Hepatitis C Prevalence and Birth Outcomes among Pregnant Women in the United States: A 2010–2020 Population Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
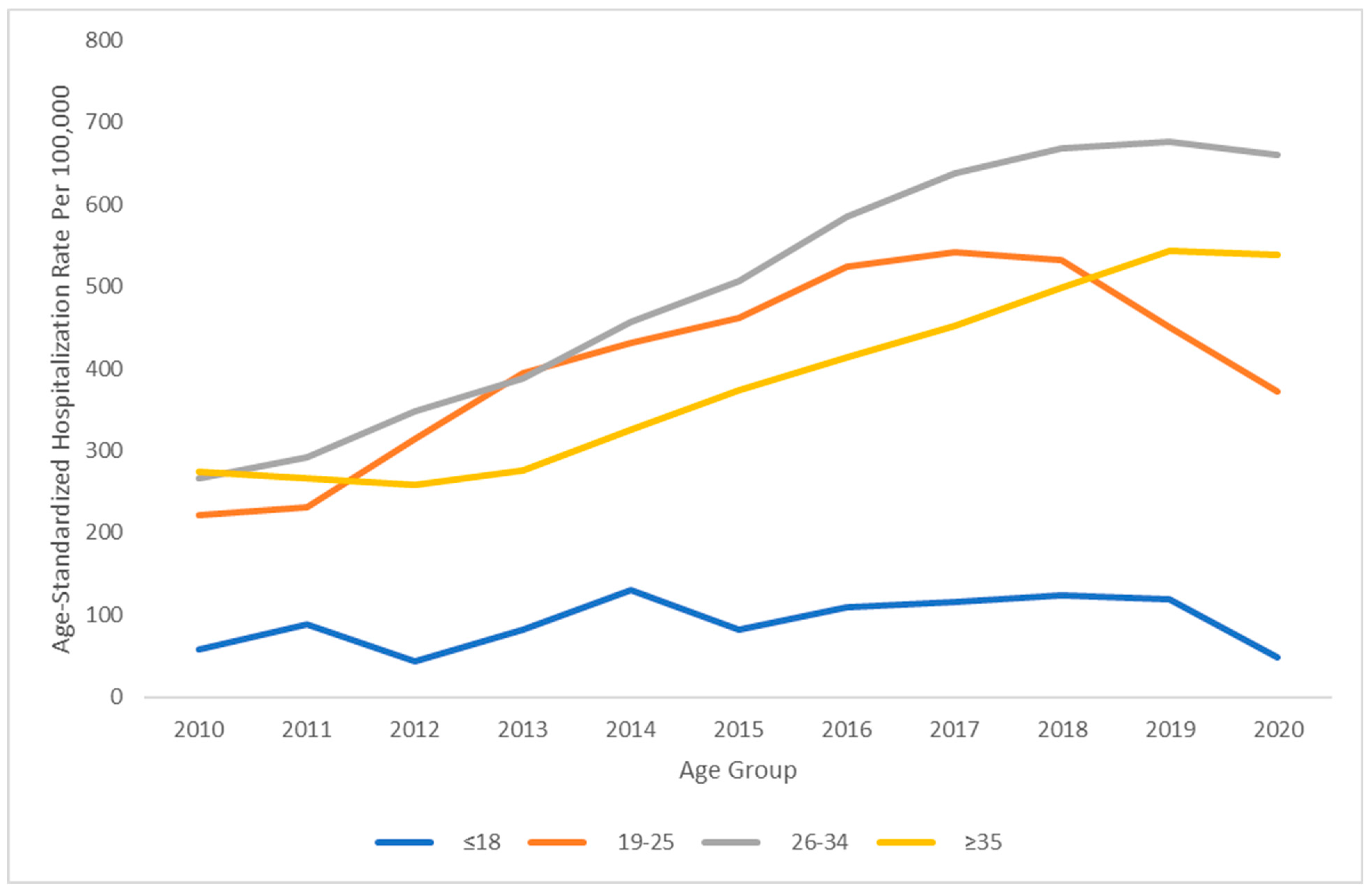
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roudot-Thoraval, F. Epidemiology of hepatitis C virus infection. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101596. [Google Scholar] [CrossRef] [PubMed]
- Edlin, B.R.; Eckhardt, B.J.; Shu, M.A.; Holmberg, S.D.; Swan, T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. J. Hepatol. 2015, 62, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.; Kruszon-Moran, D.; Ly, K.N.; Hughes, E.; Iqbal, K.; Jiles, R.B.; Holmberg, S.D. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988–2012. J. Hepatol. 2016, 63, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, J.D.; Flaxman, A.D.; Naghavi, M.; Fitzmaurice, C.; Vos, T.; Abubakar, I.; Abu-Raddad, L.J.; Assadi, R.; Bhala, N.; Cowie, B.; et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the Global Burden of Disease Study 2013. Lancet 2016, 388, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Benova, L.; Mohamoud, Y.A.; Calvert, C.; Abu-Raddad, L.J. Vertical Transmission of Hepatitis C Virus: Systematic Review and Meta-analysis. Clin. Infect. Dis. 2014, 59, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Pergam, S.A.; Wang, C.C.; Gardella, C.M.; Sandison, T.G.; Phipps, W.T.; Hawes, S.E. Pregnancy Complications Associated with Hepatitis C: Data from a 2003–2005 Washington State Birth Cohort. Am. J. Obstet. Gynecol. 2008, 199, 38.e1–38.e9. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, V.; Lebray, P.; Myers, R.P.; Pannier, E.; Moussalli, J.; Thabut, D.; Buffet, C.; Poynard, T.; Paradis, V.; Charlotte, F. Progression of liver fibrosis in women infected with hepatitis C: Long-term benefit of estrogen exposure. J. Hepatol. 2004, 40, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Gervais, A.; Bacq, Y.; Bernuau, J.; Martinot, M.; Auperin, A.; Boyer, N.; Kilani, A.; Erlinger, S.; Valla, D.; Marcellin, P. Decrease in serum ALT and increase in serum HCV RNA during pregnancy in women with chronic hepatitis C. J. Hepatol. 2000, 32, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Flemming, J.A.; Mullin, M.; Lu, J.; Sarkar, M.A.; Djerboua, M.; Velez, M.P.; Brogly, S.; Terrault, N.A. Outcomes of Pregnant Women With Cirrhosis and Their Infants in a Population-Based Study. Gastroenterology 2020, 159, 1752–1762.e10. [Google Scholar] [CrossRef]
- Hagan, L.M.; Schinazi, R.F. Best strategies for global HCV eradication. Liver Int. 2013, 33 (Suppl. S1), 68–79. [Google Scholar] [CrossRef]
