Upregulation of Neuroinflammation-Associated Genes in the Brain of SARS-CoV-2-Infected Mice
Abstract
1. Introduction
2. Results
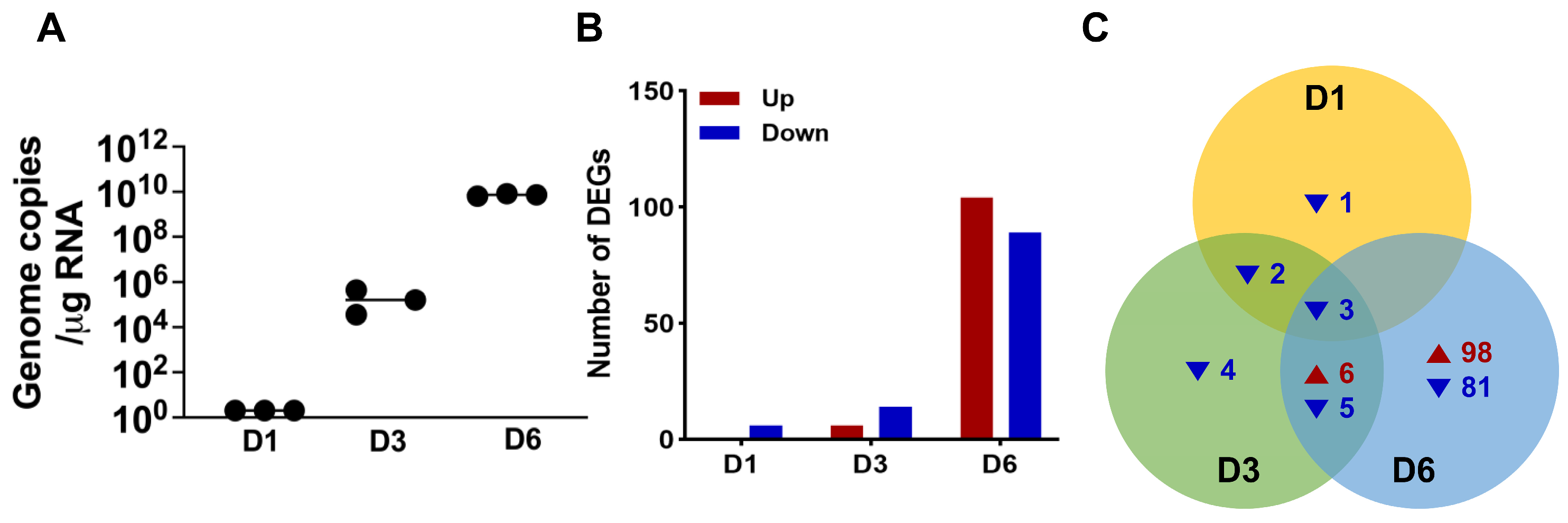
2.1. SARS-CoV-2 Infection of K18-hACE2 Mice Modulates Host Gene Expression in Brain Tissue
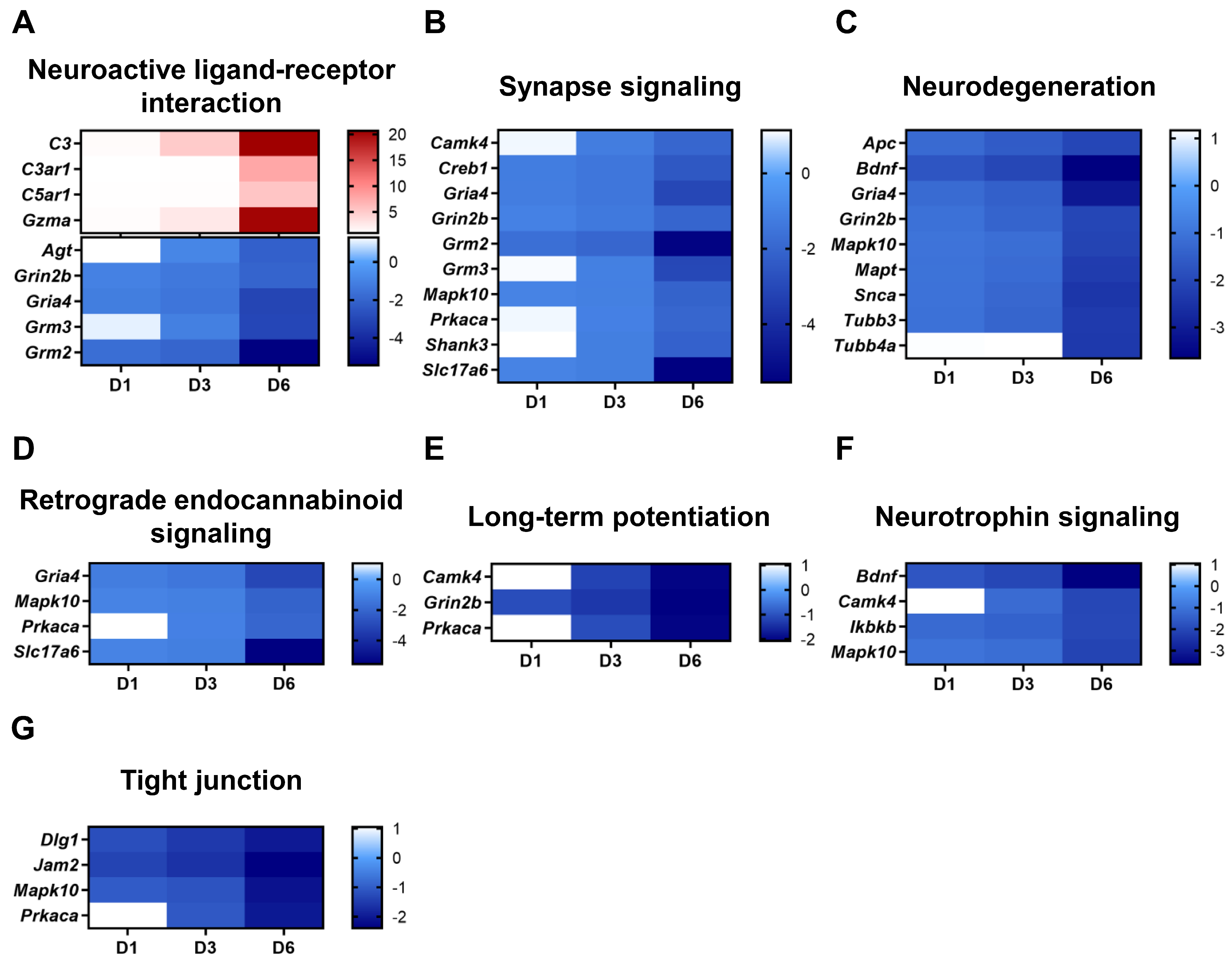
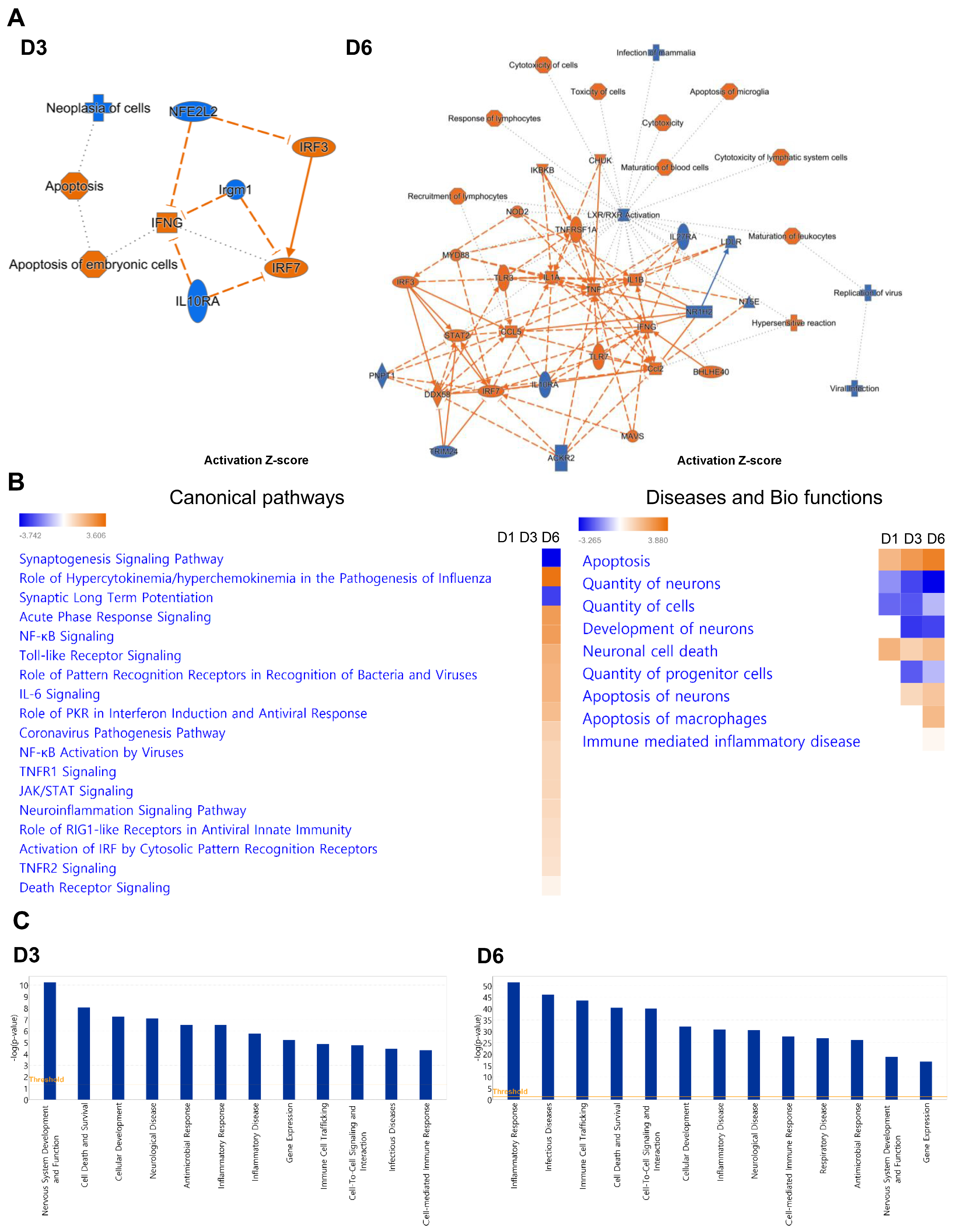
2.2. Distinct Genes Regulated by SARS-CoV-2 Infection Show Activated Viral Sensing Pathways and Neurological Dysfunction in Brain
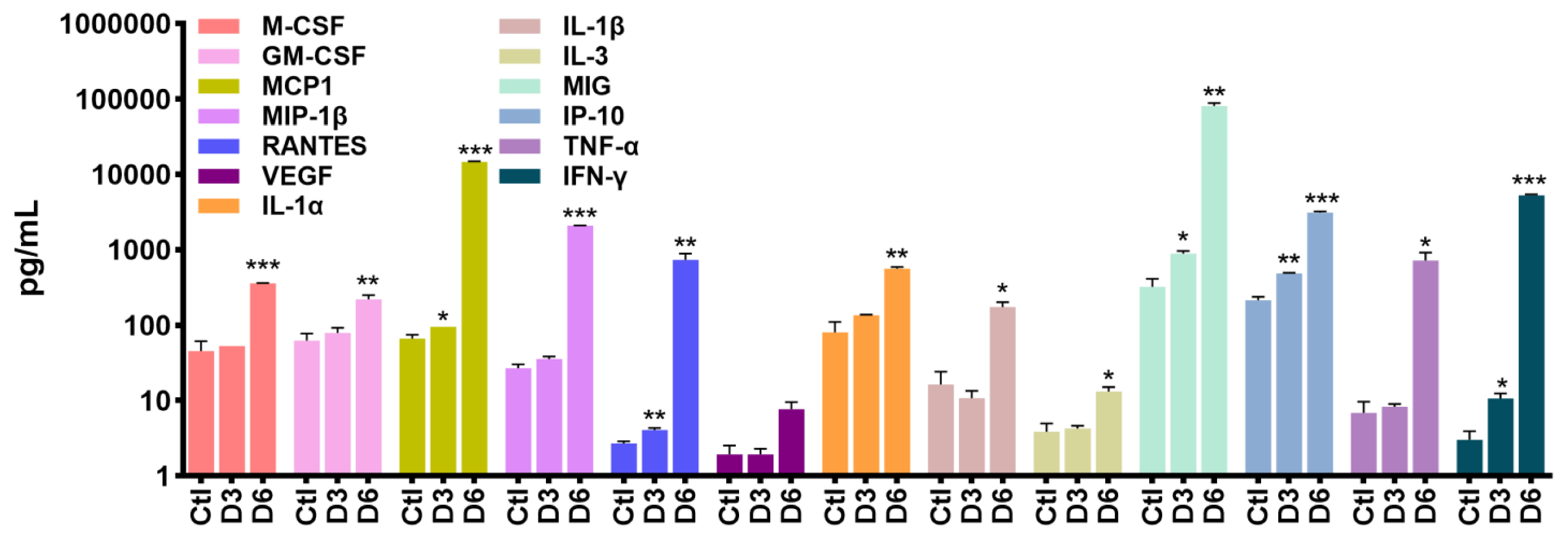
2.3. SARS-CoV-2 Infection Causes Neuro-Inflammatory Response Following Antiviral Response Activation
2.4. TLR Signaling and Programmed Cell Death Pathways Are Strongly Induced in SARS-CoV-2-Infected Brains
3. Discussion
4. Materials and Methods
4.1. SARS-CoV-2 Infection in Mice
4.2. RNA Extraction and Evaluation
4.3. Quantification of the Virus Load
4.4. NanoString nCounter® Gene Expression
4.5. Luminex Measurement of Cytokines and Chemokines
4.6. Gene Ontology (GO) and Pathway Enrichment Analysis Using Kyoto Encyclopedia of Genes and Genomes (KEGG)
4.7. Ingenuity Pathway Analysis (IPA)
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magadum, A.; Kishore, R. Cardiovascular Manifestations of COVID-19 Infection. Cells 2020, 9, 2508. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Canoll, P.D.; Klein, R.S. How COVID-19 Affects the Brain. JAMA Psychiatry 2021, 78, 682–683. [Google Scholar] [CrossRef] [PubMed]
- von Weyhern, C.H.; Kaufmann, I.; Neff, F.; Kremer, M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet 2020, 395, e109. [Google Scholar] [CrossRef] [PubMed]
- Puelles, V.G.; Lutgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Rothan, H.A.; Natekar, J.P.; Stone, S.; Pathak, H.; Strate, P.G.; Arora, K.; Brinton, M.A.; Kumar, M. Neuroinvasion and Encephalitis Following Intranasal Inoculation of SARS-CoV-2 in K18-hACE2 Mice. Viruses 2021, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Kumari, P.; Stone, S.; Natekar, J.P.; Arora, K.; Auroni, T.T.; Kumar, M. SARS-CoV-2 Infects Primary Neurons from Human ACE2 Expressing Mice and Upregulates Genes Involved in the Inflammatory and Necroptotic Pathways. Pathogens 2022, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.Z.; Cheer, J.F.; Tonini, R. Modulating the Neuromodulators: Dopamine, Serotonin, and the Endocannabinoid System. Trends Neurosci. 