Epidemiology of Hepatitis C Virus in HIV Patients from West Mexico: Implications for Controlling and Preventing Viral Hepatitis
Abstract
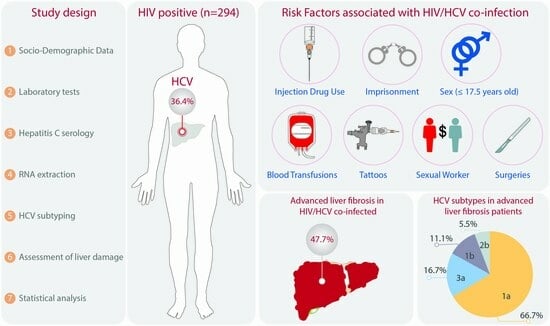
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Tests and Hepatitis C Serology
2.3. RNA Isolation and Reverse Transcription
2.4. HCV Genotyping/Subtyping
2.4.1. Line Probe Assay (LiPA)
2.4.2. DNA Sanger Sequencing
2.5. Assessment of Liver Fibrosis
2.6. Statistical Analysis
2.7. Ethics
3. Results
3.1. General Characteristics of the Study Population
3.2. HCV by Age Group and Risk Factors
3.3. HCV Subtypes in HIV Patients
3.4. HIV Groups and Liver Damage
3.5. Liver Fibrosis by HCV Subtype
3.6. Risk Factors Associated with Liver Fibrosis in HIV Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Hepatitis C. 2019, p. 1. Available online: https://www.who.int/es/news-room/fact-sheets/detail/hepatitis-c (accessed on 4 December 2023).
- Ioannou, G.N.; Bryson, C.L.; Weiss, N.S.; Miller, R.; Scott, J.D.; Boyko, E.J. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology 2013, 57, 249–257. [Google Scholar] [CrossRef]
- Platt, L.; Easterbrook, P.; Gower, E.; McDonald, B.; Sabin, K.; McGowan, C.; Yanny, I.; Razavi, H.; Vickerman, P. Prevalence and burden of HCV co-infection in people living with HIV: A global systematic review and meta-analysis. Lancet. Infect. Dis. 2016, 16, 797–808. [Google Scholar] [CrossRef]
- Alipour, A.; Rezaianzadeh, A.; Hasanzadeh, J.; Rajaeefard, A.; Davarpanah, M.A.; Hasanabadi, M. High prevalence of HCV coinfection in HIV-infected individuals in Shiraz, Islamic Republic of Iran. East. Mediterr. Health J. 2013, 19, 975–981. [Google Scholar] [CrossRef]
- Fanciulli, C.; Berenguer, J.; Busca, C.; Vivancos, M.J.; Tellez, M.J.; Dominguez, L.; Domingo, P.; Navarro, J.; Santos, J.; Iribarren, J.A.; et al. Epidemiological trends of HIV/HCV coinfection in Spain, 2015–2019. HIV Med. 2022, 23, 705–716. [Google Scholar] [CrossRef]
- Government of Mexico. The Cultures of the West. 2021; p. 1. Available online: https://nuevaescuelamexicana.sep.gob.mx/detalle-ficha/4760/ (accessed on 4 December 2023).
- Congressional Research Service. Mexico: Organized Crime and Drug Trafficking Organizations. 2020; p. 8. Available online: https://crsreports.congress.gov/product/pdf/R/R41576/45 (accessed on 4 December 2023).
- Government of Mexico. Inequality and Economy of the State of Jalisco. 2022; p. 14. Available online: https://www.economia.gob.mx/datamexico/en/profile/geo/jalisco-jc?redirect=true#equidad (accessed on 4 December 2023).
- Omland, L.H.; Osler, M.; Jepsen, P.; Krarup, H.; Weis, N.; Christensen, P.B.; Roed, C.; Sorensen, H.T.; Obel, N. Socioeconomic status in HCV infected patients-risk and prognosis. Clin. Epidemiol. 2013, 5, 163–172. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística y Geografía. Getting to Know the LGBTI+ Population in Mexico. 2021, p. 3. Available online: https://www.inegi.org.mx/tablerosestadisticos/lgbti/ (accessed on 4 December 2023).
