The Prevalence of Antibiotic Tolerance in Neisseria gonorrhoeae Varies by Anatomical Site
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
- (i)
- WHO Reference Panel
- (ii)
- Clinical Isolates
2.2. Tolerance Detection
2.3. Antimicrobial Susceptibility Testing
2.4. Induction of Resistance to Ciprofloxacin in Ceftriaxone-Tolerant Colonies
2.5. Statistical Analysis
3. Results
3.1. Detection of Tolerance and Heterotolerance in WHO Reference Strains
3.2. Differences in Azithromycin Tolerance across Infection Sites
3.3. No Difference in Tolerance to Ceftriaxone and Ciprofloxacin between the Infection Sites
3.4. Association between Ciprofloxacin and Ceftriaxone Tolerance
3.5. Induction of Resistance to Ciprofloxacin in CRO-Tolerant Isolates
4. Discussion
Impact Statement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, J.E.; Lam, H. Evolution of Bacterial Tolerance Under Antibiotic Treatment and Its Implications on the Development of Resistance. Front. Microbiol. 2021, 12, 617412. [Google Scholar] [CrossRef] [PubMed]
- Fridman, O.; Goldberg, A.; Ronin, I.; Shoresh, N.; Balaban, N.Q. Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature 2014, 513, 418–421. [Google Scholar] [CrossRef]
- Handwerger, S.; Tomasz, A. Antibiotic Tolerance Among Clinical Isolates of Bacteria. Rev. Infect. Dis. 1985, 7, 368–386. [Google Scholar] [CrossRef]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic tolerance facilitates the evolution of resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef]
- Gefen, O.; Chekol, B.; Strahilevitz, J.; Balaban, N.Q. TDtest: Easy detection of bacterial tolerance and persistence in clinical isolates by a modified disk-diffusion assay. Sci. Rep. 2017, 7, 41284. [Google Scholar] [CrossRef] [PubMed]
- Kotková, H.; Cabrnochová, M.; Lichá, I.; Tkadlec, J.; Fila, L.; Bartošová, J.; Melter, O. Evaluation of TD test for analysis of persistence or tolerance in clinical isolates of Staphylococcus aureus. J. Microbiol. Methods 2019, 167, 105705. [Google Scholar] [CrossRef]
- World Health Organisation. WHO Guidelines for the Treatment of Neisseria Gonorrhoeae; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Ohnishi, M.; Saika, T.; Hoshina, S.; Iwasaku, K.; Nakayama, S.I.; Watanabe, H.; Kitawaki, J. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg. Infect. Dis. 2011, 17, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Golparian, D.; Nicholas, R.; Ohnishi, M.; Gallay, A.; Sednaoui, P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: Novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob. Agents Chemother. 2012, 56, 1273–1280. [Google Scholar] [CrossRef]
- Balduck, M.; Laumen, J.G.E.; Abdellati, S.; De Baetselier, I.; de Block, T.; Manoharan-Basil, S.S.; Kenyon, C. Tolerance to Ceftriaxone in Neisseria gonorrhoeae: Rapid Induction in WHO P Reference Strain and Detection in Clinical Isolates. Antibiotics 2022, 11, 1480. [Google Scholar] [CrossRef]
- Ma, K.C.; Mortimer, T.D.; Hicks, A.L.; Wheeler, N.E.; Sánchez-Busó, L.; Golparian, D.; Taiaroa, G.; Rubin, D.H.; Wang, Y.; Williamson, D.A.; et al. Adaptation to the cervical environment is associated with increased antibiotic susceptibility in Neisseria gonorrhoeae. Nat. Commun. 2020, 11, 4126. [Google Scholar] [CrossRef] [PubMed]
- Morse, S.A.; Lysko, P.G.; McFarland, L.; Knapp, J.S.; Sandstrom, E.; Critchlow, C.; Holmes, K.K. Gonococcal strains from homosexual men have outer membranes with reduced permeability to hydrophobic molecules. Infect. Immun. 1982, 37, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Hauser, C.; Hirzberger, L.; Unemo, M.; Furrer, H.; Endimiani, A. In vitro activity of fosfomycin alone and in combination with ceftriaxone or azithromycin against clinical Neisseria gonorrhoeae isolates. Antimicrob. Agents Chemother. 2015, 59, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Golparian, D.; Sánchez-Busó, L.; Grad, Y.; Jacobsson, S.; Ohnishi, M.; Lahra, M.M.; Limnios, A.; Sikora, A.E.; Wi, T.; et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: Phenotypic, genetic and reference genome characterization. J. Antimicrob. Chemother. 2016, 71, 3096–3108. [Google Scholar] [CrossRef] [PubMed]
- McDermott, P.F.; White, D.G.; Zhao, S.; Simjee, S.; Walker, R.D. Antimicrobial Susceptibility Testing. In Preharvest and Postharvest Food Safety: Contemporary Issues and Future Directions; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- BioMérieux ETEST-Trusted Leader in MIC Gradient Strip Technology. Available online: https://www.biomerieux-usa.com/sites/subsidiary_us/files/prn_056750_rev_03.a_etest_brochure_final_art_2.pdf (accessed on 25 June 2024).