2021, 44, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brunink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Solomon, T. Neurological infection with SARS-CoV-2—The story so far. Nat. Rev. Neurol. 2021, 17, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Spudich, S.; Nath, A. Nervous system consequences of COVID-19. Science 2022, 375, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Seehusen, F.; Clark, J.J.; Sharma, P.; Bentley, E.G.; Kirby, A.; Subramaniam, K.; Wunderlin-Giuliani, S.; Hughes, G.L.; Patterson, E.I.; Michael, B.D.; et al. Neuroinvasion and Neurotropism by SARS-CoV-2 Variants in the K18-hACE2 Mouse. Viruses 2022, 14, 1020. [Google Scholar] [CrossRef] [PubMed]
- Thorne, L.G.; Reuschl, A.K.; Zuliani-Alvarez, L.; Whelan, M.V.X.; Turner, J.; Noursadeghi, M.; Jolly, C.; Towers, G.J. SARS-CoV-2 sensing by RIG-I and MDA5 links epithelial infection to macrophage inflammation. EMBO J. 2021, 40, e107826. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Riva, L.; Pu, Y.; Martin-Sancho, L.; Kanamune, J.; Yamamoto, Y.; Sakai, K.; Gotoh, S.; Miorin, L.; De Jesus, P.D.; et al. MDA5 Governs the Innate Immune Response to SARS-CoV-2 in Lung Epithelial Cells. Cell Rep. 2021, 34, 108628. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Karki, R.; Williams, E.P.; Yang, D.; Fitzpatrick, E.; Vogel, P.; Jonsson, C.B.; Kanneganti, T.D. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 2021, 22, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.K.; Miller, S.D. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 2004, 173, 3916–3924. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.C.; Rasley, A.; Tranguch, S.L.; Marriott, I. Cultured astrocytes express toll-like receptors for bacterial products. Glia 2003, 43, 281–291. [Google Scholar] [CrossRef]