- Mack, C.L.; Gonzalez-Peralta, R.P.; Gupta, N.; Leung, D.; Narkewicz, M.R.; Roberts, E.A.; Rosenthal, P.; Schwarz, K.B. NASPGHAN Practice guidelines: Diagnosis and management of hepatitis c infection in infants, children, and adolescents. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 838–855. [Google Scholar] [CrossRef] [PubMed]
- Omland, L.H.; Krarup, H.; Jepsen, P.; Georgsen, J.; Harritshøj, L.H.; Riisom, K.; Jacobsen, S.E.H.; Schouenborg, P.; Christensen, P.B.; Sørensen, H.T.; et al. Mortality in patients with chronic and cleared hepatitis C viral infection: A nationwide cohort study. J. Hepatol. 2010, 53, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Patrick, S.W.; Bauer, A.M.; Warren, M.D.; Jones, T.F.; Wester, C. Hepatitis C Virus Infection Among Women Giving Birth—Tennessee and United States, 2009–2014. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66, 470–473. [Google Scholar] [CrossRef]
- Shen, G.-F.; Ge, C.-H.; Shen, W.; Liu, Y.-H.; Huang, X.-Y. Association between hepatitis C infection during pregnancy with maternal and neonatal outcomes: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3475–3488. [Google Scholar] [CrossRef]
- Seto, M.T.-Y.; Cheung, K.W.; Hung, I.F. Management of viral hepatitis A, C, D and E in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 68, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Khashan, A.S.; Baker, P.N.; Kenny, L.C. Preterm birth and reduced birthweight in first and second teenage pregnancies: A register-based cohort study. BMC Pregnancy Childbirth 2010, 10, 36. [Google Scholar] [CrossRef]
- Baker, A.M.; Haeri, S. Estimating risk factors for spontaneous preterm delivery in teen pregnancies. Arch. Gynecol. Obstet. 2014, 289, 1203–1206. [Google Scholar] [CrossRef]
- Prediction and Prevention of Spontaneous Preterm Birth: ACOG Practice Bulletin, Number 234. Obstet. Gynecol. 2021, 138, e65–e90. [CrossRef]
- Freeman, A.J.; Dore, G.J.; Law, M.G.; Thorpe, M.; Von Overbeck, J.; Lloyd, A.R.; Marinos, G.; Kaldor, J.M. Estimating progression to cirrhosis in chronic hepatitis C virus infection. J. Hepatol. 2001, 34, 809–816. [Google Scholar] [CrossRef]
- Hagström, H.; Höijer, J.; Marschall, H.; Williamson, C.; Heneghan, M.A.; Westbrook, R.H.; Ludvigsson, J.F.; Stephansson, O. Outcomes of Pregnancy in Mothers With Cirrhosis: A National Population-Based Cohort Study of 1.3 Million Pregnancies. Hepatol. Commun. 2018, 2, 1299–1305. [Google Scholar] [CrossRef]
- Venkatesh, K.K.; Lynch, C.D.; Powe, C.E.; Costantine, M.M.; Thung, S.F.; Gabbe, S.G.; Grobman, W.A.; Landon, M.B. Risk of Adverse Pregnancy Outcomes Among Pregnant Individuals With Gestational Diabetes by Race and Ethnicity in the United States, 2014–2020. JAMA 2022, 327, 1356–1367. [Google Scholar] [CrossRef] [PubMed]