- Cirz, R.T.; Romesberg, F.E. Induction and inhibition of ciprofloxacin resistance-conferring mutations in hypermutator bacteria. Antimicrob. Agents Chemother. 2006, 50, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Raisman, J.C.; Fiore, M.A.; Tomin, L.; Adjei, J.K.; Aswad, V.X.; Chu, J.; Domondon, C.J.; Donahue, B.A.; Masciotti, C.A.; McGrath, C.G.; et al. Evolutionary paths to macrolide resistance in a Neisseria commensal converge on ribosomal genes through short sequence duplications. PLoS ONE 2022, 17, e0262370. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.P.; Camilleri, S.; Ward, C.; Huffam, S.; Chen, M.Y.; Bradshaw, C.S.; Fairley, C.K. Duration of gonorrhoea and chlamydia infection at the pharynx and rectum among men who have sex with men: A systematic review. Sex. Health 2016, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Dekaboruah, E.; Suryavanshi, M.V.; Chettri, D.; Verma, A.K. Human microbiome: An academic update on human body site specific surveillance and its possible role. Arch. Microbiol. 2020, 202, 2147–2167. [Google Scholar] [CrossRef]
- Galiwango, R.M.; Park, D.E.; Huibner, S.; Onos, A.; Aziz, M.; Roach, K.; Anok, A.; Nnamutete, J.; Isabirye, Y.; Wasswa, J.B.; et al. Immune milieu and microbiome of the distal urethra in Ugandan men: Impact of penile circumcision and implications for HIV susceptibility. Microbiome 2022, 10, 7. [Google Scholar] [CrossRef]
- Akomoneh, E.A.; Laumen, J.G.E.; Abdellati, S.; Van Dijck, C.; Vanbaelen, T.; Britto, X.B.; Manoharan-Basil, S.S.; Kenyon, C. The Discovery of Oropharyngeal Microbiota with Inhibitory Activity against Pathogenic Neisseria gonorrhoeae and Neisseria meningitidis: An In Vitro Study of Clinical Isolates. Microorganisms 2022, 10, 2497. [Google Scholar] [CrossRef]
- Baquero, F.; Moreno, F. The microcins. FEMS Microbiol. Lett. 1984, 23, 117–124. [Google Scholar] [CrossRef]
- Simpson, D.M.; Davis, C.P. Properties of a gonococcal inhibitor produced by Escherichia coli. J. Gen. Microbiol. 1979, 115, 471–477. [Google Scholar] [CrossRef] [PubMed]
- McBride, M.E.; Duncan, W.C.; Knox, J.M. Bacterial interference of Neisseria gonorrhoeae by alpha-haemolytic streptococci. Br. J. Vener. Dis. 1980, 56, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.C.-H.; Wang, J.-T.; Shu-Chen, W.; Ni, Y.-H. Host-microbial interactions and regulation of intestinal epithelial barrier function: From physiology to pathology. World J. Gastrointest. Pathophysiol. 2012, 3, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Tedijanto, C.; Olesen, S.W.; Grad, Y.H.; Lipsitch, M. Estimating the proportion of bystander selection for antibiotic resistance among potentially pathogenic bacterial flora. Proc. Natl. Acad. Sci. USA 2018, 115, E11988–E11995. