The Last Mile in Polio Eradication: Program Challenges and Perseverance
Abstract
:1. Introduction
2. Current Status of Polio Eradication
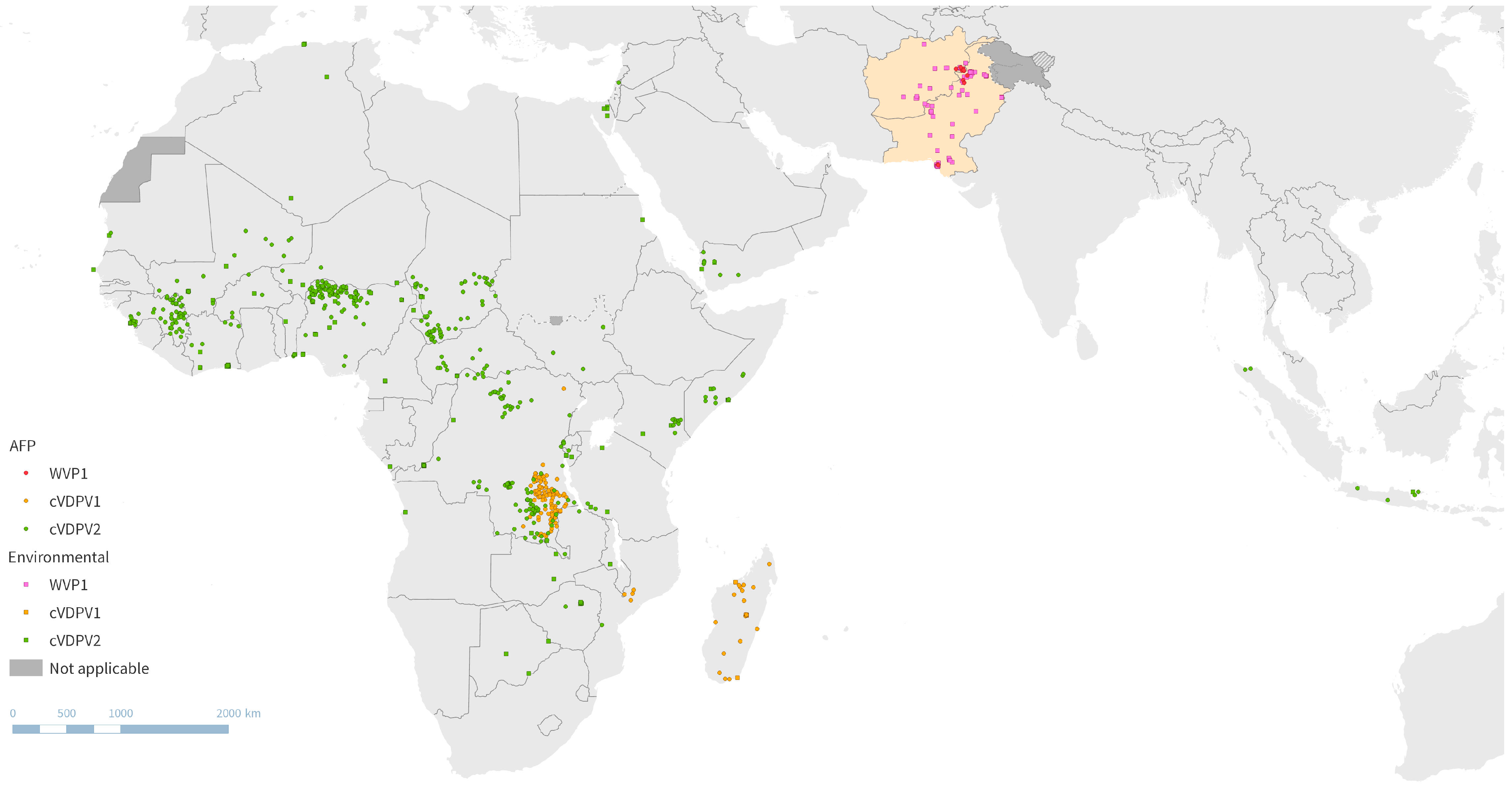
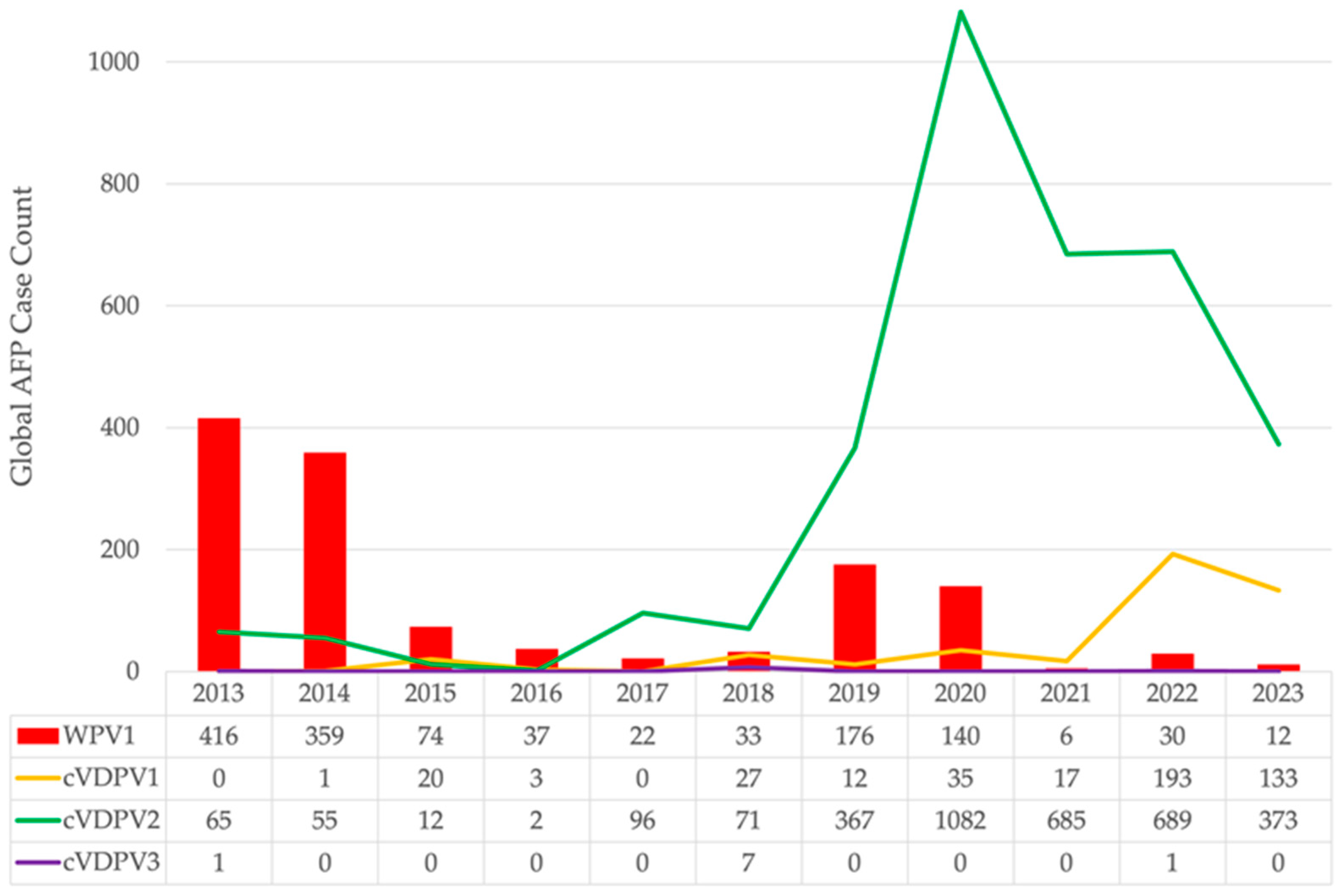
2.1. Global Polio Epidemiology
2.2. Poliovirus Surveillance
2.3. Challenges and the Current GPEI Polio Eradication Strategy