- Rivas-Estilla, A.M.; Ramirez-Valles, E.; Martinez-Hernandez, R.; Charles-Nino, C.; Ramirez-Camacho, E.; Rositas-Noriega, F.; Garza-Rodriguez, M.L.; Barrera-Saldana, H.A.; Trujillo-Murillo, K.; Ramos-Jimenez, J. Hepatitis C virus infection among HIV-1 infected individuals from northern Mexico. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2007, 37, 311–316. [Google Scholar] [CrossRef]
- Caceres, C.F.; Aggleton, P.; Galea, J.T. Sexual diversity, social inclusion and HIV/AIDS. AIDS 2008, 22 (Suppl. S2), S45–S55. [Google Scholar] [CrossRef]
- Daniel, W.W. Biostatistics: A Foundation for Analysis in the Health Sciences Instructors Solutions Manual; John Wiley & Sons Canada, Limited: Montreal, QC, Canada, 2008. [Google Scholar]
- Li, D.; Long, Y.; Wang, T.; Xiao, D.; Zhang, J.; Guo, Z.; Wang, B.; Yan, Y. Epidemiology of hepatitis C virus infection in highly endemic HBV areas in China. PLoS ONE 2013, 8, e54815. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Hawkins, C.; Agbaji, O.; Ugoagwu, P.; Thio, C.L.; Auwal, M.M.; Ani, C.; Okafo, C.; Wallender, E.; Murphy, R.L. Assessment of liver fibrosis by transient elastography in patients with HIV and hepatitis B virus coinfection in Nigeria. Clin. Infect. Dis. 2013, 57, e189–e192. [Google Scholar] [CrossRef]
- Sandrin, L.; Fourquet, B.; Hasquenoph, J.M.; Yon, S.; Fournier, C.; Mal, F.; Christidis, C.; Ziol, M.; Poulet, B.; Kazemi, F.; et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef]
- Borsoi Viana, M.S.; Takei, K.; Collarile Yamaguti, D.C.; Guz, B.; Strauss, E. Use of AST platelet ratio index (APRI Score) as an alternative to liver biopsy for treatment indication in chronic hepatitis C. Ann. Hepatol. 2009, 8, 26–31. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, D.Y.; Park, J.Y.; Ahn, S.H.; Chon, C.Y.; Kim, J.K.; Paik, Y.H.; Lee, K.S.; Park, Y.N.; Han, K.H. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. Off. J. Int. Assoc. Study Liver 2010, 30, 546–553. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Lin, Z.H.; Xin, Y.N.; Dong, Q.J.; Wang, Q.; Jiang, X.J.; Zhan, S.H.; Sun, Y.; Xuan, S.Y. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology 2011, 53, 726–736. [Google Scholar] [CrossRef]
- Jose-Abrego, A.; Panduro, A.; Fierro, N.A.; Roman, S. High prevalence of HBV infection, detection of subgenotypes F1b, A2, and D4, and differential risk factors among Mexican risk populations with low socioeconomic status. J. Med. Virol. 2017, 89, 2149–2157. [Google Scholar] [CrossRef]
- Jose-Abrego, A.; Roman, S.; Rebello Pinho, J.R.; de Castro, V.F.D.; Panduro, A. Hepatitis B Virus (HBV) Genotype Mixtures, Viral Load, and Liver Damage in HBV Patients Co-infected With Human Immunodeficiency Virus. Front. Microbiol. 2021, 12, 640889. [Google Scholar] [CrossRef]
- Sedeno-Monge, V.; Laguna-Meraz, S.; Santos-Lopez, G.; Panduro, A.; Sosa-Jurado, F.; Jose-Abrego, A.; Melendez-Mena, D.; Munoz-Ramirez, M.A.; Cosme-Chavez, M.; Roman, S. A comprehensive update of the status of hepatitis C virus (HCV) infection in Mexico-A systematic review and meta-analysis (2008–2019). Ann. Hepatol. 2021, 20, 100292. [Google Scholar] [CrossRef]
- Government of Mexico. National Program for the Elimination of Hepatitis C. 2021; p. 1. Available online: https://www.gob.mx/cms/uploads/attachment/file/689905/Boleti_n_VHC_DICIEM (accessed on 10 January 2024).
- Government of Mexico. Results of the Hepatitis C Program from Mexico City. 2022; p. 1. Available online: https://condesa.cdmx.gob.mx/pdf/ResultadosProgramaHepatitisC_2020a2022_CDMXvF.pdf (accessed on 10 January 2024).
- Centers for Disease Control and Prevention. HIV and Viral Hepatitis. 2017; p. 1. Available online: https://stacks.cdc.gov/view/cdc/48584/cdc_48584_DS1.pdf (accessed on 10 January 2024).
- Akhtar, A.; Fatima, S.; Saeed, H.; Soo, C.T.; Khan, A.H. HIV-HCV Coinfection: Prevalence and Treatment Outcomes in Malaysia. Intervirology 2022, 65, 87–93. [Google Scholar] [CrossRef]
- Freitas, S.Z.; Teles, S.A.; Lorenzo, P.C.; Puga, M.A.; Tanaka, T.S.; Thomaz, D.Y.; Martins, R.M.; Druzian, A.F.; Lindenberg, A.S.; Torres, M.S.; et al. HIV and HCV coinfection: Prevalence, associated factors and genotype characterization in the Midwest Region of Brazil. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 517–524. [Google Scholar] [CrossRef]
- Centro de Investigacion y Docencia Economica A.C. Resultados de la Primera Encuesta Realizada a Población Interna en Centros Federales de Readaptación Social. 2012, p. 24. Available online: https://publiceconomics.files.wordpress.com/2013/01/encuesta_internos_cefereso_2012.pdf (accessed on 12 January 2024).