- Lehnardt, S.; Henneke, P.; Lien, E.; Kasper, D.L.; Volpe, J.J.; Bechmann, I.; Nitsch, R.; Weber, J.R.; Golenbock, D.T.; Vartanian, T. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J. Immunol. 2006, 177, 583–592. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Batista, C.R.A.; Saliba, S.W.; Yousif, N.M.; de Oliveira, A.C.P. Role of Microglia TLRs in Neurodegeneration. Front. Cell Neurosci. 2018, 12, 329. [Google Scholar] [CrossRef]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-kappaB pathway. Elife 2021, 10, e68563. [Google Scholar] [CrossRef] [PubMed]
- van der Sluis, R.M.; Cham, L.B.; Gris-Oliver, A.; Gammelgaard, K.R.; Pedersen, J.G.; Idorn, M.; Ahmadov, U.; Hernandez, S.S.; Cemalovic, E.; Godsk, S.H.; et al. TLR2 and TLR7 mediate distinct immunopathological and antiviral plasmacytoid dendritic cell responses to SARS-CoV-2 infection. EMBO J. 2022, 41, e109622. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.B.; Jana, M.; Roy, A.; Corbett, G.T.; Kundu, M.; Chandra, S.; Mondal, S.; Dasarathi, S.; Mufson, E.J.; Mishra, R.K.; et al. Selective disruption of TLR2-MyD88 interaction inhibits inflammation and attenuates Alzheimer’s pathology. J. Clin. Investig. 2018, 128, 4297–4312. [Google Scholar] [CrossRef] [PubMed]
- Dzamko, N.; Gysbers, A.; Perera, G.; Bahar, A.; Shankar, A.; Gao, J.; Fu, Y.; Halliday, G.M. Toll-like receptor 2 is increased in neurons in Parkinson’s disease brain and may contribute to alpha-synuclein pathology. Acta Neuropathol. 2017, 133, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168 e117. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Guan, Z.; Li, H.; Ye, M.; Chen, X.; Shen, J.; Zhou, Y.; Shi, Z.L.; Zhou, P.; et al. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct. Target. Ther. 2020, 5, 235. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Soares, V.C.; de Azevedo-Quintanilha, I.G.; Dias, S.; Fintelman-Rodrigues, N.; Sacramento, C.Q.; Mattos, M.; de Freitas, C.S.; Temerozo, J.R.; Teixeira, L.; et al. SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes. Cell Death Discov. 