- Dongarwar, D.; Ajewole, V.; Spooner, K.K.; Tran, V.; Adebusuyi, T.; Onyenaka, C.; Bakare, O.; Emeh, C.; Baines, K.; Boua, D.; et al. Racial and Ethnic Disparities in Stillbirth among Pregnant Women with Obesity. Am. J. Perinatol. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- MacDorman, M.F.; Thoma, M.; Declcerq, E.; Howell, E.A. Racial and Ethnic Disparities in Maternal Mortality in the United States Using Enhanced Vital Records, 2016–2017. Am. J. Public Health 2021, 111, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Wedi, C.O.O.; Kirtley, S.; Hopewell, S.; Corrigan, R.; Kennedy, S.H.; Hemelaar, J. Perinatal outcomes associated with maternal HIV infection: A systematic review and meta-analysis. Lancet HIV 2016, 3, e33–e48. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Richardson, B.A.; Kinuthia, J.; Unger, J.A.; Drake, A.L.; Osborn, L.; Matemo, D.; Patterson, J.; McClelland, R.S.; John-Stewart, G. Chlamydia, Gonorrhea, and Incident HIV Infection During Pregnancy Predict Preterm Birth Despite Treatment. J. Infect. Dis. 2021, 224, 2085–2093. [Google Scholar] [CrossRef]
- Malaba, T.R.; Phillips, T.; Le Roux, S.; Brittain, K.; Zerbe, A.; Petro, G.; Ronan, A.; McIntyre, J.A.; Abrams, E.J.; Myer, L. Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int. J. Epidemiol. 2017, 46, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Mugo, C.; Nduati, R.; Osoro, E.; Nyawanda, B.O.; Mirieri, H.; Hunsperger, E.; Verani, J.R.; Jin, H.; Mwaengo, D.; Maugo, B.; et al. Comparable Pregnancy Outcomes for HIV-Uninfected and HIV-Infected Women on Antiretroviral Treatment in Kenya. J. Infect. Dis. 2022, 226, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Pol, S.; Haour, G.; Fontaine, H.; Dorival, C.; Petrov-Sanchez, V.; Bourliere, M.; Capeau, J.; Carrieri, P.; Larrey, D.; Larsen, C.; et al. The negative impact of HBV/HCV coinfection on cirrhosis and its consequences. Aliment. Pharmacol. Ther. 2017, 46, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Pineda, J.A.; Romero-Gómez, M.; Díaz-García, F.; Girón-González, J.A.; Montero, J.L.; Torre-Cisneros, J.; Andrade, R.J.; González-Serrano, M.; Aguilar, J.; Aguilar-Guisado, M.; et al. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. J. Hepatol. 2005, 41, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.S.; Baden, L.R.; Yu, E.; Mrus, J.M.; Carnie, J.; Heeren, T.; Koziel, M.J. Influence of Human Immunodeficiency Virus Infection on the Course of Hepatitis C Virus Infection: A Meta-Analysis. Clin. Infect. Dis. 2001, 33, 562–569. [Google Scholar] [CrossRef]