- Instituto Nacional de Estadistica y Geografia. Encuesta Nacional de Adolescentes en el Sistema de Justicia Penal (ENASJUP). 2022, p. 39. Available online: https://www.inegi.org.mx/contenidos/saladeprensa/boletines/2023/ENASJUP/ENASJUP2022.pdf (accessed on 12 January 2024).
- World Health Organization. Elimination of Hepatitis by 2030. 2023, p. 1. Available online: https://www.who.int/health-topics/hepatitis/elimination-of-hepatitis-by-2030#tab=tab_1 (accessed on 22 February 2024).
- Revill, P.A.; Chisari, F.V.; Block, J.M.; Dandri, M.; Gehring, A.J.; Guo, H.; Hu, J.; Kramvis, A.; Lampertico, P.; Janssen, H.L.A.; et al. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol. Hepatol. 2019, 4, 545–558. [Google Scholar] [CrossRef]
- Atkins, R.; Sulik, M.J.; Hart, D.; Ayres, C.; Read, N. The effects of school poverty on adolescents’ sexual health knowledge. Res. Nurs. Health 2012, 35, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.M.; Smith, M.J.; del Rio, C. Low educational level: A risk factor for sexually transmitted infections among commercial sex workers in Quito, Ecuador. Int. J. STD AIDS 2008, 19, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Gonzalez, L.E.; Camiro-Zuniga, A.; Pineirua-Menendez, A.; Sanchez-Avila, J.F.; Hirata-Hernandez, A.H.; Maranon-Solorio, K.A.; Zamora-Tapia, I.; Perez-Carrizosa, A.; Simental-Aldaba, E.; Sierra-Madero, J.G. Risk factors associated with HCV co-infection amongst MSM and transgender women living with HIV in Mexico City: A case-control study. Ann. Hepatol. 2022, 27, 100758. [Google Scholar] [CrossRef] [PubMed]
- Alter, H.J.; Klein, H.G. The hazards of blood transfusion in historical perspective. Blood 2008, 112, 2617–2626. [Google Scholar] [CrossRef] [PubMed]
- Elgharably, A.; Gomaa, A.I.; Crossey, M.M.; Norsworthy, P.J.; Waked, I.; Taylor-Robinson, S.D. Hepatitis C in Egypt—Past, present, and future. Int. J. Gen. Med. 2017, 10, 1–6. [Google Scholar] [CrossRef]
- World Health Organization. Access to Treatment and Care for All: The Path to Eliminate Hepatitis C in Egypt. 2023, p. 1. Available online: https://www.who.int/news-room/feature-stories/detail/access-to-treatment-and-care-for-all--the-path-to-eliminate-hepatitis-c-in-egypt (accessed on 16 January 2024).
- StatPearls. Infectious Complications of Blood Transfusion. 2023; p. 1. Available online: https://www.ncbi.nlm.nih.gov/books/NBK585035/#:~:text=Numerous%20important%20viral%20infections%20were,first%20recognized%20in%20the%201990s (accessed on 16 January 2024).
- Novelo-Garza, B.; Duque-Rodriguez, J.; Mejia-Dominguez, A.M.; Rivas-Gonzalez, M.R.; Torres-Torres, O. Blood safety in Mexico and a perspective on Latin America. Transfus. Apher. Sci. 2019, 58, 102661. [Google Scholar] [CrossRef]
- Government of Mexico. Draft Official Mexican Standard for the Disposal of Human Blood and Its Components for Therapeutic Purposes. 1993; p. 1. Available online: https://dof.gob.mx/nota_detalle.php?codigo=4810828&fecha=08/12/1993#gsc.tab=0 (accessed on 16 January 2024).
- Said, Z.N.A.; El-Sayed, M.H. Challenge of managing hepatitis B virus and hepatitis C virus infections in resource-limited settings. World J. Hepatol. 2022, 14, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Copes, R.; Baharlou, S.; Etminan, M.; Buxton, J. Tattooing and the risk of transmission of hepatitis C: A systematic review and meta-analysis. Int. J. Infect. Dis. 2010, 14, e928–e940. [Google Scholar] [CrossRef]
- Simmonds, P.; Bukh, J.; Combet, C.; Deleage, G.; Enomoto, N.; Feinstone, S.; Halfon, P.; Inchauspe, G.; Kuiken, C.; Maertens, G.; et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 2005, 42, 962–973. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses (ICTV). Confirmed HCV Genotypes/Subtypes. 2022, p. 1. Available online: https://ictv.global/sg_wiki/flaviviridae/hepacivirus/table1 (accessed on 9 April 2024).
- Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.G.; Sablon, E.; Chamberland, J.; Fournier, E.; Dandavino, R.; Tremblay, C.L. Hepatitis C virus genotype 7, a new genotype originating from central Africa. J. Clin. Microbiol. 2015, 53, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Borgia, S.M.; Hedskog, C.; Parhy, B.; Hyland, R.H.; Stamm, L.M.; Brainard, D.M.; Subramanian, M.G.; McHutchison, J.G.; Mo, H.; Svarovskaia, E.; et al. Identification of a Novel Hepatitis C Virus Genotype from Punjab, India: Expanding Classification of Hepatitis C Virus into 8 Genotypes. J. Infect. Dis. 2018, 218, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Avila-Rios, S.; Garcia-Morales, C.; Reyes-Teran, G.; Gonzalez-Rodriguez, A.; Matias-Florentino, M.; Mehta, S.R.; Chaillon, A. Phylodynamics of HIV in the Mexico City Metropolitan Region. J. Virol. 2022, 96, e0070822. [Google Scholar] [CrossRef] [PubMed]
- Torres-Valadez, R.; Roman, S.; Jose-Abrego, A.; Sepulveda-Villegas, M.; Ojeda-Granados, C.; Rivera-Iniguez, I.; Panduro, A. Early Detection of Liver Damage in Mexican Patients with Chronic Liver Disease. J. Transl. Intern. Med. 2017, 5, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Truong, T.; Laureillard, D.; Lacombe, K.; Duong Thi, H.; Pham Thi Hanh, P.; Truong Thi Xuan, L.; Chu Thi, N.; Luong Que, A.; Vu Hai, V.; Nagot, N.; et al. High Proportion of HIV-HCV Coinfected Patients with Advanced Liver Fibrosis Requiring Hepatitis C Treatment in Haiphong, Northern Vietnam (ANRS 12262). PLoS ONE 2016, 11, e0153744. [Google Scholar] [CrossRef] [PubMed]
- Pineda, J.A.; Gonzalez, J.; Ortega, E.; Tural, C.; Macias, J.; Griffa, L.; Burgos, A.; on behalf of the Grafihco Study Team. Prevalence and factors associated with significant liver fibrosis assessed by transient elastometry in HIV/hepatitis C virus-coinfected patients. J. Viral Hepat. 2010, 17, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Schiavini, M.; Angeli, E.; Mainini, A.; Zerbi, P.; Duca, P.G.; Gubertini, G.; Vago, L.; Fociani, P.; Giorgi, R.; Cargnel, A. Risk factors for fibrosis progression in HIV/HCV coinfected patients from a retrospective analysis of liver biopsies in 1985–2002. HIV Med 2006, 7, 331–337. [Google Scholar] [CrossRef]
- Chahal, H.S.; Marseille, E.A.; Tice, J.A.; Pearson, S.D.; Ollendorf, D.A.; Fox, R.K.; Kahn, J.G. Cost-effectiveness of Early Treatment of Hepatitis C Virus Genotype 1 by Stage of Liver Fibrosis in a US Treatment-Naive Population. JAMA Int. Med. 2016, 176, 65–73. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Aronsohn, A.; Price, J.; Lo Re, V.; Panel, A.-I.H.G. Hepatitis C Guidance 2023 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin. Infect. Dis. 2023. [Google Scholar] [CrossRef]
- Bruno, S.; Crosignani, A.; Maisonneuve, P.; Rossi, S.; Silini, E.; Mondelli, M.U. Hepatitis C virus genotype 1b as a major risk factor associated with hepatocellular carcinoma in patients with cirrhosis: A seventeen-year prospective cohort study. Hepatology 2007, 46, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, S.; Aghemo, A.; Rumi, M.G.; Colombo, M. HCV genotype 3: An independent predictor of fibrosis progression in chronic hepatitis C. J. Hepatol. 2009, 51, 964–966. [Google Scholar] [CrossRef]