2021, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Lee, S.; Mall, R.; Pandian, N.; Wang, Y.; Sharma, B.R.; Malireddi, R.S.; Yang, D.; Trifkovic, S.; Steele, J.A.; et al. ZBP1-dependent inflammatory cell death, PANoptosis, and cytokine storm disrupt IFN therapeutic efficacy during coronavirus infection. Sci. Immunol. 2022, 7, eabo6294. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- McKenzie, B.A.; Dixit, V.M.; Power, C. Fiery Cell Death: Pyroptosis in the Central Nervous System. Trends Neurosci. 2020, 43, 55–73. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Vince, J.E. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ. 2019, 26, 99–114. [Google Scholar] [CrossRef]
- Zelic, M.; Pontarelli, F.; Woodworth, L.; Zhu, C.; Mahan, A.; Ren, Y.; LaMorte, M.; Gruber, R.; Keane, A.; Loring, P.; et al. RIPK1 activation mediates neuroinflammation and disease progression in multiple sclerosis. Cell Rep. 2021, 35, 109112. [Google Scholar] [CrossRef] [PubMed]
- Ofengeim, D.; Mazzitelli, S.; Ito, Y.; DeWitt, J.P.; Mifflin, L.; Zou, C.; Das, S.; Adiconis, X.; Chen, H.; Zhu, H.; et al. RIPK1 mediates a disease-associated microglial response in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, E8788–E8797. [Google Scholar] [CrossRef] [PubMed]
- Mifflin, L.; Hu, Z.; Dufort, C.; Hession, C.C.; Walker, A.J.; Niu, K.; Zhu, H.; Liu, N.; Liu, J.S.; Levin, J.Z.; et al. A RIPK1-regulated inflammatory microglial state in amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2025102118. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Amin, P.; Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 2019, 20, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Levinson, J.N.; El-Husseini, A. Building excitatory and inhibitory synapses: Balancing neuroligin partnerships. Neuron 2005, 48, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Niciu, M.J.; Kelmendi, B.; Sanacora, G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol. Biochem. Behav. 2012, 100, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Stone, S.; Natekar, J.; Kumari, P.; Arora, K.; Kumar, M. The FDA-approved gold drug auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. Virology 2020, 547, 7–11. [Google Scholar] [CrossRef]
