Sunflower Oil and Cholesterol Nanoemulsion: A Novel Carrier for Micafungin to Combat Multi-Resistant Candida auris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Development and Characterization of the Nanoemulsion
2.2. Fungal Strains
2.3. Evaluation of Antibiofilm Efficacy
2.4. Confocal Laser Scanning Microscopy
2.4.1. In Vivo Antifungal Activity
2.4.2. Histopathology
3. Results
3.1. Development and Characterization
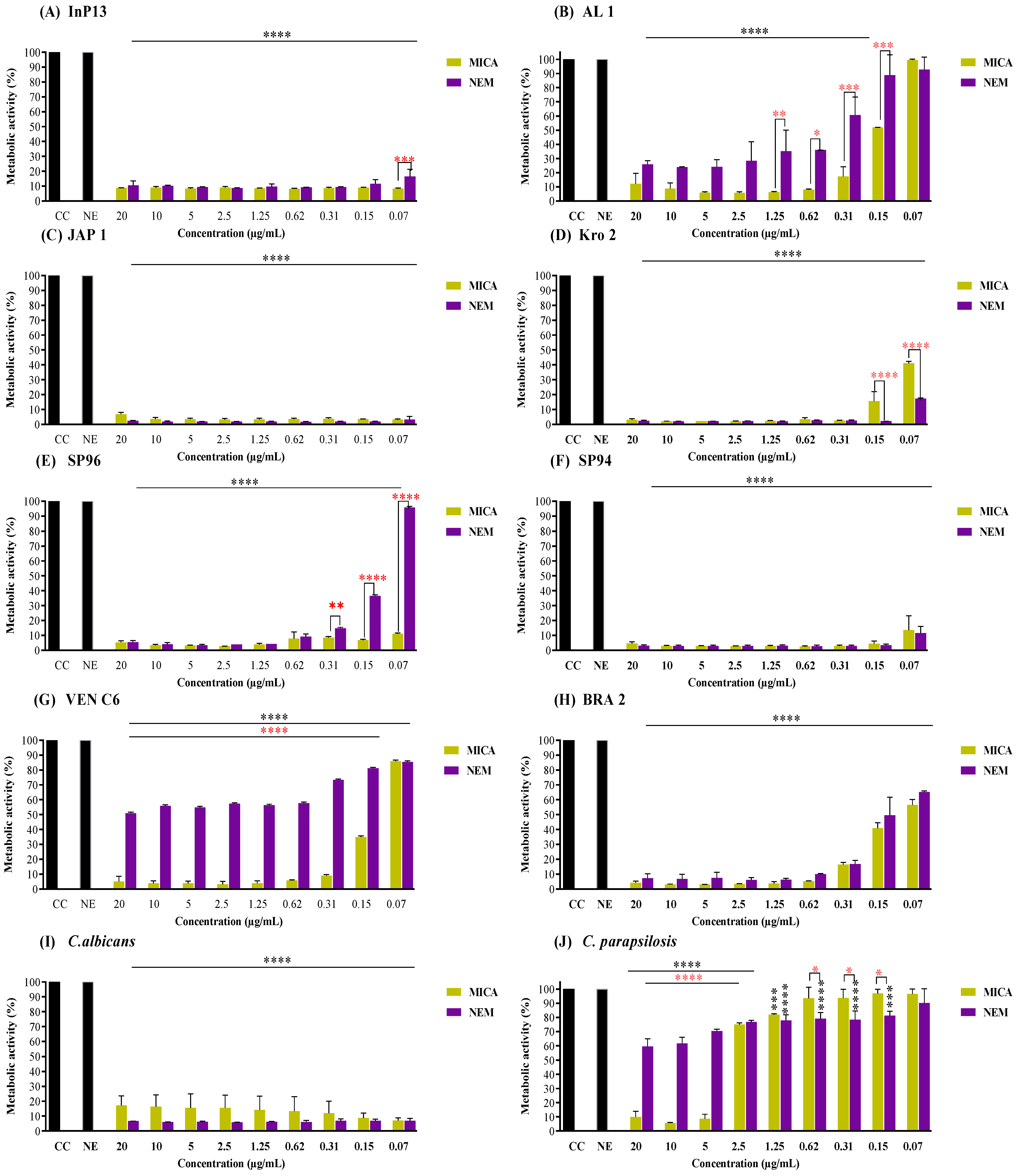
3.2. Pre-Adherent Antibiofilm Activity
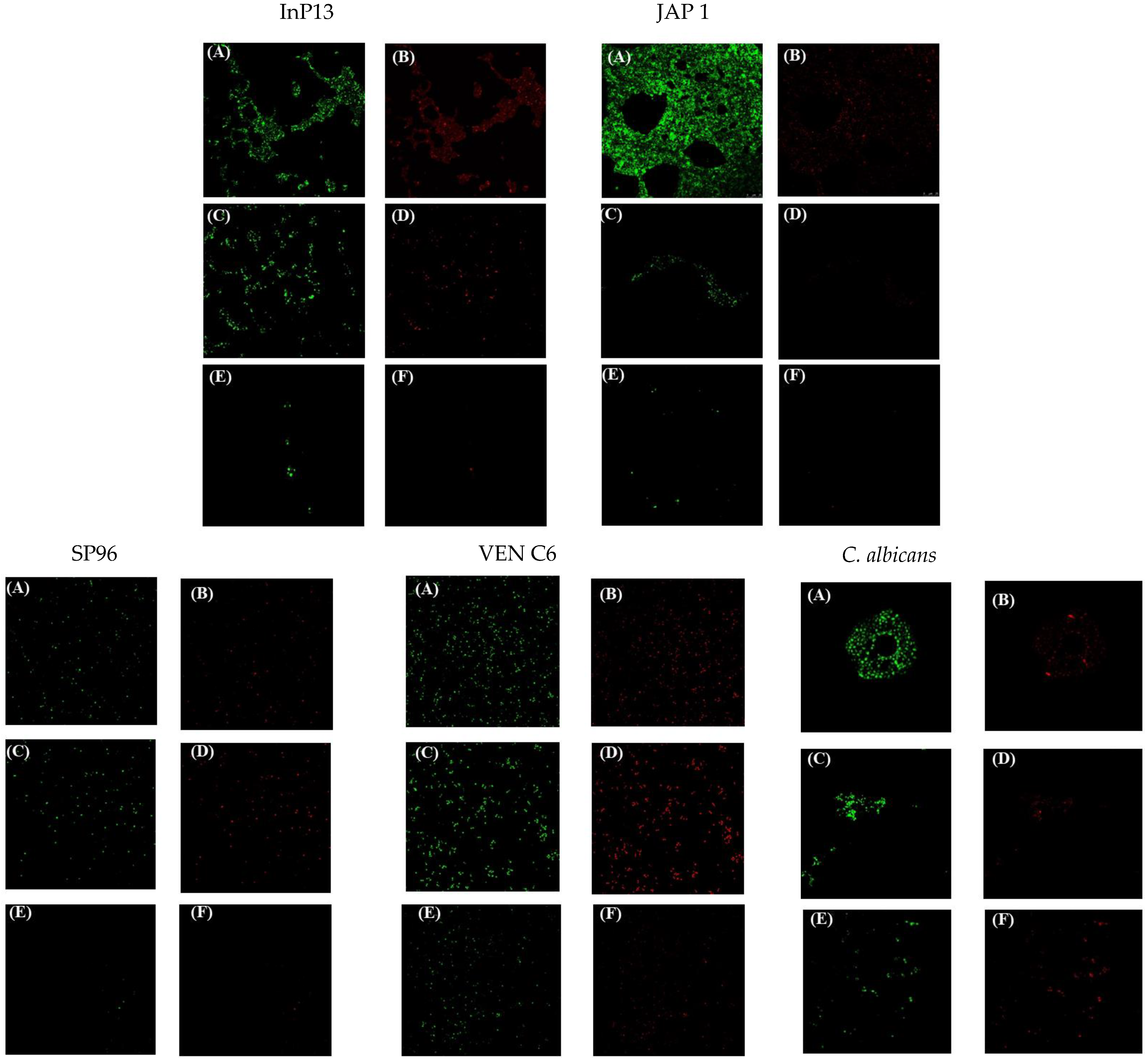
3.3. Confocal Laser Microscopy
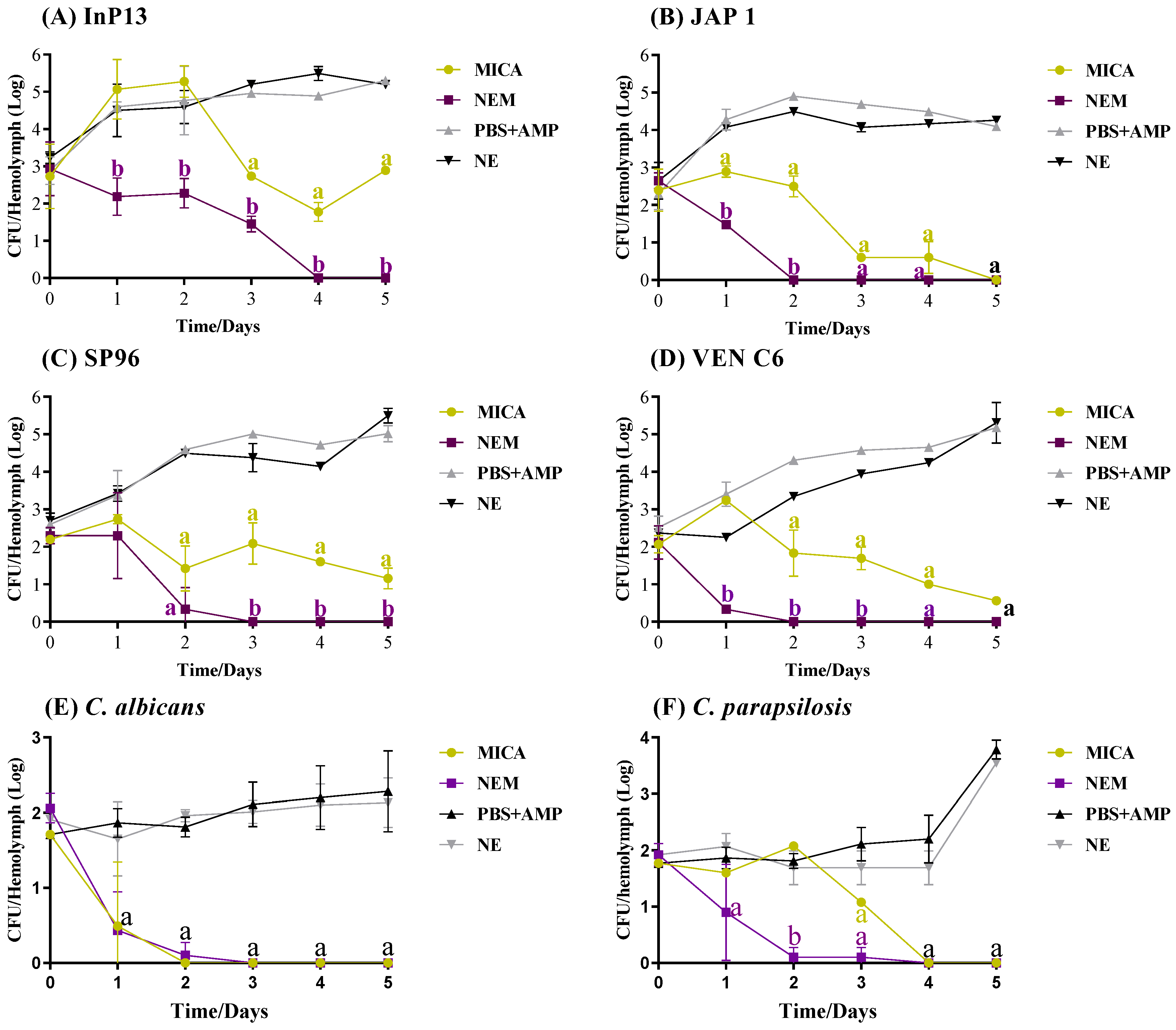
3.4. In Vivo Antifungal Activity
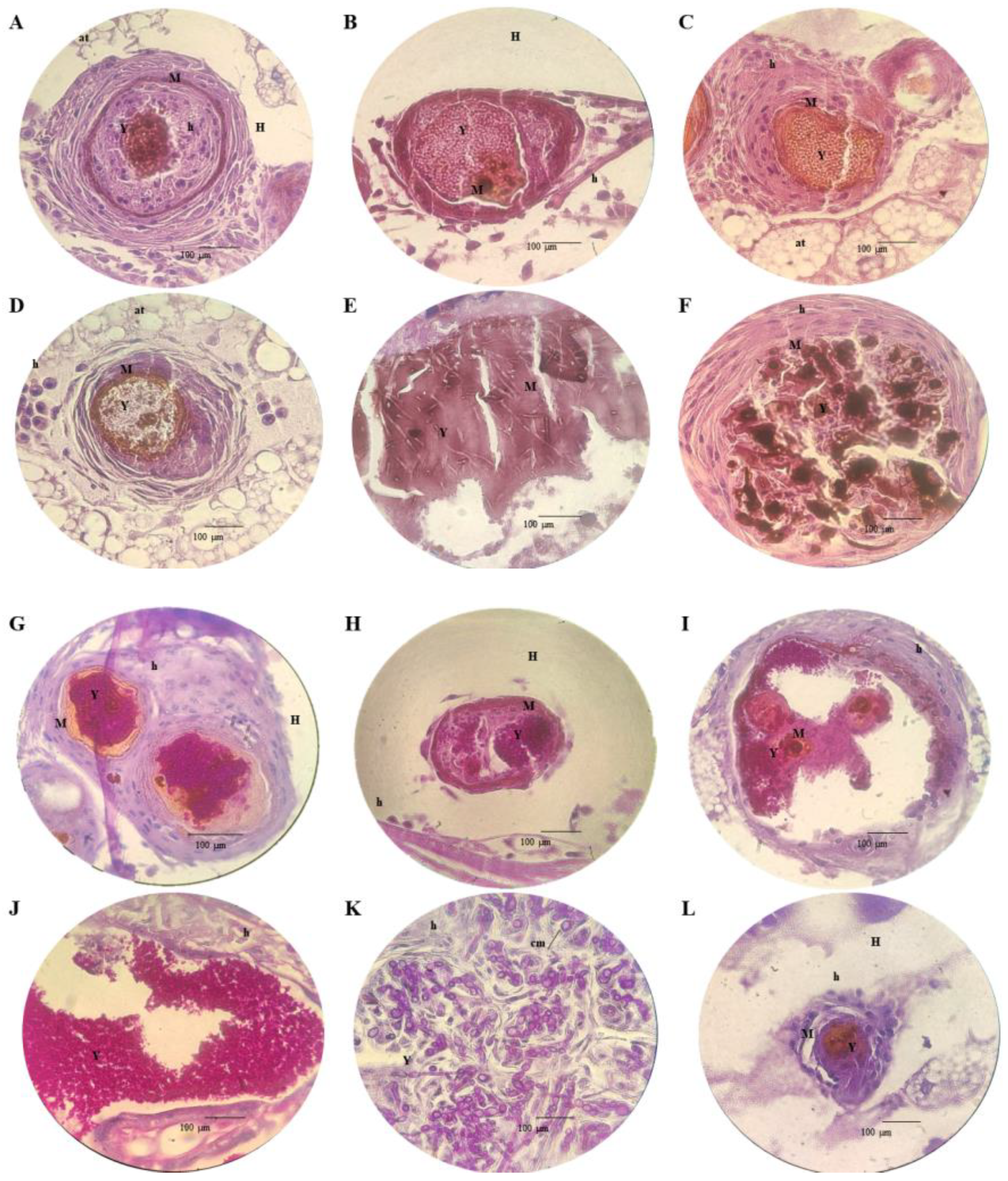
3.5. Histopathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, H.; Bing, J.; Nobile, C.J.; Huang, G. Candida auris Infections in China. Virulence 2022, 13, 589–591. [Google Scholar] [CrossRef]