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.; Zaidi, A.; Asbel, L.; Baldwin, T.; Gratzer, B.; Guerry, S.; Kerani, R.P.; Pathela, P.; Pettus, K.; Soge, O.O.; et al. Comparison of antimicrobial susceptibilities of pharyngeal, rectal, and urethral Neisseria gonorrhoeae isolates among men who have sex with men. Antimicrob. Agents Chemother. 2015, 59, 2588–2595. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, S.; Cole, M.J.; Spiteri, G.; Day, M.; Unemo, M. Associations between antimicrobial susceptibility/resistance of Neisseria gonorrhoeae isolates in European Union/European Economic Area and gender, sexual orientation and anatomical site of infection, 2009–2016. BMC Infect. Dis. 2021, 21, 273. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.; Hatzis, C.L.; Lau, A.; Williamson, D.A.; Chow, E.P.; Fairley, C.K.; Hocking, J.S. Treatment efficacy for pharyngeal Neisseria gonorrhoeae: A systematic review and meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 2020, 75, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Levin-Reisman, I.; Brauner, A.; Ronin, I.; Balaban, N.Q. Epistasis between antibiotic tolerance, persistence, and resistance mutations. Proc. Natl. Acad. Sci. USA 2019, 116, 14734–14739. [Google Scholar] [CrossRef]
- Khan, M.; Ma, K.; Wan, I.; Willcox, M.D. Ciprofloxacin resistance and tolerance of Pseudomonas aeruginosa ocular isolates. Contact Lens Anterior Eye 2023, 46, 101819. [Google Scholar] [CrossRef]
- Liu, J.; Gefen, O.; Ronin, I.; Bar-Meir, M.; Balaban, N.Q. Effect of tolerance on the evolution of antibiotic resistance under drug combinations. Science 2020, 367, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.R.; Burns, J.L.; Lory, S.; Lewis, K. Emergence of Pseudomonas aeruginosa Strains Producing High Levels of Persister Cells in Patients with Cystic Fibrosis. J. Bacteriol. 2010, 192, 6191–6199. [Google Scholar] [CrossRef] [PubMed]
- Honsa, E.S.; Cooper, V.S.; Mhaissen, M.N.; Frank, M.; Shaker, J.; Iverson, A.; Rubnitz, J.; Hayden, R.T.; Lee, R.E.; Rock, C.O.; et al. RelA Mutant Enterococcus faecium with Multiantibiotic Tolerance Arising in an Immunocompromised Host. MBio 2017, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Lazarovits, G.; Gefen, O.; Cahanian, N.; Adler, K.; Fluss, R.; Levin-Reisman, I.; Ronin, I.; Motro, Y.; Moran-Gilad, J.; Balaban, N.Q.; et al. Prevalence of Antibiotic Tolerance and Risk for Reinfection Among Escherichia coli Bloodstream Isolates: A Prospective Cohort Study. Clin. Infect. Dis. 2022, 75, 1706–1713. [Google Scholar] [CrossRef]