3. Closing in on Poliovirus Eradication
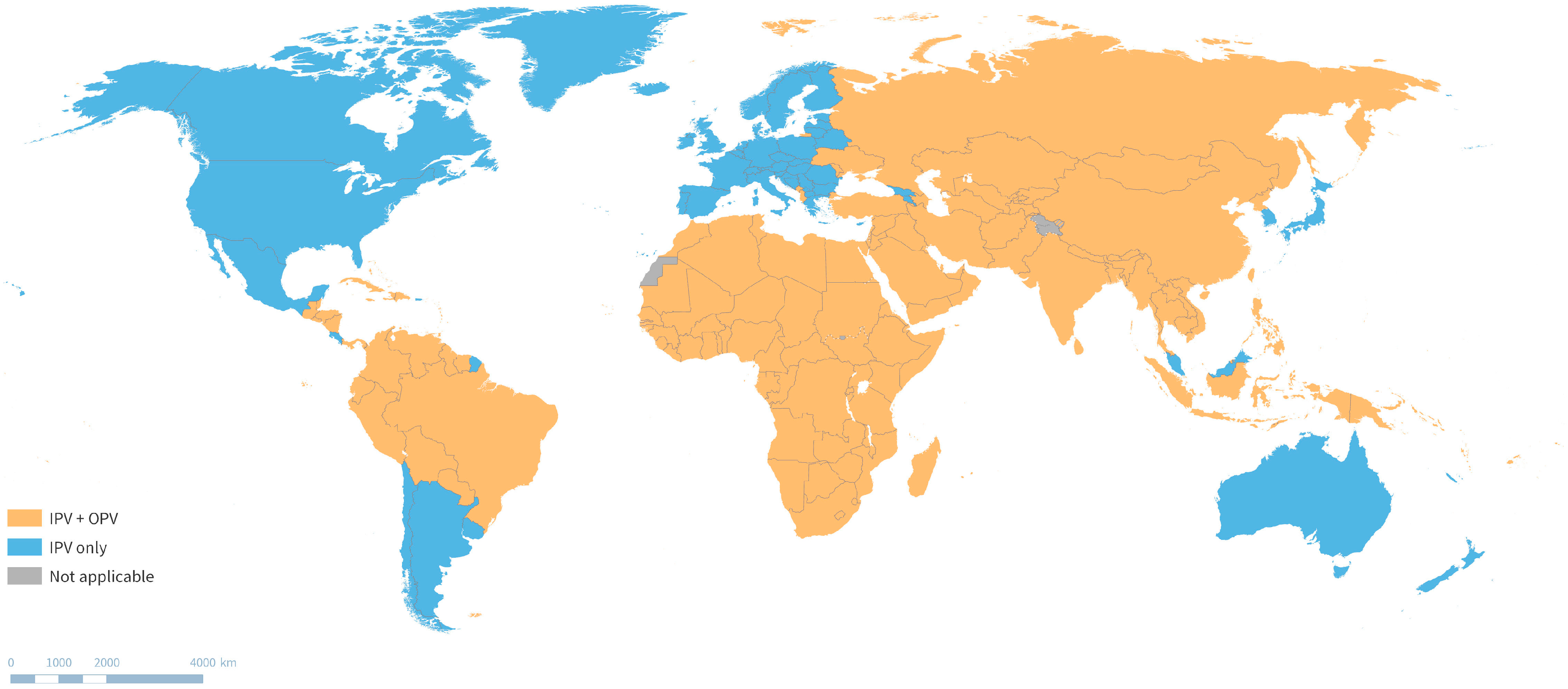
3.1. Evolving Routine Immunization Schedules
3.2. New Tools and Strategies: Outbreak Response
3.3. New Tools and Strategies: Surveillance and Timeliness of Detection

4. Preparing for a Polio-Free World
4.1. Challenges and the GPEI Post-Certification Strategy
4.2. Polio Vaccination in the Post-Certification Era
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- History of the Polio Vaccine. Available online: https://www.who.int/news-room/spotlight/history-of-vaccination/history-of-polio-vaccination?topicsurvey=ht7j2q)&gclid=CjwKCAiAhJWsBhAaEiwAmrNyq96p4otvLmTvsY_CT9YnLuQo-9VdI3OTAlb5SQaKrl8Wlq-WGGasARoCBYoQAvD_BwE (accessed on 22 December 2023).