- Stone, S.; Rothan, H.A.; Natekar, J.P.; Kumari, P.; Sharma, S.; Pathak, H.; Arora, K.; Auroni, T.T.; Kumar, M. SARS-CoV-2 Variants of Concern Infect the Respiratory Tract and Induce Inflammatory Response in Wild-Type Laboratory Mice. Viruses 2021, 14, 27. [Google Scholar] [CrossRef]
- Azouz, F.; Arora, K.; Krause, K.; Nerurkar, V.R.; Kumar, M. Integrated MicroRNA and mRNA Profiling in Zika Virus-Infected Neurons. Viruses 2019, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Lim, S.; Song, M.J.; Ahn, J.H.; Lee, C.H.; Shin, O.S. Whole Transcriptome Analyses Reveal Differential mRNA and microRNA Expression Profiles in Primary Human Dermal Fibroblasts Infected with Clinical or Vaccine Strains of Varicella Zoster Virus. Pathogens 2019, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kumar, M.; Kwon, H.I.; Seong, R.K.; Han, K.; Song, J.M.; Kim, C.J.; Choi, Y.K.; Shin, O.S. Dynamic changes in host gene expression associated with H5N8 avian influenza virus infection in mice. Sci. Rep. 2015, 5, 16512. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Belcaid, M.; Nerurkar, V.R. Identification of host genes leading to West Nile virus encephalitis in mice brain using RNA-seq analysis. Sci. Rep. 2016, 6, 26350. [Google Scholar] [CrossRef]


| Upregulated Genes | Downregulated Genes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D3 | D6 | D1 | D3 | D6 | ||||||
| Gene | FC | Gene | FC | Gene | FC | Gene | FC | Gene | FC | Gene | FC |
| Cxcl10 | 15.27 | Cxcl10 | 1393.91 | Eomes | −9.00 | Eomes | −14.51 | Gpr34 | −10.18 | ||
| Lcn2 | 9.45 | Ccl2 | 651.09 | Arc | −3.12 | Dlx1 | −4.10 | Eomes | −7.81 | ||
| Irf7 | 6.29 | Lcn2 | 426.24 | Dlx1 | −2.97 | Arc | −3.92 | Ttr | −7.23 | ||
| Zbp1 | 6.11 | Ccl5 | 328.46 | Cdkn1a | −2.78 | Reln | −2.76 | Cdc7 | −5.84 | ||
| Ccl2 | 5.89 | Rsad2 | 219.17 | Dlx2 | −2.58 | Dlx2 | −2.53 | Slc17a6 | −5.55 | ||