- Horton, M.V.; Nett, J.E. Candida auris Infection and Biofilm Formation: Going Beyond the Surface. Curr. Clin. Microbiol. Rep. 2020, 7, 51–56. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.; Chen, X.; Li, H.; Yin, Z.; Zhang, B.; Xu, Y.; Zhang, Y.; Zhang, R.; Huang, X.; et al. Innate Immune Responses against the Fungal Pathogen Candida auris. Nat. Commun. 2022, 13, 3553. [Google Scholar] [CrossRef]
- Vinayagamoorthy, K.; Pentapati, K.C.; Prakash, H. Prevalence, Risk Factors, Treatment and Outcome of Multidrug Resistance Candida auris Infections in Coronavirus Disease (COVID-19) Patients: A Systematic Review. Mycoses 2022, 65, 613–624. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, Development and Applications in Drug Delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Marena, G.D.; Ramos, M.A.D.S.; Lima, L.C.; Chorilli, M.; Bauab, T.M. Galleria Mellonella for Systemic Assessment of Anti-Candida auris Using Amphotericin B Loaded in Nanoemulsion. Sci. Total Environ. 2022, 807, 151023. [Google Scholar] [CrossRef]
- Duarte, A.B.S.; Perez-Castillo, Y.; da Nóbrega Alves, D.; de Castro, R.D.; de Souza, R.L.; de Sousa, D.P.; Oliveira, E.E. Antifungal Activity against Candida Albicans of Methyl 3,5-Dinitrobenzoate Loaded Nanoemulsion. Braz. J. Microbiol. 2024, 55, 25–39. [Google Scholar] [CrossRef]
- Gomes, S.I.L.; Guimarães, B.; Gasco, P.; Blosi, M.; Costa, A.L.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Nanoemulsion Carriers for Drug Delivery: Assessment of Environmental Hazards. Environ. Pollut. 2023, 328, 121669. [Google Scholar] [CrossRef]