- Hird, T.R.; Grassly, N.C. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012, 8, e1002599. [Google Scholar] [CrossRef] [PubMed]
- GPEI Polio Eradication Strategy 2022–2026: Delivering on a Promise. Available online: https://polioeradication.org/wp-content/uploads/2021/10/9789240031937-eng.pdf (accessed on 6 January 2021).
- Global Wild Poliovirus 2017–2023—Data in WHO HQ as of 19 December 2023. Available online: https://polioeradication.org/polio-today/polio-now/wild-poliovirus-list/ (accessed on 22 December 2023).
- Global Polio Eradication. Available online: https://www.cdc.gov/polio/global-polio-eradication.html (accessed on 14 December 2023).
- Global Eradication of Wild Poliovirus Type 2 Declared. Available online: https://polioeradication.org/news-post/global-eradication-of-wild-poliovirus-type-2-declared/ (accessed on 6 December 2023).
- Global Certification of Eradication of Indigenous Wild Poliovirus Type 3. Available online: https://www.cdc.gov/globalhealth/immunization/stories/global-certification-of-eradication-of-indigenous-wild-poliovirus-type-3.html (accessed on 6 December 2023).
- WHO. Global Wild Poliovirus 2015–2020. Available online: https://polioeradication.org/wp-content/uploads/2020/12/weekly-polio-analyses-WPV-20201222.pdf (accessed on 22 December 2023).
- WHO AFRO. Polio Eradication: Africa Regional Certification Commission begins Verification Visit to Nigeria; WHO AFRO: Abuja, Nigeria, 2019. [Google Scholar]
- GPEI; WHO. Variant Poliovirus (cVDPV). Available online: https://polioeradication.org/polio-today/polio-prevention/the-virus/variant-poliovirus-cvdpv/ (accessed on 5 April 2024).
- World Health Organization. Statement of the Thirty-Sixth Meeting of the Polio IHR Emergency Committee; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Global Circulating Vaccine-Derived Poliovirus (cVDPV)—Data in WHO HQ as of 19 December 2023. Available online: https://polioeradication.org/this-week/variant-poliovirus-cvdpv/ (accessed on 22 December 2023).
- Bjork, A.; Akbar, I.; Chaudhury, S.; Wadood, M.Z.; Ather, F.; Jorba, J.; Martinez, M. Progress Toward Poliomyelitis Eradication—Afghanistan, January 2022–June 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Sutter, R.W.; Platt, L.; Mach, O.; Jafari, H.; Aylward, R. The new polio eradication end game: Rationale and supporting evidence. J. Infect. Dis. 2014, 210, S434–S438. [Google Scholar] [CrossRef] [PubMed]
- Sutter, R.W.; Cochi, S.L. Inactivated Poliovirus Vaccine Supply Shortage: Is There Light at the End of the Tunnel? J. Infect. Dis. 2019, 220, 1545–1546. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Greene, S.; Burns, C.C.; Tallis, G.; Wassilak, S.; Bolu, O. Progress Toward Poliomyelitis Eradication—Worldwide, January 2021–March 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 517–522. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Polio Surveillance Action Plan 2022–2024; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Tuma, J.; Wilkinson, A.L.; Diop, O.M.; Jorba, J.; Gardner, T.; Snider, C.J.; Anand, A.; Ahmed, J. Surveillance to Track Progress Toward Polio Eradication—Worldwide, 2019–2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 667–673. [Google Scholar] [CrossRef]