- Manoharan-Basil, S.; Balduck, M.; Laumen, J.; Kenyon, C. Transcriptomic profiling of ceftriaxone-tolerant phenotypes of Neisseria gonorrhoeae WHO P reference strain. In Proceedings of the ECCMID, Copenhagen, Denmark, 15–18 April 2023. accepted abstract (eposter number ALP1205). [Google Scholar]
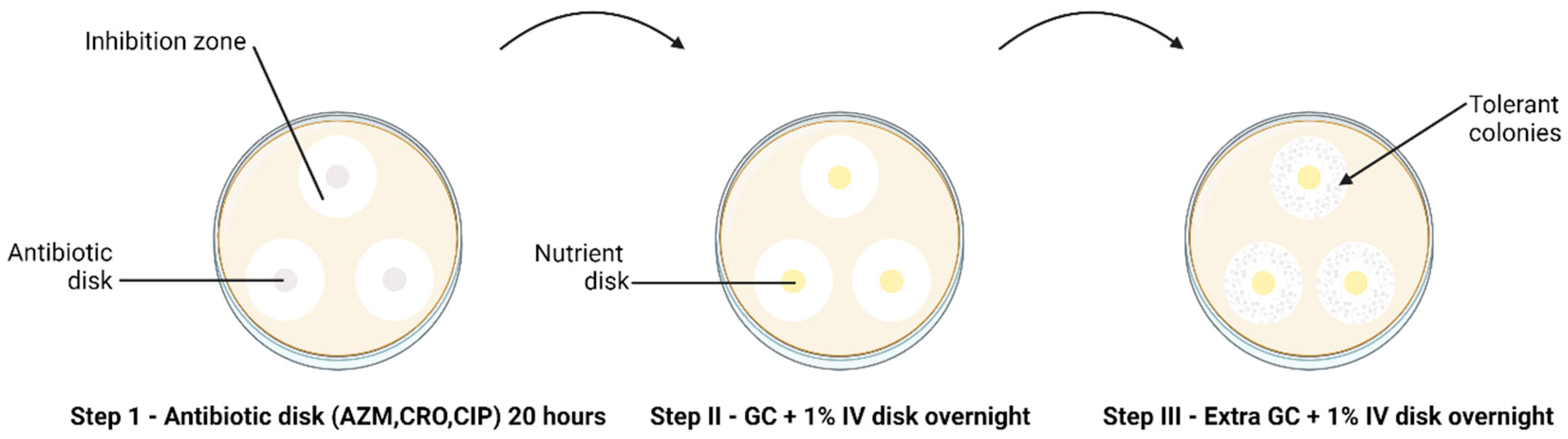



| ANTIBIOTICS | TOLERANCE | |
|---|---|---|
| Number | WHO Isolates | |
| AZITHROMYCIN | 1 | Z |
| CIPROFLOXACIN | 1 | P |
| CEFTRIAXONE | 7 | K, M, N, O, P, U, W |
| ANTIBIOTICS | TOLERANCE | POTENTIAL TOLERANCE | ||||
|---|---|---|---|---|---|---|
| Yes | No | Percent | Yes | No | Percent | |
| AZITHROMYCIN | ||||||
| ANORECTAL | 4 | 26 | 13.3% | 9 | 21 | 30% |
| UROGENITAL | 0 | 32 | 0% | 1 | 31 | 3.1% |
| p value | 0.033 | p value | 0.004 | |||
| CIPROFLOXACIN | ||||||
| ANORECTAL | 2 | 28 | 6.7% | 4 | 26 | 13.3% |
| UROGENITAL | 2 | 30 | 6.3% | 7 | 25 | 21.9% |
| p value | 0.947 | p value | 0.370 | |||
| CEFTRIAXONE | ||||||
| ANORECTAL | 6 | 24 | 20% | 10 | 30 | 33.3% |
| UROGENITAL | 2 | 30 | 6.3% | 2 | 30 | 6.3% |
| p value | 0.107 | p value | 0.007 | |||
| Tolerance | Ceftriaxone | Ciprofloxacin | ||
|---|---|---|---|---|
| No | Yes | No | Yes | |
| Azithromycin | ||||
| No | 51 (88%) | 7 (12%) | 54 (93%) | 4 (7%) |
| Yes | 3 (75%) | 1 (25%) | 4 (100%) | 0 (0%) |
| p-value 0.433 | p-value 1.0 | |||
| Ciprofloxacin | ||||
| No | 52 (89%) | 6 (10%) | - | - |
| Yes | 2 (50%) | 2 (50%) | ||
| p-value 0.077 | ||||
| COEF. | 95% CI | p-VALUE | |
|---|---|---|---|
| DAY | 0.007 | 0.002–0.011 | 0.002 |
| Tolerance | −0.004 | −0.018–0.010 | 0.556 |
| Random effects Strain id | |||
| 1.85 × 10−10 | 2.7 × 10−17–0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balduck, M.; Strikker, A.; Gestels, Z.; Abdellati, S.; Van den Bossche, D.; De Baetselier, I.; Kenyon, C.; Manoharan-Basil, S.S. The Prevalence of Antibiotic Tolerance in Neisseria gonorrhoeae Varies by Anatomical Site. Pathogens 2024, 13, 538. https://doi.org/10.3390/pathogens13070538
Balduck M, Strikker A, Gestels Z, Abdellati S, Van den Bossche D, De Baetselier I, Kenyon C, Manoharan-Basil SS. The Prevalence of Antibiotic Tolerance in Neisseria gonorrhoeae Varies by Anatomical Site. Pathogens. 2024; 13(7):538. https://doi.org/10.3390/pathogens13070538
Chicago/Turabian StyleBalduck, Margaux, Akim Strikker, Zina Gestels, Saïd Abdellati, Dorien Van den Bossche, Irith De Baetselier, Chris Kenyon, and Sheeba Santhini Manoharan-Basil. 2024. "The Prevalence of Antibiotic Tolerance in Neisseria gonorrhoeae Varies by Anatomical Site" Pathogens 13, no. 7: 538. https://doi.org/10.3390/pathogens13070538