- Shahid, M.; Hussain, A.; Khan, A.A.; Ramzan, M.; Alaofi, A.L.; Alanazi, A.M.; Alanazi, M.M.; Rauf, M.A. Ketoconazole-Loaded Cationic Nanoemulsion: In Vitro-Ex Vivo-In Vivo Evaluations to Control Cutaneous Fungal Infections. ACS Omega 2022, 7, 20267–20279. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, T.; Alaseem, A.M.; Khan, M.M.; Mukhtar, B.; Kamal, M.; Anwer, R.; Ahmed, S.; Alam, A. Preparation and Evaluation of Nanoemulsion of Citronella Essential Oil with Improved Antimicrobial and Anti-Cancer Properties. Antibiotics 2023, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Marena, G.D.; Ramos, M.A.d.S.; Carvalho, G.C.; de Lima, L.C.; do Nascimento, A.L.C.S.; Sábio, R.M.; Rodero, C.F.; Spósito, L.; Bauab, T.M.; Chorilli, M. Development and Characterization of an Amphotericin B—Loaded Nanoemulsion Applied to Candida auris Biofilms Control. J. Drug Deliv. Sci. Technol. 2022, 74, 103566. [Google Scholar] [CrossRef]
- Garcia-Bustos, V.; Pemán, J.; Ruiz-Gaitán, A.; Cabañero-Navalon, M.D.; Cabanilles-Boronat, A.; Fernández-Calduch, M.; Marcilla-Barreda, L.; Sigona-Giangreco, I.A.; Salavert, M.; Tormo-Mas, M.Á.; et al. Host–Pathogen Interactions upon Candida auris Infection: Fungal Behaviour and Immune Response in Galleria Mellonella. Emerg. Microbes Infect. 2022, 11, 136–146. [Google Scholar] [CrossRef]
- Szymański, M.; Chmielewska, S.; Czyżewska, U.; Malinowska, M.; Tylicki, A. Echinocandins–Structure, Mechanism of Action and Use in Antifungal Therapy. J. Enzym. Inhib. Med. Chem. 2022, 37, 876–894. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to Azoles and Echinocandins Worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Centers for Desease Control and Prevention. Clinical Treatment of C. auris infections. Available online: https://www.cdc.gov/candida-auris/hcp/clinical-care/index.html (accessed on 10 June 2023).