- Zomahoun, D.; Burman, A.; Snider, C.J.; Chauvin, C.; Gardner, T.; Lickness, J.S.; Ahmed, J.A.; Diop, O.M.; Gerber, S.; Anand, A. Impact of COVID-19 Pandemic on Global Poliovirus Surveillance. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Stehling-Ariza, T.; Wilkinson, A.L.; Diop, O.M.; Jorba, J.; Asghar, H.; Avagnan, T.; Grabovac, V.; Johnson, T.; Joshi, S.; Kfutwah, A.; et al. Surveillance to Track Progress toward Poliomyelitis Eradication—Worldwide, 2021–2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. CDC to Expand Wastewater Testing for Poliovirus. JAMA 2023, 329, 116–117. [Google Scholar] [CrossRef]
- Expansion of Polio Sewage Surveillance to Areas Outside London. UK Health Security Agency: Health and Social Care, Public Health. Published 2 September 2022. Available online: https://www.gov.uk/government/news/expansion-of-polio-sewage-surveillance-to-areas-outside-london#:~:text=We%20are%20now%20expanding%20the,areas%20as%20the%20need%20arises (accessed on 5 April 2024).
- Independent Monitoring Board: Global Polio Eradication Initiative. Closing in on Zero: Adapting to Complexity and Risk on the Path to End Polio; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- World Health Organization. Polio Eradication Strategy: GPEI Response to the Midterm Review; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Immunization Systems Management Group of the Global Polio Eradication Initiative. Introduction of Inactivated Poliovirus Vaccine and Switch from Trivalent to Bivalent Oral Poliovirus Vaccine—Worldwide, 2013–2016. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 699–702. [Google Scholar]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, November 2012—Conclusions and Recommendations. Wkly. Epidemiol. Rec. 2013, 88, 1–16. [Google Scholar]
- WHO. Meeting of the Strategic Advisory Group of Experts on Immunization, October 2020—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2020, 95, 585–608. [Google Scholar]
- World Health Organization. 15th Meeting of the SAGE Polio Working Group—Conclusions and Recommendations; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Ahmad, M.; Verma, H.; Deshpande, J.; Kunwar, A.; Bavdekar, A.; Mahantashetti, N.S.; Krishnamurthy, B.; Jain, M.; Mathew, M.A.; Pawar, S.D.; et al. Immunogenicity of Fractional Dose Inactivated Poliovirus Vaccine in India. J. Pediatr. Infect. Dis. Soc. 2022, 11, 60–68. [Google Scholar] [CrossRef]
- Anand, A.; Molodecky, N.A.; Pallansch, M.A.; Sutter, R. Immunogenicity to poliovirus type 2 following two doses of fractional intradermal inactivated poliovirus vaccine: A novel dose sparing immunization schedule. Vaccine 2017, 35, 2993–2998. [Google Scholar] [CrossRef] [PubMed]
- Trueba, G.; Jeyaseelan, V.; Lopez, L.; Mainou, B.A.; Zhang, Y.; Whittembury, A.; Olmedo Valarezo, A.; Baquero, G.; Romero de Aguinaga, R.; Zurita Salinas, L.; et al. Achieving high immunogenicity against poliovirus with fractional doses of inactivated poliovirus vaccine in Ecuador-results from a cross-sectional serological survey. Lancet Reg. Health Am. 2022, 11, 100235. [Google Scholar] [CrossRef] [PubMed]
- Resik, S.; Mach, O.; Tejeda, A.; Jeyaseelan, V.; Fonseca, M.; Diaz, M.; Alemany, N.; Hung, L.H.; Aleman, Y.; Mesa, I.; et al. Immunogenicity of Intramuscular Fractional Dose of Inactivated Poliovirus Vaccine. J. Infect. Dis. 2020, 221, 895–901. [Google Scholar] [CrossRef]
- WHO Immunization Data Portal. Available online: https://immunizationdata.who.int/ (accessed on 18 December 2023).