- Probst, A.; Dang, T.; Bochud, M.; Egger, M.; Negro, F.; Bochud, P.Y. Role of hepatitis C virus genotype 3 in liver fibrosis progression--a systematic review and meta-analysis. J. Viral Hepat. 2011, 18, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.K.; Yadav, U.N.; Khatiwada, S.; Tamang, M.K.; Dahal, S.; Li, Y.P. Hepatitis C virus genotype and its correlation with viral load in patients from Kathmandu, Nepal. J. Infect. Dev. Ctries. 2020, 14, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- McMahon, B.J.; Bruden, D.; Townshend-Bulson, L.; Simons, B.; Spradling, P.; Livingston, S.; Gove, J.; Hewitt, A.; Plotnik, J.; Homan, C.; et al. Infection with Hepatitis C Virus Genotype 3 Is an Independent Risk Factor for End-Stage Liver Disease, Hepatocellular Carcinoma, and Liver-Related Death. Clin. Gastroenterol. Hepatol. 2017, 15, 431–437.e432. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Li, J.; Zhang, Y.; Ji, S.; Li, Y.; Zhao, Y.; Li, B.; Li, W.; Quan, M.; Duan, Y.; et al. Real-World Effectiveness of Direct-Acting Antiviral Regimens against Hepatitis C Virus (HCV) Genotype 3 Infection: A Systematic Review and Meta-Analysis. Ann. Hepatol. 2021, 23, 100268. [Google Scholar] [CrossRef]
- Smith, D.A.; Fernandez-Antunez, C.; Magri, A.; Bowden, R.; Chaturvedi, N.; Fellay, J.; McLauchlan, J.; Foster, G.R.; Irving, W.L.; Consortium, S.-H.; et al. Viral genome wide association study identifies novel hepatitis C virus polymorphisms associated with sofosbuvir treatment failure. Nat. Commun. 2021, 12, 6105. [Google Scholar] [CrossRef]
- Jacobson, I.M.; Gordon, S.C.; Kowdley, K.V.; Yoshida, E.M.; Rodriguez-Torres, M.; Sulkowski, M.S.; Shiffman, M.L.; Lawitz, E.; Everson, G.; Bennett, M.; et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N. Engl. J. Med. 2013, 368, 1867–1877. [Google Scholar] [CrossRef]
- Cavalcante, L.N.; Lyra, A.C. Predictive factors associated with hepatitis C antiviral therapy response. World J. Hepatol. 2015, 7, 1617–1631. [Google Scholar] [CrossRef]
- Dar, G.A.; Yattoo, G.N.; Gulzar, G.M.; Sodhi, J.S.; Gorka, S.; Laway, M.A. Treatment of Chronic Hepatitis C Genotype 3 With Ledipasvir and Sofosbuvir: An Observational Study. J. Clin. Exp. Hepatol. 2021, 11, 227–231. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, S. Antiviral treatment of hepatitis C virus infection and factors affecting efficacy. World J. Gastroenterol. 2013, 19, 8963–8973. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Kemmer, N.; Neff, G.W. Predicting the probable outcome of treatment in HCV patients. Ther. Adv. Gastroenterol. 2009, 2, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Revised surveillance case definition for HIV infection—United States, 2014. MMWR. Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2014, 63, 1–10. [Google Scholar]
- Sullivan, T. The Implications of Low Absolute CD4 Counts in Patients with Cirrhosis and Human Immunodeficiency Virus. Open Forum Infect. Dis. 2016, 3, ofw060. [Google Scholar] [CrossRef]
- Lin, W.; Zhong, H.; Wen, C.; He, Y.; Zheng, X.; Li, H.; Chen, X.; He, H.; Chen, J.; Chen, L.; et al. Persistently low CD4 cell counts are associated with hepatic events in HCV/HIV coinfected patients: Data from the national free antiretroviral treatment program of China. Chin. Med. J. 2022, 135, 2699–2705. [Google Scholar] [CrossRef] [PubMed]