- Fioriti, S.; Brescini, L.; Pallotta, F.; Canovari, B.; Morroni, G.; Barchiesi, F. Antifungal Combinations against Candida Species: From Bench to Bedside. J. Fungi 2022, 8, 1077. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.M.; Abik, F.; Mikkonen, K.S. An Overview of Nanoemulsion Characterization via Atomic Force Microscopy. Crit. Rev. Food Sci. Nutr. 2022, 62, 4908–4928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yi, Y.; Qi, J.; Lu, Y.; Tian, Z.; Xie, Y.; Yuan, H.; Wu, W. Controlled Release of Cyclosporine A Self-Nanoemulsifying Systems from Osmotic Pump Tablets: Near Zero-Order Release and Pharmacokinetics in Dogs. Int. J. Pharm. 2013, 452, 233–240. [Google Scholar] [CrossRef]
- Marena, G.D.; Carvalho, G.C.; Ramos, M.A.d.S.; Chorilli, M.; Bauab, T.M. Anti-Candida auris Activity in Vitro and in Vivo of Micafungin Loaded Nanoemulsions. Med. Mycol. 2023, 62, myac090. [Google Scholar] [CrossRef]
- Atiencia-Carrera, M.B.; Cabezas-Mera, F.S.; Vizuete, K.; Debut, A.; Tejera, E.; Machado, A. Evaluation of the Biofilm Life Cycle between Candida Albicans and Candida Tropicalis. Front. Cell Infect. Microbiol. 2022, 12, 953168. [Google Scholar] [CrossRef]
- Alves, R.; Barata-Antunes, C.; Casal, M.; Brown, A.J.P.; van Dijck, P.; Paiva, S. Adapting to Survive: How Candida Overcomes Host-Imposed Constraints during Human Colonization. PLoS Pathog. 2020, 16, e1008478. [Google Scholar] [CrossRef]
- Eix, E.F.; Nett, J.E. How Biofilm Growth Affects Candida-Host Interactions. Front. Microbiol. 2020, 11, 542412. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef]
- Cateau, E.; Rodier, M.H.; Imbert, C. In Vitro Efficacies of Caspofungin or Micafungin Catheter Lock Solutions on Candida Albicans Biofilm Growth. J. Antimicrob. Chemother. 2008, 62, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Miyagawa, S.; Takeda, O.; Hakariya, M.; Matsumoto, S.; Ohno, H.; Miyazaki, Y. Real-Time Microscopic Observation of Candida Biofilm Development and Effects Due to Micafungin and Fluconazole. Antimicrob. Agents Chemother. 2013, 57, 2226–2230. [Google Scholar] [CrossRef]
- Singh, R.; Nadhe, S.; Wadhwani, S.; Shedbalkar, U.; Chopade, B.A. Nanoparticles for Control of Biofilms of Acinetobacter Species. Materials 2016, 9, 383. [Google Scholar] [CrossRef]
- Dos Santos Ramos, M.A.; Da Silva, P.B.; Spósito, L.; De Toledo, L.G.; Bonifácio, B.v.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-Based Drug Delivery Systems for Control of Microbial Biofilms: A Review. Int. J. Nanomed. 2018, 13, 1179–1213. [Google Scholar] [CrossRef]
- Lee, Y.; Robbins, N.; Cowen, L.E. Molecular Mechanisms Governing Antifungal Drug Resistance. npj Antimicrob. Resist. 2023, 1, 5. [Google Scholar] [CrossRef]
- Giongo, J.L.; de Almeida Vaucher, R.; Fausto, V.P.; Quatrin, P.M.; Lopes, L.Q.S.; Santos, R.C.V.; Gündel, A.; Gomes, P.; Steppe, M. Anti-Candida Activity Assessment of Pelargonium Graveolens Oil Free and Nanoemulsion in Biofilm Formation in Hospital Medical Supplies. Microb. Pathog. 2016, 100, 170–178. [Google Scholar] [CrossRef]