- Hexavalent Vaccine Programme Information. Available online: https://www.gavi.org/our-support/guidelines/hexavalent-vaccine-programme-information (accessed on 31 January 2024).
- WHO. Polio vaccines: WHO position paper—June 2022. Wkly. Epidemiol. Rec. 2022, 97, 277–300. [Google Scholar]
- Macklin, G.R.; Goel, A.; Mach, O.; Tallis, G.; Ahmed, J.; O’Reilly, K.M.; Grassly, N.C.; Diop, O.M. Epidemiology of type 2 vaccine-derived poliovirus outbreaks between 2016 and 2020. Vaccine 2023, 41, A19–A24. [Google Scholar] [CrossRef]
- Yeh, M.T.; Bujaki, E.; Dolan, P.T.; Smith, M.; Wahid, R.; Konz, J.; Weiner, A.J.; Bandyopadhyay, A.; Van Damme, P.; De Coster, I.; et al. Engineering the Live-Attenuated Polio Vaccine to Prevent Reversion to Virulence. Cell Host Microbe 2020, 27, 736–751. [Google Scholar] [CrossRef]
- Macklin, G.; Peak, C.; Eisenhawer, M.; Kurji, F.; Mach, O.; Konz, J.; Gast, C.; Bachtiar, N.S.; Bandyopadhyay, A.; Zipursky, S. Enabling accelerated vaccine roll-out for Public Health Emergencies of International Concern (PHEICs): Novel Oral Polio Vaccine type 2 (nOPV2) experience. Vaccine 2023, 41, A122–A127. [Google Scholar] [CrossRef]
- GPEI Press Release on nOPV2 Prequalification. Available online: https://polioeradication.org/news-post/gpei-press-release-on-nopv2-prequalification/ (accessed on 5 April 2024).
- Bandyopadhyay, A.; Cooper, L.; Zipursky, S. One billion doses and WHO Prequalification of nOPV2: Implications for the global polio situation and beyond. PLoS Glob. Public Health 2024, 4, e0002920. [Google Scholar] [CrossRef] [PubMed]
- Peak, C.M.; Lyons, H.; Cooper, L.V.; Voorman, A.; Hawes, K.; Bandyopadhyay, A. Monitoring Risk of Type-2 Circulating Vaccine-Derived Poliovirus Emergence during Roll-Out of Type-2 Novel Oral Polio Vaccine. In Proceedings of the 9th International Conference on Infectious Disease Dynamics, Bologna, Italy, 28 November–1 December 2023. [Google Scholar]
- Davlantes, E.; Jorba, J.; Henderson, E.; Bullard, K.; Deka, M.A.; Kfutwah, A.; Martin, J.; Bessaud, M.; Shulman, L.; Diop, O.M.; et al. Notes from the Field: Circulating Vaccine-Derived Poliovirus Type 2 Emergences Linked to Novel Oral Poliovirus Vaccine Type 2 Use—Six African Countries, 2021–2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1041–1042. [Google Scholar] [CrossRef] [PubMed]
- WHO. Meeting of the Strategic Advisory Group of Experts on Immunization, September 2023: Conclusions and recommendations. Wkly. Epidemiol. Rec. 2023, 95, 599–620. [Google Scholar]
- Bandyopadhyay, A.; Lopez Cavestany, R.; Blake, I.; Macklin, G.; Cooper, L.V.; Grassly, N.; Melquiades dos Santos Nery, A.; Mach, O. Use of inactivated poliovirus vaccine for poliovirus outbreak response. Lancet Infect. Dis. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Shaw, A.G.; Majumdar, M.; Troman, C.; O’Toole, Á.; Benny, B.; Abraham, D.; Praharaj, I.; Kang, G.; Sharif, S.; Alam, M.M.; et al. Rapid and Sensitive Direct Detection and Identification of Poliovirus from Stool and Environmental Surveillance Samples by Use of Nanopore Sequencing. J. Clin. Microbiol. 2020, 58, e00920. [Google Scholar] [CrossRef]
- Harrington, C.; Sun, H.; Jeffries-Miles, S.; Gerloff, N.; Mandelbaum, M.; Pang, H.; Collins, N.; Burns, C.C.; Vega, E. Culture-Independent Detection of Poliovirus in Stool Samples by Direct RNA Extraction. Microbiol. Spectr. 2021, 9, e0066821. [Google Scholar] [CrossRef]
- Lab Sample Transport for Polio Eradication. Available online: https://www.villagereach.org/project/polio-laboratory-sample-transport/ (accessed on 19 December 2023).