| Rsad2 | 5.83 | Cxcl9 | 186.42 | Fos | −2.05 | Map3k1 | −2.30 | Grm2 | −5.44 | ||
| Il1rn | 167.77 | Homer1 | −2.27 | Cx3cr1 | −4.45 | ||||||
| Gbp2 | 134.63 | Ago4 | −2.24 | Sall1 | −4.20 | ||||||
| Ccl3 | 126.66 | Sox4 | −2.23 | Ugt8a | −4.19 | ||||||
| Zbp1 | 99.06 | Fos | −2.09 | Chn2 | −4.06 | ||||||
| Irf7 | 81.45 | Dot1l | −2.07 | Fcrls | −4.04 | ||||||
| Ccl7 | 80.87 | Bdnf | −2.05 | P2ry12 | −3.90 | ||||||
| Lilrb4a | 55.60 | Sall1 | −2.04 | Dlx1 | −3.79 | ||||||
| Ccl4 | 54.35 | Grm2 | −2.01 | Slc2a5 | −3.67 | ||||||
| Psmb8 | 53.35 | Bdnf | −3.66 | ||||||||
| Bcl2a1a | 43.68 | Adamts16 | −3.41 | ||||||||
| Sell | 39.69 | Stmn1 | −3.30 | ||||||||
| Casp4 | 35.66 | Ago4 | −3.25 | ||||||||
| Ptx3 | 35.53 | Gria4 | −3.12 | ||||||||
| Slfn8 | 34.66 | Reln | −3.09 | ||||||||
| Category | GO ID | Term | # Genes (%) | p Value |
|---|---|---|---|---|
| Day 3 | ||||
| Biological process | GO:0051607 | defense response to virus | 4 (66.67%) | 1.52 × 10−5 |
| GO:0002376 | immune system process | 4 (66.67%) | 1.62 × 10−4 | |
| GO:0045087 | innate immune response | 4 (66.67%) | 3.98 × 10−4 | |
| GO:0009617 | response to bacterium | 3 (50.00%) | 2.01 × 10−3 | |
| GO:0060340 | positive regulation of type I interferon-mediated signaling pathway | 2 (33.33%) | 0.005811579 | |
| GO:0090026 | positive regulation of monocyte chemotaxis | 2 (33.33%) | 0.005822179 | |
| GO:0070098 | chemokine-mediated signaling pathway | 2 (33.33%) | 0.012371086 | |
| GO:0031640 | killing of cells of other organism | 2 (33.33%) | 0.018885435 | |
| GO:0030593 | neutrophil chemotaxis | 2 (33.33%) | 0.020134244 | |
| Cellular component | GO:0005576 | extracellular region | 3 (50.00%) | 0.065899318 |
| GO:0005615 | extracellular space | 3 (50.00%) | 0.072143421 | |
| Molecular function | GO:0005515 | protein binding | 5 (83.33%) | 0.027720426 |
| GO:0008009 | chemokine activity | 2 (33.33%) | 0.011893042 | |
| GO:0008201 | heparin binding | 2 (33.33%) | 0.046110545 | |
| GO:0005125 | cytokine activity | 2 (33.33%) | 0.061699185 | |
| Day 6 | ||||
| Biological process | GO:0002376 | immune system process | 28 (27.18%) | 1.01 × 10−20 |