- Junqueira, J.C.; Jorge, A.O.C.; Barbosa, J.O.; Rossoni, R.D.; Vilela, S.F.G.; Costa, A.C.B.P.; Primo, F.L.; Gonçalves, J.M.; Tedesco, A.C.; Suleiman, J.M.A.H. Photodynamic Inactivation of Biofilms Formed by Candida Spp., Trichosporon Mucoides, and Kodamaea Ohmeri by Cationic Nanoemulsion of Zinc 2,9,16,23-Tetrakis(Phenylthio)-29H, 31H-Phthalocyanine (ZnPc). Lasers Med. Sci. 2012, 27, 1205–1212. [Google Scholar] [CrossRef]
- Jemel, S.; Guillot, J.; Kallel, K.; Botterel, F.; Dannaoui, E. Galleria mellonella for the Evaluation of Antifungal Efficacy against Medically Important Fungi, a Narrative Review. Microorganisms 2020, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.; Binder, U.; Kavanagh, K. Galleria Mellonella Larvae as a Model for Investigating Fungal—Host Interactions. Front. Fungal Biol. 2022, 3, 893494. [Google Scholar] [CrossRef]
- Fan, S.; Li, C.; Bing, J.; Huang, G.; Du, H. Discovery of the Diploid Form of the Emerging Fungal Pathogen Candida auris. ACS Infect. Dis. 2020, 6, 2641–2646. [Google Scholar] [CrossRef]
- Facchini, N.; Wernli, L.; Rieken, M.; Bonkat, G.; Wirz, D.; Braissant, O. Again and Again—Survival of Candida Albicans in Urine Containing Antifungals. Pharmaceutics 2024, 16, 605. [Google Scholar] [CrossRef]
- Muñoz, J.E.; Ramirez, L.M.; Dias, L.d.S.; Rivas, L.A.; Ramos, L.S.; Santos, A.L.S.; Taborda, C.P.; Parra-Giraldo, C.M. Pathogenicity Levels of Colombian Strains of Candida auris and Brazilian Strains of Candida Haemulonii Species Complex in Both Murine and Galleria Mellonella Experimental Models. J. Fungi 2020, 6, 104. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marena, G.D.; López, A.; Carvalho, G.C.; Marín, M.d.P.; Pérez Ruiz, M.D.; Pérez-Royo, J.M.; Tormo-Mas, M.Á.; Bernabé, P.; Valentín, E.; Bauab, T.M.; et al. Sunflower Oil and Cholesterol Nanoemulsion: A Novel Carrier for Micafungin to Combat Multi-Resistant Candida auris. Pathogens 2024, 13, 549. https://doi.org/10.3390/pathogens13070549
Marena GD, López A, Carvalho GC, Marín MdP, Pérez Ruiz MD, Pérez-Royo JM, Tormo-Mas MÁ, Bernabé P, Valentín E, Bauab TM, et al. Sunflower Oil and Cholesterol Nanoemulsion: A Novel Carrier for Micafungin to Combat Multi-Resistant Candida auris. Pathogens. 2024; 13(7):549. https://doi.org/10.3390/pathogens13070549
Chicago/Turabian StyleMarena, Gabriel Davi, Alejandro López, Gabriela Corrêa Carvalho, María del Pilar Marín, María Dolores Pérez Ruiz, Jose Manuel Pérez-Royo, María Ángeles Tormo-Mas, Patricia Bernabé, Eulogio Valentín, Taís Maria Bauab, and et al. 2024. "Sunflower Oil and Cholesterol Nanoemulsion: A Novel Carrier for Micafungin to Combat Multi-Resistant Candida auris" Pathogens 13, no. 7: 549. https://doi.org/10.3390/pathogens13070549








