casalino, E.; Lucet, J.C.; Descamps, D.; et al. Viral epidemiology and SARS-CoV-2 co-infections with other respiratory viruses during the first COVID-19 wave in Paris, France. Influenza Other Respir. Viruses 2021, 15, 425–428. [Google Scholar] [CrossRef]
- Cong, B.; Deng, S.; Wang, X.; Li, Y. The role of respiratory co-infection with influenza or respiratory syncytial virus in the clinical severity of COVID-19 patients: A systematic review and meta-analysis. J. Global Health 2022, 12, 05040. [Google Scholar] [CrossRef]
- Trifonova, I.; Christova, I.; Madzharova, I.; Angelova, S.; Voleva, S.; Yordanova, R.; Tcherveniakova, T.; Krumova, S.; Korsun, N. Clinical significance and role of coinfections with respiratory pathogens among individuals with confirmed severe acute respiratory syndrome coronavirus-2 infection. Front. Public Health 2022, 10, 959319. [Google Scholar] [CrossRef]
- Kinoshita, T.; Watanabe, K.; Sakurai, Y.; Nishi, K.; Yoshikawa, R.; Yasuda, J. Co-infection of SARS-CoV-2 and influenza virus causes more severe and prolonged pneumonia in hamsters. Sci. Rep. 2021, 11, 21259. [Google Scholar] [CrossRef]
- Hartwig, S.M.; Miller, A.M.; Varga, S.M. Respiratory Syncytial Virus Provides Protection against a Subsequent Influenza A Virus Infection. J. Immunol. 2022, 208, 720–731. [Google Scholar] [CrossRef]
- George, J.A.; AlShamsi, S.H.; Alhammadi, M.H.; Alsuwaidi, A.R. Exacerbation of Influenza A Virus Disease Severity by Respiratory Syncytial Virus Co-Infection in a Mouse Model. Viruses 2021, 13, 1630. [Google Scholar] [CrossRef]
- Cox, G.; Gonzalez, A.J.; Ijezie, E.C.; Rodriguez, A.; Miller, C.R.; Van Leuven, J.T.; Miura, T.A. Priming with Rhinovirus Protects Mice Against a Lethal Pulmonary Coronavirus Infection. Front. Immunol. 2022, 13, 886611. [Google Scholar] [CrossRef]
- Pinky, L.; Dobrovolny, H.M. Epidemiological Consequences of Viral Interference: A Mathematical Modeling Study of Two Interacting Viruses. Front. Microbiol. 2022, 13, 830423. [Google Scholar] [CrossRef]
- Morris, D.R.; Qu, Y.; Thomason, K.S.; Haas De Mello, A.; Preble, R.; Menachery, V.D.; Casola, A.; Garofalo, R.P. The impact of RSV/SARS-CoV-2 co-infection on clinical disease and viral replication: Insights from a BALB/c mouse model. bioRxiv 2023. [Google Scholar] [CrossRef]
- Alvares, P.A. SARS-CoV-2 and Respiratory Syncytial Virus Coinfection in Hospitalized Pediatric Patients. Pediatr. Infect. Dis. J. 2021, 40, e164–e166. [Google Scholar] [CrossRef]
- Kaida, A.; Kubo, H.; Goto, K.; Shiomi, M.; Kohdera, U.; Iritani, N. Co-Infection of Human Metapneumovirus with Adenovirus or Respiratory Syncytial Virus among Children in Japan. Microbiol. Immunol. 2007, 51, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Calvo, C.; García-García, M.L.; Pozo, F.; Paula, G.; Molinero, M.; Calderón, A.; González-Esguevillas, M.; Casas, I. Respiratory Syncytial Virus Coinfections with Rhinovirus and Human Bocavirus in Hospitalized Children. Medicine 2015, 94, e1788. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sharma, S.; Barua, S.; Tripathi, B.N.; Rouse, B.T. Virological and Immunological Outcomes of Coinfections. Clin. Microbiol. Rev. 2018, 31, 10–128. [Google Scholar] [CrossRef]