| GO:0006954 | inflammatory response | 26 (25.24%) | 2.05 × 10−21 | |
| GO:0045087 | innate immune response | 23 (22.33%) | 2.19 × 10−12 | |
| GO:0006955 | immune response | 21 (20.39%) | 1.52 × 10−13 | |
| GO:0045944 | positive regulation of transcription from RNA polymerase II promoter | 19 (18.45%) | 2.68 × 10−5 | |
| GO:0051607 | defense response to virus | 18 (17.48%) | 1.45 × 10−15 | |
| GO:0071222 | cellular response to lipopolysaccharide | 17 (16.50%) | 1.40 × 10−12 | |
| GO:0010628 | positive regulation of gene expression | 17 (16.50%) | 2.75 × 10−8 | |
| GO:0032760 | positive regulation of tumor necrosis factor production | 16 (15.53%) | 2.14 × 10−17 | |
| GO:0009617 | response to bacterium | 15 (14.56%) | 1.34 × 10−10 | |
| Cellular component | GO:0016020 | membrane | 52 (50.49%) | 1.54 × 10−4 |
| GO:0016021 | integral component of membrane | 48 (46.60%) | 0.004153123 | |
| GO:0005886 | plasma membrane | 46 (44.66%) | 2.11 × 10−4 | |
| GO:0005737 | cytoplasm | 45 (43.69%) | 0.047232 | |
| GO:0005576 | extracellular region | 36 (34.95%) | 8.19 × 10−13 | |
| GO:0005615 | extracellular space | 31 (30.10%) | 7.79 × 10−9 | |
| GO:0005829 | cytosol | 31 (30.10%) | 0.011572543 | |
| GO:0009897 | external side of plasma membrane | 27 (26.21%) | 1.29 × 10−16 | |
| GO:0009986 | cell surface | 25 (24.27%) | 2.53 × 10−13 | |
| GO:0005887 | integral component of plasma membrane | 13 (12.62%) | 0.02582786 | |
| Molecular function | GO:0005515 | protein binding | 48 (46.60%) | 1.65 × 10−5 |
| GO:0042802 | identical protein binding | 31 (30.10%) | 2.21 × 10−8 | |
| GO:0005125 | cytokine activity | 13 (12.62%) | 2.43 × 10−9 | |
| GO:0003700 | transcription factor activity, sequence-specific DNA binding | 9 (8.74%) | 0.0153461 | |
| GO:0042803 | protein homodimerization activity | 9 (8.74%) | 0.053333585 | |
| GO:0004888 | transmembrane signaling receptor activity | 8 (7.77%) | 1.38 × 10−4 | |
| GO:0008009 | chemokine activity | 7 (6.80%) | 9.10 × 10−8 | |
| GO:0002020 | protease binding | 7 (6.80%) | 1.70 × 10−4 | |