- WHO; GPEI. Polio Post-Certification Strategy. Available online: http://polioeradication.org/polio-today/preparing-for-a-polio-free-world/transition-planning/polio-post-certification-strategy (accessed on 10 May 2023).
- Duizer, E.; Ruijs, W.L.M.; Hintaran, A.D.P.; Hafkamp, M.C.; van der Veer, M.; te Wierik, M.J. Wild poliovirus type 3 (WPV3)-shedding event following detection in environmental surveillance of poliovirus essential facilities, The Netherlands, November 2022 to January 2023. Eurosurveillance 2023, 28, 2300049. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Action Plan for Poliovirus Containment; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- WHO. Meeting of the Strategic Advisory Group of Experts on immunization, October 2016—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2016, 91, 561–584. [Google Scholar]
- Polio Antivirals Initiative. Available online: https://www.taskforce.org/polio-antivirals-initiative/ (accessed on 19 December 2023).
- Sherry, L.; Swanson, J.J.; Grehan, K.; Xu, H.; Uchida, M.; Jones, I.M.; Stonehouse, N.J.; Rowlands, D.J. Protease-Independent Production of Poliovirus Virus-like Particles in Pichia pastoris: Implications for Efficient Vaccine Development and Insights into Capsid Assembly. Microbiol. Spectr. 2023, 11, e0430022. [Google Scholar] [CrossRef]
- A Study to Evaluate the Safety and Immunogenicity of Recombinant Trivalent Poliomyelitis Vaccine (Sf-RVN Cell) in Healthy Adults, CanSino Biologics Inc. 2024. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT06101173 (accessed on 5 April 2024).
- World Health Organization: Containment Advisory Group on Novel Poliovirus Strains. Report: CAG TC2 on Novel Poliovirus Strains. Available online: https://polioeradication.org/wp-content/uploads/2018/05/containment-advisory-group-novel-poliovirus-strains-8-march-2018-20180518.pdf (accessed on 8 March 2018).
- WHO. Meeting of the Strategic Advisory Group of Experts on immunization, April 2017—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2017, 92, 301–320. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez Cavestany, R.; Eisenhawer, M.; Diop, O.M.; Verma, H.; Quddus, A.; Mach, O. The Last Mile in Polio Eradication: Program Challenges and Perseverance. Pathogens 2024, 13, 323. https://doi.org/10.3390/pathogens13040323
Lopez Cavestany R, Eisenhawer M, Diop OM, Verma H, Quddus A, Mach O. The Last Mile in Polio Eradication: Program Challenges and Perseverance. Pathogens. 2024; 13(4):323. https://doi.org/10.3390/pathogens13040323
Chicago/Turabian StyleLopez Cavestany, Rocio, Martin Eisenhawer, Ousmane M. Diop, Harish Verma, Arshad Quddus, and Ondrej Mach. 2024. "The Last Mile in Polio Eradication: Program Challenges and Perseverance" Pathogens 13, no. 4: 323. https://doi.org/10.3390/pathogens13040323
APA StyleLopez Cavestany, R., Eisenhawer, M., Diop, O. M., Verma, H., Quddus, A., & Mach, O. (2024). The Last Mile in Polio Eradication: Program Challenges and Perseverance. Pathogens, 13(4), 323. https://doi.org/10.3390/pathogens13040323