- Forbester, J.L.; Humphreys, I.R. Genetic influences on viral-induced cytokine responses in the lung. Mucosal Immunol. 2021, 14, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, E.; Panera, N.; Alisi, A.; Carloni, E.; Russo, L.; Campagna, I.; Rizzo, C.; Concato, C.; Linardos, G.; Piccioni, L.; et al. Cytokine expression patterns in hospitalized children with Bordetella pertussis, Rhinovirus or co-infection. Sci. Rep. 2021, 11, 10948. [Google Scholar] [CrossRef]
- Devi, P.; Khan, A.; Chattopadhyay, P.; Mehta, P.; Sahni, S.; Sharma, S.; Pandey, R. Co-infections as Modulators of Disease Outcome: Minor Players or Major Players? Front. Microbiol. 2021, 12, 664386. [Google Scholar] [CrossRef]
- Gonzalez, A.J.; Ijezie, E.C.; Balemba, O.B.; Miura, T.A. Attenuation of Influenza A Virus Disease Severity by Viral Coinfection in a Mouse Model. J. Virol. 2018, 92, 10–128. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Winkler, E.S.; Bailey, A.L.; Kafai, N.M.; Nair, S.; McCune, B.T.; Yu, J.; Fox, J.M.; Chen, R.E.; Earnest, J.T.; Keeler, S.P.; et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 2020, 21, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Mead, H.; Tian, L.; Park, J.G.; Garcia, J.I.; Jaramillo, S.; Barr, T.; Kollath, D.S.; Coyne, V.K.; Stone, N.E.; et al. The K18-Human ACE2 Transgenic Mouse Model Recapitulates Non-severe and Severe COVID-19 in Response to an Infectious Dose of the SARS-CoV-2 Virus. J. Virol. 2022, 96, e0096421. [Google Scholar] [CrossRef]
- Belser, J.A.; Katz, J.M.; Tumpey, T.M. The ferret as a model organism to study influenza A virus infection. Dis. Model. Mech. 2011, 4, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Rennick, L.J.; Nambulli, S.; Lemon, K.; Olinger, G.Y.; Crossland, N.A.; Millar, E.L.; Duprex, W.P. Recombinant subtype A and B human respiratory syncytial virus clinical isolates co-infect the respiratory tract of cotton rats. J. Gen. Virol. 2020, 101, 1056–1068. [Google Scholar] [CrossRef]
- Bourzac, K. Respiratory syncytial virus co-infections might conspire to worsen disease. Nature 2023, 621, S60–S61. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Barrero Guevara, L.A.; Goult, E.; Briga, M.; Kramer, S.C.; Kovacevic, A.; Opatowski, L.; Domenech De Cellès, M. The interactions of SARS-CoV-2 with cocirculating pathogens: Epidemiological implications and current knowledge gaps. PLoS Pathog. 2023, 19, e1011167. [Google Scholar] [CrossRef]
- Martin, B.E.; Harris, J.D.; Sun, J.; Koelle, K.; Brooke, C.B. Cellular co-infection can modulate the efficiency of influenza A virus production and shape the interferon response. PLoS Pathog. 2020, 16, e1008974. [Google Scholar] [CrossRef]