| GO:0019899 | enzyme binding | 7 (6.80%) | 0.036582315 | |
| GO:0008233 | peptidase activity | 7 (6.80%) | 0.048234665 | |
| Sample | Entry | KEGG Pathway | # Genes |
|---|---|---|---|
| Day 1 | mmu04921 | Oxytocin signaling pathway | 2 |
| Day 3 | mmu04657 | IL-17 signaling pathway | 4 |
| mmu05171 | Coronavirus disease—COVID-19 | 3 | |
| mmu04623 | Cytosolic DNA-sensing pathway | 3 | |
| mmu04622 | RIG-I-like receptor signaling pathway | 3 | |
| mmu04668 | TNF signaling pathway | 3 | |
| mmu04620 | Toll-like receptor signaling pathway | 3 | |
| mmu04010 | MAPK signaling pathway | 3 | |
| Day 6 | mmu04060 | Cytokine–cytokine receptor interaction | 25 |
| mmu04010 | MAPK signaling pathway | 21 | |
| mmu05171 | Coronavirus disease—COVID-19 | 20 | |
| mmu04620 | Toll-like receptor signaling pathway | 20 | |
| mmu04668 | TNF signaling pathway | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.-J.; Kumari, P.; Auroni, T.T.; Stone, S.; Pathak, H.; Elsharkawy, A.; Natekar, J.P.; Shin, O.S.; Kumar, M. Upregulation of Neuroinflammation-Associated Genes in the Brain of SARS-CoV-2-Infected Mice. Pathogens 2024, 13, 528. https://doi.org/10.3390/pathogens13070528
Oh S-J, Kumari P, Auroni TT, Stone S, Pathak H, Elsharkawy A, Natekar JP, Shin OS, Kumar M. Upregulation of Neuroinflammation-Associated Genes in the Brain of SARS-CoV-2-Infected Mice. Pathogens. 2024; 13(7):528. https://doi.org/10.3390/pathogens13070528
Chicago/Turabian StyleOh, Soo-Jin, Pratima Kumari, Tabassum Tasnim Auroni, Shannon Stone, Heather Pathak, Amany Elsharkawy, Janhavi Prasad Natekar, Ok Sarah Shin, and Mukesh Kumar. 2024. "Upregulation of Neuroinflammation-Associated Genes in the Brain of SARS-CoV-2-Infected Mice" Pathogens 13, no. 7: 528. https://doi.org/10.3390/pathogens13070528
APA StyleOh, S.-J., Kumari, P., Auroni, T. T., Stone, S., Pathak, H., Elsharkawy, A., Natekar, J. P., Shin, O. S., & Kumar, M. (2024). Upregulation of Neuroinflammation-Associated Genes in the Brain of SARS-CoV-2-Infected Mice. Pathogens, 13(7), 528. https://doi.org/10.3390/pathogens13070528