- Iacob, D.G.; Luminos, M.; Benea, O.E.; Tudor, A.M.; Olariu, C.M.; Iacob, S.A.; Ruta, S. Liver fibrosis progression in a cohort of young HIV and HIV/ HBV co-infected patients: A longitudinal study using noninvasive APRI and Fib-4 scores. Front. Med. 2022, 9, 888050. [Google Scholar] [CrossRef] [PubMed]
- Belinskaia, D.A.; Voronina, P.A.; Goncharov, N.V. Integrative Role of Albumin: Evolutionary, Biochemical and Pathophysiological Aspects. J. Evol. Biochem. Physiol. 2021, 57, 1419–1448. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef]
- Nagao, Y.; Sata, M. Serum albumin and mortality risk in a hyperendemic area of HCV infection in Japan. Virol. J. 2010, 7, 375. [Google Scholar] [CrossRef]
- Ripoll, C.; Bari, K.; Garcia-Tsao, G. Serum Albumin Can Identify Patients with Compensated Cirrhosis with a Good Prognosis. J. Clin. Gastroenterol. 2015, 49, 613–619. [Google Scholar] [CrossRef]
- Leal, J.A.; Fausto, M.A.; Carneiro, M.; Tubinambas, U. Prevalence of hypoalbuminemia in outpatients with HIV/AIDS. Rev. Soc. Bras. Med. Trop. 2018, 51, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Sticova, E.; Jirsa, M. New insights in bilirubin metabolism and their clinical implications. World J. Gastroenterol. 2013, 19, 6398–6407. [Google Scholar] [CrossRef] [PubMed]
- Roche, S.P.; Kobos, R. Jaundice in the adult patient. Am. Fam. Physician 2004, 69, 299–304. [Google Scholar] [PubMed]
- Du, M.; Zhang, S.; Xiao, L.; Xu, Y.; Liu, P.; Tang, Y.; Wei, S.; Xing, M.; Miao, X.; Yao, P. The Relationship between Serum Bilirubin and Elevated Fibrotic Indices among HBV Carriers: A Cross-Sectional Study of a Chinese Population. Int. J. Mol. Sci. 2016, 17, 2057. [Google Scholar] [CrossRef]
- Fujita, K.; Oura, K.; Yoneyama, H.; Shi, T.; Takuma, K.; Nakahara, M.; Tadokoro, T.; Nomura, T.; Morishita, A.; Tsutsui, K.; et al. Albumin-bilirubin score indicates liver fibrosis staging and prognosis in patients with chronic hepatitis C. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2019, 49, 731–742. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- McDonald, C.; Uy, J.; Hu, W.; Wirtz, V.; Juethner, S.; Butcher, D.; McGrath, D.; Farajallah, A.; Moyle, G. Clinical significance of hyperbilirubinemia among HIV-1-infected patients treated with atazanavir/ritonavir through 96 weeks in the CASTLE study. AIDS Patient Care STDS 2012, 26, 259–264. [Google Scholar] [CrossRef]
- Memon, N.; Weinberger, B.I.; Hegyi, T.; Aleksunes, L.M. Inherited disorders of bilirubin clearance. Pediatr. Res. 2016, 79, 378–386. [Google Scholar] [CrossRef]
- Erlinger, S.; Arias, I.M.; Dhumeaux, D. Inherited disorders of bilirubin transport and conjugation: New insights into molecular mechanisms and consequences. Gastroenterology 2014, 146, 1625–1638. [Google Scholar] [CrossRef]




| Variable | Category or Measure | Total (N = 294) | HIV (N = 187) | HIV/HCV (N = 107) | p-Value |
|---|---|---|---|---|---|
| Gender | Female | 35 (11.9%) | 20 (10.7%) | 15 (14.0%) | 0.455 1 |
| Male | 259 (88.1%) | 167 (89.3%) | 92 (86.0%) | 0.397 1 | |
| Age (year) | Median (Q1, Q3) | 38.0 (32.2, 46.8) | 38.0 (31.0, 46.2) | 39.5 (34.2, 46.8) | 0.131 2 |
| Onset of sexual life | Median (Q1, Q3) | 17.0 (15.0, 18.0) | 18.0 (15.0, 19.0) | 15.0 (13.0, 18.0) | <0.001 2 |
| Sexual preference | Heterosexual | 121 (43.1%) | 64 (36.2%) | 57 (54.8%) | <0.001 1 |
| Bisexual | 61 (21.7%) | 41 (23.2%) | 20 (19.2%) | 0.459 1 | |