- Malausse, N.; van der Werf, S.; Naffakh, N.; Munier, S. Influenza B Virus Infection Is Enhanced Upon Heterotypic Co-infection with Influenza A Virus. Front. Microbiol. 2021, 12, 631346. [Google Scholar] [CrossRef]
- Liu, Y.; Ling, L.; Wong, S.H.; Wang, M.H.; Fitzgerald, J.R.; Zou, X.; Fang, S.; Liu, X.; Wang, X.; Hu, W.; et al. Outcomes of respiratory viral-bacterial co-infection in adult hospitalized patients. eClinicalMedicine 2021, 37, 100955. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Papanikolopoulou, A.; Vassiliu, S.; Theodoridou, K.; Nikolopoulou, G.; Sipsas, N.V. COVID-19 and Respiratory Virus Co-Infections: A Systematic Review of the Literature. Viruses 2023, 15, 865. [Google Scholar] [CrossRef]
- Baldassi, D.; Gabold, B.; Merkel, O.M. Air−Liquid Interface Cultures of the Healthy and Diseased Human Respiratory Tract: Promises, Challenges, and Future Directions. Adv. NanoBiomed Res. 2021, 1, 2000111. [Google Scholar] [CrossRef]
- Dee, K.; Schultz, V.; Haney, J.; Bissett, L.A.; Magill, C.; Murcia, P.R. Influenza A and Respiratory Syncytial Virus Trigger a Cellular Response That Blocks Severe Acute Respiratory Syndrome Virus 2 Infection in the Respiratory Tract. J. Infect. Dis. 2022, 227, 1396–1406. [Google Scholar] [CrossRef]
- Cheemarla, N.R.; Watkins, T.A.; Mihaylova, V.T.; Foxman, E.F. Viral interference during influenza A-SARS-CoV-2 coinfection of the human airway epithelium and reversal by oseltamivir. J. Infect. Dis. 2023, jiad402. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, A.; Padey, B.; Duliere, V.; Mouton, W.; Oliva, J.; Laurent, E.; Milesi, C.; Lina, B.; Traversier, A.; Julien, T.; et al. Interactions between Severe Acute Respiratory Syndrome Coronavirus 2 Replication and Major Respiratory Viruses in Human Nasal Epithelium. J. Infect. Dis. 2022, 226, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Bosakova, V.; De Zuani, M.; Sladkova, L.; Garlikova, Z.; Jose, S.S.; Zelante, T.; Hortova Kohoutkova, M.; Fric, J. Lung Organoids—The Ultimate Tool to Dissect Pulmonary Diseases? Front. Cell Dev. Biol. 2022, 10, 899368. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.; Kim, H.; Gil, D.; Han, H.J.; Thimmulappa, R.K.; Choi, J.H.; Kim, J.H. Reciprocal enhancement of SARS-CoV-2 and influenza virus replication in human pluripotent stem cell-derived lung organoids. Emerg. Microbes Infect. 2023, 12, 2211685. [Google Scholar] [CrossRef]
- Hye, M.A.; Biswas MA, H.A.; Uddin, M.F.; Saifuddin, M. Mathematical Modeling of COVID-19 and Dengue Co-Infection Dynamics in Bangladesh: Optimal Control and Data-Driven Analysis. Comput. Math. Model. 2022, 33, 173–192. [Google Scholar] [CrossRef]
- Pinky, L.; Dobrovolny, H.M. Coinfections of the Respiratory Tract: Viral Competition for Resources. PLoS ONE 2016, 11, e0155589. [Google Scholar] [CrossRef]
- Dobrovolny, H.M.; Reddy, M.B.; Kamal, M.A.; Rayner, C.R.; Beauchemin, C.A. Assessing Mathematical Models of Influenza Infections Using Features of the Immune Response. PLoS ONE 2013, 8, e57088. [Google Scholar] [CrossRef]