- Keikha, M.; Eslami, M.; Yousefi, B.; Ali-Hassanzadeh, M.; Kamali, A.; Yousefi, M.; Karbalaei, M. HCV genotypes and their determinative role in hepatitis C treatment. VirusDisease 2020, 31, 235–240. [Google Scholar] [CrossRef]
- Farci, P.; Purcell, R.H. Clinical significance of hepatitis C virus genotypes and quasispecies. Semin. Liver Dis. 2000, 20, 103–126. [Google Scholar] [PubMed]
- Chilaka, V.N.; Konje, J.C. Viral Hepatitis in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 287–296. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Hepatitis C | Non-Hepatitis C | p-Value |
|---|---|---|---|
| Number of Hospitalizations, N | 195,851 | 44,440,393 | |
| Age, Years, Median (IQR) | 29 (25–32) | 28 (24–33) | 0.021 |
| Length of Stay, Days, Median (IQR) | 3 (2–3) | 2 (2–3) | <0.001 |
| Mortality, N (%) | 209 (0.1) | 8211 (<0.1) | <0.001 |
| Race/Ethnicity | <0.001 | ||
| Non-Hispanic White, N (%) | 150,801 (77.0) | 21,676,105 (48.8) | |
| Non-Hispanic Black, N (%) | 11,131 (5.7) | 6,588,504 (14.8) | |
| Hispanic, N (%) | 14,383 (7.3) | 8,892,075 (20.0) | |
| Asian or Pacific Islander, N (%) | 2207 (1.1) | 2,354,304 (5.3) | |
| Native American, N (%) | 3126 (1.6) | 327,003 (0.7) | |
| Other/Unknown, N (%) | 14,203 (7.3) | 4,602,402 (10.4) | |
| Region of Hospital | <0.001 | ||
| Northeast, N (%) | 39,777 (20.3) | 7,132,807 (16.1) | |
| Midwest, N (%) | 40,467 (20.7) | 9,305,085 (20.9) | |
| South, N (%) | 87,959 (44.9) | 17,272,945 (38.9) | |
| West, N (%) | 27,647 (14.1) | 10,729,556 (24.1) | |
| Type of Hospital | <0.001 | ||
| Rural, N (%) | 26,802 (13.7) | 4,307,036 (9.7) | |
| Urban Non-Teaching, N (%) | 34,438 (17.6) | 12,549,502 (28.2) | |
| Urban Teaching, N (%) | 133,737 (68.3) | 27,444,930 (61.8) | |
| Unknown, N (%) | 874 (0.4) | 138,925 (0.3) |
| Risk Factors | Crude Odds Ratio (95% CI) | Crude p-Value | Adjusted Odds Ratio (95% CI) | Adjusted p-Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | High (vs. Low) | 1.04 (1.04–1.04) | <0.001 | 1.04 (1.04–1.04) | <0.001 |
| Race/Ethnicity | White (vs. Other) | 1.30 (1.23–1.37) | <0.001 | 0.86 (0.81–0.91) | <0.001 |
| Co-Infection | |||||
| HIV | Yes/No | 0.78 (0.54–1.13) | 0.190 | 0.92 (0.62–1.36) | 0.666 |
| HBV | Yes/No | 2.43 (1.68–3.50) | <0.001 | 2.57 (1.75–3.77) | <0.001 |
| Co-Morbidity | |||||
| Diabetes | Yes/No | 1.58 (1.50–1.67) | <0.001 | 1.03 (0.97–1.09) | 0.326 |
| Obesity | Yes/No | 0.73 (0.67–0.80) | <0.001 | 0.70 (0.64–0.77) | <0.001 |
| Risk Factors | Crude Odds Ratio (95% CI) | Crude p-Value | Adjusted Odds Ratio (95% CI) | Adjusted p-Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | High (vs. Low) | 1.02 (1.02–1.02) | <0.001 | 1.01 (1.01–1.02) | <0.001 |