| Homosexual | 99 (35.2%) | 72 (40.7%) | 27 (26.0%) | 0.008 1 | |
| Education | High school/university | 101 (35.6%) | 77 (43.0%) | 24 (22.9%) | <0.001 1 |
| Basic/none | 183 (64.4%) | 102 (57.0%) | 81 (77.1%) | <0.001 1 | |
| Hepatitis B status | HBsAg (−)/anti-HBc (−) | 149 (50.7%) | 84 (44.9%) | 65 (60.7%) | 0.012 1 |
| HBsAg (−)/anti-HBc (+) | 55 (18.7%) | 21 (11.2%) | 34 (31.8%) | <0.001 1 | |
| HBsAg (+)/anti-HBc (−) | 13 (4.4%) | 12 (6.4%) | 1 (0.9%) | 0.036 1 | |
| HBsAg (+)/anti-HBc (+) | 53 (18.0%) | 49 (26.2%) | 4 (3.7%) | <0.001 1 | |
| HBsAg (+)/anti-HBc not tested | 24 (8.2%) | 21 (11.2%) | 3 (2.8%) | <0.001 1 | |
| CD8+ cells/mm3 | Median (Q1, Q3) | 895.0 (585.0, 1187.0) | 884.0 (599.5, 1212.0) | 917.0 (560.0, 1126.5) | 0.555 2 |
| CD4+ cells/mm3 | Median (Q1, Q3) | 315.0 (172.0, 531.0) | 316.0 (198.2, 543.0) | 314.0 (135.5, 509.5) | 0.707 2 |
| ALT IU/L | Median (Q1, Q3) | 28.0 (21.0, 50.0) | 27.0 (20.0, 40.0) | 36.0 (24.0, 59.0) | 0.004 2 |
| AST IU/L | Median (Q1, Q3) | 32.0 (25.0, 48.0) | 29.0 (24.0, 40.0) | 38.0 (30.5, 60.5) | <0.001 2 |
| GGT IU/L | Median (Q1, Q3) | 44.0 (26.2, 77.8) | 39.0 (25.0, 60.0) | 55.0 (31.0, 175.0) | 0.003 2 |
| Platelet cells/µL | Median (Q1, Q3) | 219.0 (174.8, 279.0) | 229.0 (184.0, 289.0) | 208.0 (151.5, 263.0) | 0.094 2 |
| HIV viral load (copies/mL) | Median (Q1, Q3) | 40.0 (34.8, 225.2) | 40.0 (31.0, 213.0) | 42.0 (40.0, 225.5) | 0.422 2 |
| HIV viral load class * | Unavailable | 78 (26.5%) | 50 (26.7%) | 28 (26.2%) | 1.000 1 |
| Suppressed < 1000 copies/mL | 169 (57.5%) | 104 (55.6%) | 65 (60.7%) | 0.463 1 | |
| Unsuppressed ≥ 1000 copies/mL | 47 (16.0%) | 33 (17.6%) | 14 (13.1%) | 0.388 1 | |
| HCV viral load (IU/mL) | Median (Q1, Q3) | 2,165,000.0 (106,374.2, 11,800,000.0) | - | 2,165,000.0 (106,374.2, 11,800,000.0) |
| Liver Stiffness | Variable | F1–F2 | F3–F4 | OR (Univariable) | OR (Multivariable) |
|---|---|---|---|---|---|
| Age (year) | Age < 41.5 | 63 (67.7) | 20 (43.5) | - | - |
| Age ≥ 41.5 | 30 (32.3) | 26 (56.5) | 2.73 (1.33–5.71; p = 0.007) | - | |
| DB (mg/dL) | DB < 0.16 | 57 (78.1) | 16 (48.5) | - | - |
| DB ≥ 0.16 | 16 (21.9) | 17 (51.5) | 3.79 (1.58–9.28; p = 0.003) | - | |
| AST (IU/L) | AST < 98.0 | 73 (96.1) | 25 (75.8) | - | - |
| AST ≥ 98.0 | 3 (3.9) | 8 (24.2) | 7.79 (2.08–37.70; p = 0.004) | 9.75 (2.01–72.88; p = 0.009) | |
| Viral hepatitis | HIV/HBsAg | 44 (58.7) | 10 (25.0) | - | - |
| HIV/HCV | 31 (41.3) | 30 (75.0) | 4.26 (1.87–10.36; p = 0.001) | 3.69 (1.38–10.79; p = 0.012) |
| APRI | Variable | Without SLF | With SLF | OR (Univariable) | OR (Multivariable) |
|---|---|---|---|---|---|
| Platelet cells/µL | Platelets > 157.5 | 155 (95.1) | 14 (29.8) | - | - |
| Platelets ≤ 157.5 | 8 (4.9) | 33 (70.2) | 45.67 (18.60–125.18; p < 0.001) | - | |
| DB (mg/dL) | DB < 0.16 | 120 (75.5) | 13 (28.3) | - | - |
| DB ≥ 0.16 | 39 (24.5) | 33 (71.7) | 7.81 (3.82–16.81; p < 0.001) | - | |
| IB (mg/dL) | IB < 0.78 | 137 (86.2) | 28 (60.9) | - | - |
| IB ≥ 0.78 | 22 (13.8) | 18 (39.1) | 4.00 (1.90–8.46; p < 0.001) | 5.12 (1.65–17.00; p = 0.005) | |
| ALT (IU/L) | ALT < 54.5 | 146 (89.6) | 18 (38.3) | - | - |
| ALT ≥ 54.5 | 17 (10.4) | 29 (61.7) | 13.84 (6.51–30.74; p < 0.001) | 16.56 (6.30–48.58; p < 0.001) | |
| AST (IU/L) | AST < 43.5 | 141 (86.5) | 6 (12.8) | - | - |