- Dwomoh, D.; Iddi, S.; Adu, B.; Aheto, J.M.; Sedzro, K.M.; Fobil, J.; Bosomprah, S. Mathematical modeling of COVID-19 infection dynamics in Ghana: Impact evaluation of integrated government and individual level interventions. Infect. Dis. Model. 2021, 6, 381–397. [Google Scholar] [CrossRef]
- Kapoor, V.; Briese, T.; Ranjan, A.; Donovan, W.M.; Mansukhani, M.M.; Chowdhary, R.; Lipkin, W.I. Validation of the VirCapSeq-VERT system for differential diagnosis, detection, and surveillance of viral infections. J. Clin. Microbiol. 2024, 62, e0061223. [Google Scholar] [CrossRef]
- Kim, K.W.; Deveson, I.W.; Pang, C.N.; Yeang, M.; Naing, Z.; Adikari, T.; Hammond, J.M.; Stevanovski, I.; Beukers, A.G.; Verich, A.; et al. Respiratory viral co-infections among SARS-CoV-2 cases confirmed by virome capture sequencing. Sci. Rep. 2021, 11, 3934. [Google Scholar] [CrossRef]
- Vazquez-Armendariz, A.I.; Tata, P.R. Recent advances in lung organoid development and applications in disease modeling. J. Clin. Investig. 2023, 133, e170500. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Olivieri, E.A.; Razooky, B.; Le Pen, J.; De Santis, R.; Barrows, D.; Sabry, Z.; Hoffmann, H.H.; Park, J.; Carroll, T.S.; Poirier, J.T.; et al. Organotypic human lung bud microarrays identify BMP-dependent SARS-CoV-2 infection in lung cells. Stem Cell Rep. 2023, 18, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
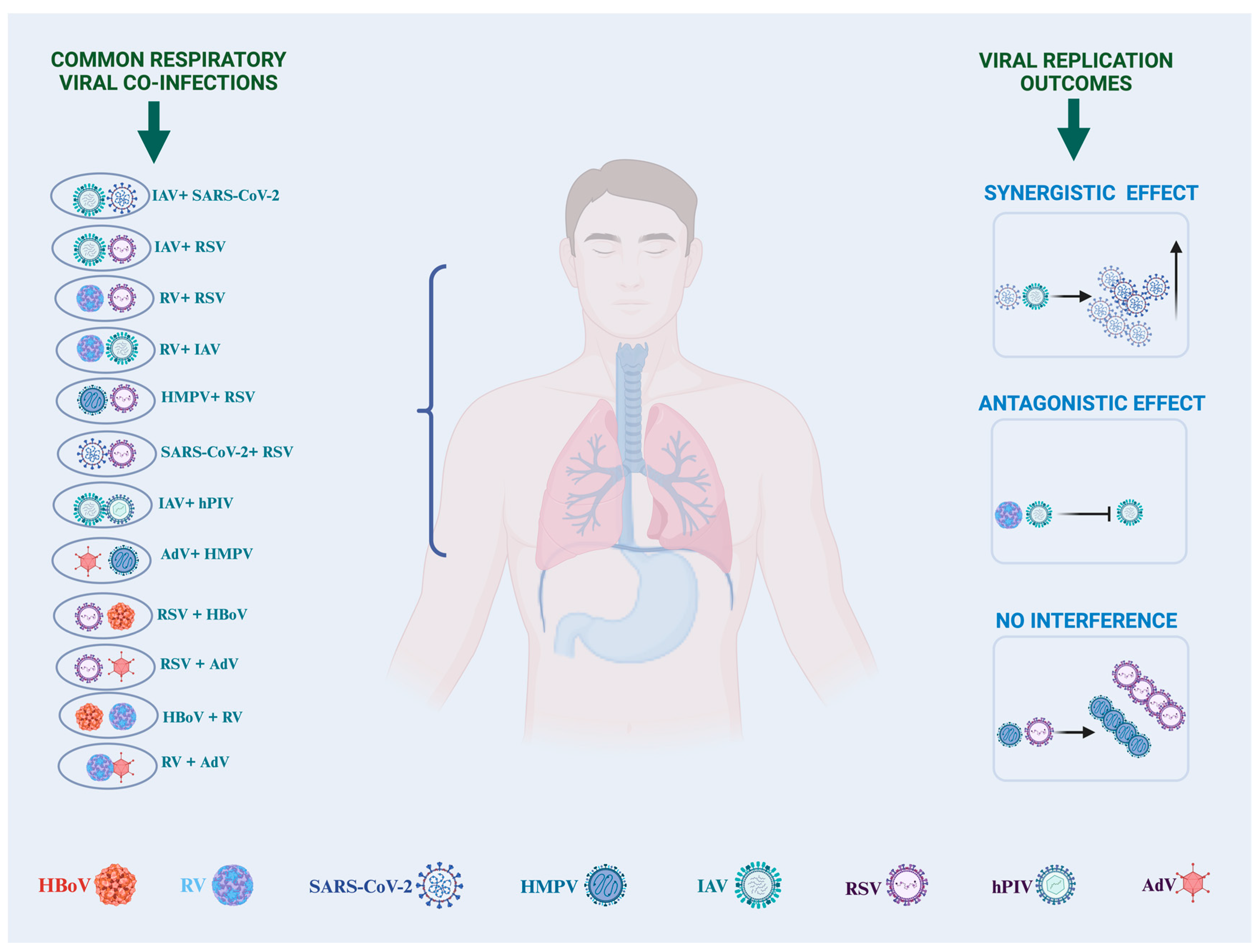
| Respiratory Viral Coinfection | Disease Outcome | References |
|---|---|---|
| HMPV and RSV | Severe bronchiolitis, | [51] |
| Increased chance of admission into the pediatric intensive care unit | ||
| Pneumonia | [71] | |
| IAV and SARS-CoV-2 | Severe and prolonged pneumonia | [64] |
| Alveolar necrosis | [55] | |
| RSV and IAV | Severe airway obstruction | [65] |
| HMPV and AdV | Bronchitis, Bronchiolitis, Asthmatic bronchiolitis | [71] |
| RSV and RV | Fever and hypoxia, | [72] |
| Pediatric intensive care unit admission |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babawale, P.I.; Guerrero-Plata, A. Respiratory Viral Coinfections: Insights into Epidemiology, Immune Response, Pathology, and Clinical Outcomes. Pathogens 2024, 13, 316. https://doi.org/10.3390/pathogens13040316
Babawale PI, Guerrero-Plata A. Respiratory Viral Coinfections: Insights into Epidemiology, Immune Response, Pathology, and Clinical Outcomes. Pathogens. 2024; 13(4):316. https://doi.org/10.3390/pathogens13040316
Chicago/Turabian StyleBabawale, Pius I., and Antonieta Guerrero-Plata. 2024. "Respiratory Viral Coinfections: Insights into Epidemiology, Immune Response, Pathology, and Clinical Outcomes" Pathogens 13, no. 4: 316. https://doi.org/10.3390/pathogens13040316
APA StyleBabawale, P. I., & Guerrero-Plata, A. (2024). Respiratory Viral Coinfections: Insights into Epidemiology, Immune Response, Pathology, and Clinical Outcomes. Pathogens, 13(4), 316. https://doi.org/10.3390/pathogens13040316