| Race/Ethnicity | White (vs. Other) | 1.02 (0.96–1.08) | 0.475 | 0.91 (0.86–0.97) | 0.003 |
| Co-Infection | |||||
| HIV | Yes/No | 2.85 (2.27–3.57) | <0.001 | 2.22 (1.72–2.88) | <0.001 |
| HBV | Yes/No | 15.70 (12.92–19.09) | <0.001 | 13.41 (10.85–16.57) | <0.001 |
| Co-Morbidity | |||||
| Diabetes | Yes/No | 2.36 (2.23–2.49) | <0.001 | 1.83 (1.72–1.95) | <0.001 |
| Obesity | Yes/No | 1.20 (1.10–1.30) | <0.001 | 0.98 (0.89–1.07) | 0.602 |
| Risk Factors | Crude Odds Ratio (95% CI) | Crude p-Value | Adjusted Odds Ratio (95% CI) | Adjusted p-Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | High (vs. Low) | 0.96 (0.96–0.96) | <0.001 | 0.96 (0.96–0.96) | <0.001 |
| Race/Ethnicity | White (vs. Other) | 0.49 (0.46–0.52) | <0.001 | 0.74 (0.69–0.78) | <0.001 |
| Co-Infection | |||||
| HIV | Yes/No | 0.17 (0.06–0.44) | <0.001 | 0.24 (0.09–0.65) | 0.005 |
| HBV | Yes/No | 0.68 (0.30–1.52) | 0.346 | 1.49 (0.67–3.33) | 0.328 |
| Co-Morbidity | |||||
| Diabetes | Yes/No | 0.10 (0.09–0.12) | <0.001 | 0.30 (0.25–0.37) | <0.001 |
| Obesity | Yes/No | 0.71 (0.63–0.80) | <0.001 | 1.65 (1.46–1.88) | <0.001 |
| Risk Factors | Crude Odds Ratio (95% CI) | Crude p-Value | Adjusted Odds Ratio (95% CI) | Adjusted p-Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | High (vs. Low) | 0.97 (0.97–0.98) | <0.001 | 0.98 (0.98–0.98) | <0.001 |
| Race/Ethnicity | White (vs. Other) | 0.41 (0.31–0.53) | <0.001 | 0.52 (0.39–0.69) | <0.001 |
| Co-Infection | |||||
| HIV | Yes/No | 1.61 (0.40–6.47) | 0.504 | 1.65 (0.40–6.75) | 0.484 |
| HBV | Yes/No | N/A | N/A | N/A | N/A |
| Co-Morbidity | |||||
| Diabetes | Yes/No | 0.13 (0.07–0.27) | <0.001 | 0.23 (0.11–0.49) | <0.001 |
| Obesity | Yes/No | 0.73 (0.45–1.20) | 0.215 | 1.21 (0.72–2.04) | 0.467 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasuwanich, P.; Rajborirug, S.; Egerman, R.S.; Wen, T.S.; Karnsakul, W. Hepatitis C Prevalence and Birth Outcomes among Pregnant Women in the United States: A 2010–2020 Population Study. Pathogens 2024, 13, 321. https://doi.org/10.3390/pathogens13040321
Wasuwanich P, Rajborirug S, Egerman RS, Wen TS, Karnsakul W. Hepatitis C Prevalence and Birth Outcomes among Pregnant Women in the United States: A 2010–2020 Population Study. Pathogens. 2024; 13(4):321. https://doi.org/10.3390/pathogens13040321
Chicago/Turabian StyleWasuwanich, Paul, Songyos Rajborirug, Robert S. Egerman, Tony S. Wen, and Wikrom Karnsakul. 2024. "Hepatitis C Prevalence and Birth Outcomes among Pregnant Women in the United States: A 2010–2020 Population Study" Pathogens 13, no. 4: 321. https://doi.org/10.3390/pathogens13040321