| AST ≥ 43.5 | 22 (13.5) | 41 (87.2) | 43.80 (17.78–126.25; p < 0.001) | - | |
| GGT (IU/L) | GGT < 70.5 | 120 (78.9) | 22 (50.0) | - | - |
| GGT ≥ 70.5 | 32 (21.1) | 22 (50.0) | 3.75 (1.85–7.67; p < 0.001) | - | |
| Albumin (g/dL) | Albumin > 3.4 | 124 (80.0) | 16 (37.2) | - | - |
| Albumin ≤ 3.4 | 31 (20.0) | 27 (62.8) | 6.75 (3.29–14.32; p < 0.001) | 5.60 (2.04–16.69; p = 0.001) | |
| CD4+ cells/mm3 | CD4+ > 191.5 | 130 (80.2) | 20 (43.5) | - | - |
| CD4+ ≤ 191.5 | 32 (19.8) | 26 (56.5) | 5.28 (2.64–10.76; p < 0.001) | 3.41 (1.27–9.48; p = 0.016) | |
| Viral hepatitis | HIV/HBsAg | 56 (53.3) | 9 (32.1) | - | - |
| HIV/HCV | 49 (46.7) | 19 (67.9) | 2.41 (1.02–6.06; p = 0.050) | - |
| FIB4 | Variable | Without AF | With AF | OR (Univariable) | OR (Multivariable) |
|---|---|---|---|---|---|
| Platelet cells/µL | Platelets > 124.5 | 179 (95.7) | 3 (14.3) | - | - |
| Platelets ≤ 124.5 | 8 (4.3) | 18 (85.7) | 134.25 (37.04–668.41; p < 0.001) | - | |
| DB (mg/dL) | DB < 0.20 | 148 (80.9) | 3 (15.0) | - | - |
| DB ≥ 0.20 | 35 (19.1) | 17 (85.0) | 23.96 (7.55–106.76; p < 0.001) | - | |
| IB (mg/dL) | IB < 0.78 | 155 (84.7) | 8 (40.0) | - | - |
| IB ≥ 0.78 | 28 (15.3) | 12 (60.0) | 8.30 (3.16–22.99; p < 0.001) | 13.32 (4.00–50.49; p < 0.001) | |
| ALT (IU/L) | ALT < 47.5 | 145 (77.5) | 7 (33.3) | - | - |
| ALT ≥ 47.5 | 42 (22.5) | 14 (66.7) | 6.90 (2.69–19.27; p < 0.001) | - | |
| AST (IU/L) | AST < 48.5 | 154 (82.4) | 3 (14.3) | - | - |
| AST ≥ 48.5 | 33 (17.6) | 18 (85.7) | 28.00 (8.86–124.55; p < 0.001) | - | |
| GGT (IU/L) | GGT < 82.5 | 138 (79.3) | 8 (40.0) | - | - |
| GGT ≥ 82.5 | 36 (20.7) | 12 (60.0) | 5.75 (2.22–15.70; p < 0.001) | - | |
| Albumin (g/dL) | Albumin > 3.7 | 102 (57.0) | 1 (5.9) | - | - |
| Albumin ≤ 3.7 | 77 (43.0) | 16 (94.1) | 21.19 (4.19–386.68; p = 0.003) | 18.98 (3.19–367.86; p = 0.007) | |
| CD4+ cells/mm3 | CD4+ > 191.5 | 141 (75.8) | 7 (35.0) | - | - |
| CD4+ ≤ 191.5 | 45 (24.2) | 13 (65.0) | 5.82 (2.24–16.33; p < 0.001) | 4.04 (1.17–15.80; p = 0.033) | |
| Viral hepatitis | HIV/HBsAg | 61 (52.1) | 3 (21.4) | - | - |
| HIV/HCV | 56 (47.9) | 11 (78.6) | 3.99 (1.18–18.33; p = 0.041) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jose-Abrego, A.; Trujillo-Trujillo, M.E.; Laguna-Meraz, S.; Roman, S.; Panduro, A. Epidemiology of Hepatitis C Virus in HIV Patients from West Mexico: Implications for Controlling and Preventing Viral Hepatitis. Pathogens 2024, 13, 360. https://doi.org/10.3390/pathogens13050360
Jose-Abrego A, Trujillo-Trujillo ME, Laguna-Meraz S, Roman S, Panduro A. Epidemiology of Hepatitis C Virus in HIV Patients from West Mexico: Implications for Controlling and Preventing Viral Hepatitis. Pathogens. 2024; 13(5):360. https://doi.org/10.3390/pathogens13050360
Chicago/Turabian StyleJose-Abrego, Alexis, Maria E. Trujillo-Trujillo, Saul Laguna-Meraz, Sonia Roman, and Arturo Panduro. 2024. "Epidemiology of Hepatitis C Virus in HIV Patients from West Mexico: Implications for Controlling and Preventing Viral Hepatitis" Pathogens 13, no. 5: 360. https://doi.org/10.3390/pathogens13050360
APA StyleJose-Abrego, A., Trujillo-Trujillo, M. E., Laguna-Meraz, S., Roman, S., & Panduro, A. (2024). Epidemiology of Hepatitis C Virus in HIV Patients from West Mexico: Implications for Controlling and Preventing Viral Hepatitis. Pathogens, 13(5), 360. https://doi.org/10.3390/pathogens13050360