Genetic Characterization of Palyam Serogroup Viruses Isolated in Japan from 1984 to 2018 and Development of a Real-Time RT-PCR Assay for Broad Detection of Palyam Serogroup Viruses and Specific Detection of Chuzan (Kasba) and D’Aguilar Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Full-Length Amplification of cDNAs (FLAC), Next-Generation Sequencing (NGS) and Sanger Sequencing
2.3. Phylogenetic Analysis
2.4. Primer and Probe Design for a Real-Time RT-PCR Assay
2.5. A Real-Time RT-PCR Assay
2.6. Evaluation of Analytical Specificity and Sensitivity of the Real-Time RT-PCR Assay
2.7. Validation of the Real-Time RT-PCR Assay Using Field-Collected Samples
3. Results
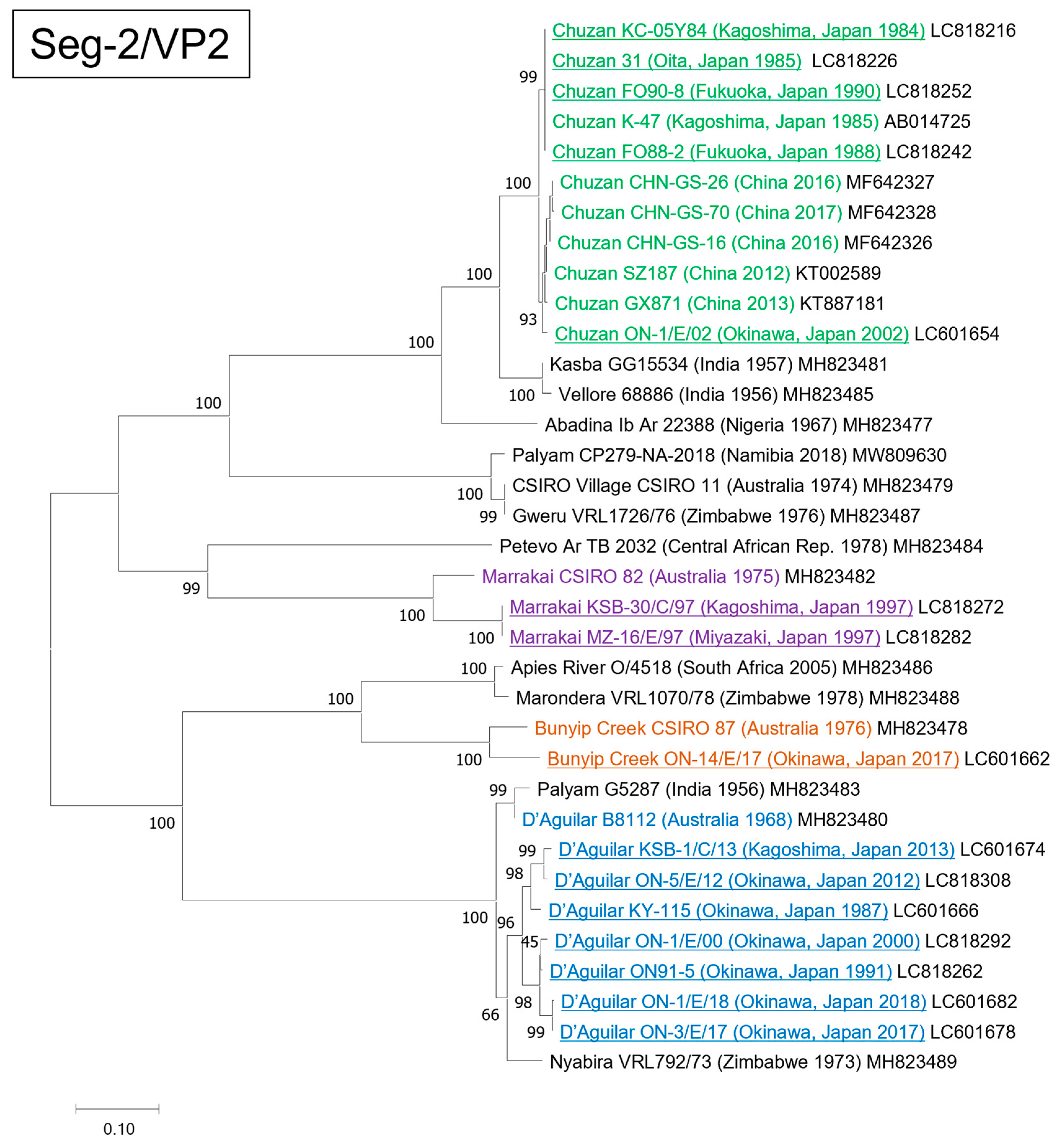
3.1. Genetic and Phylogenetic Characteristics of Seg-2
3.2. Genetic and Phylogenetic Characteristics of the Other Genome Segments
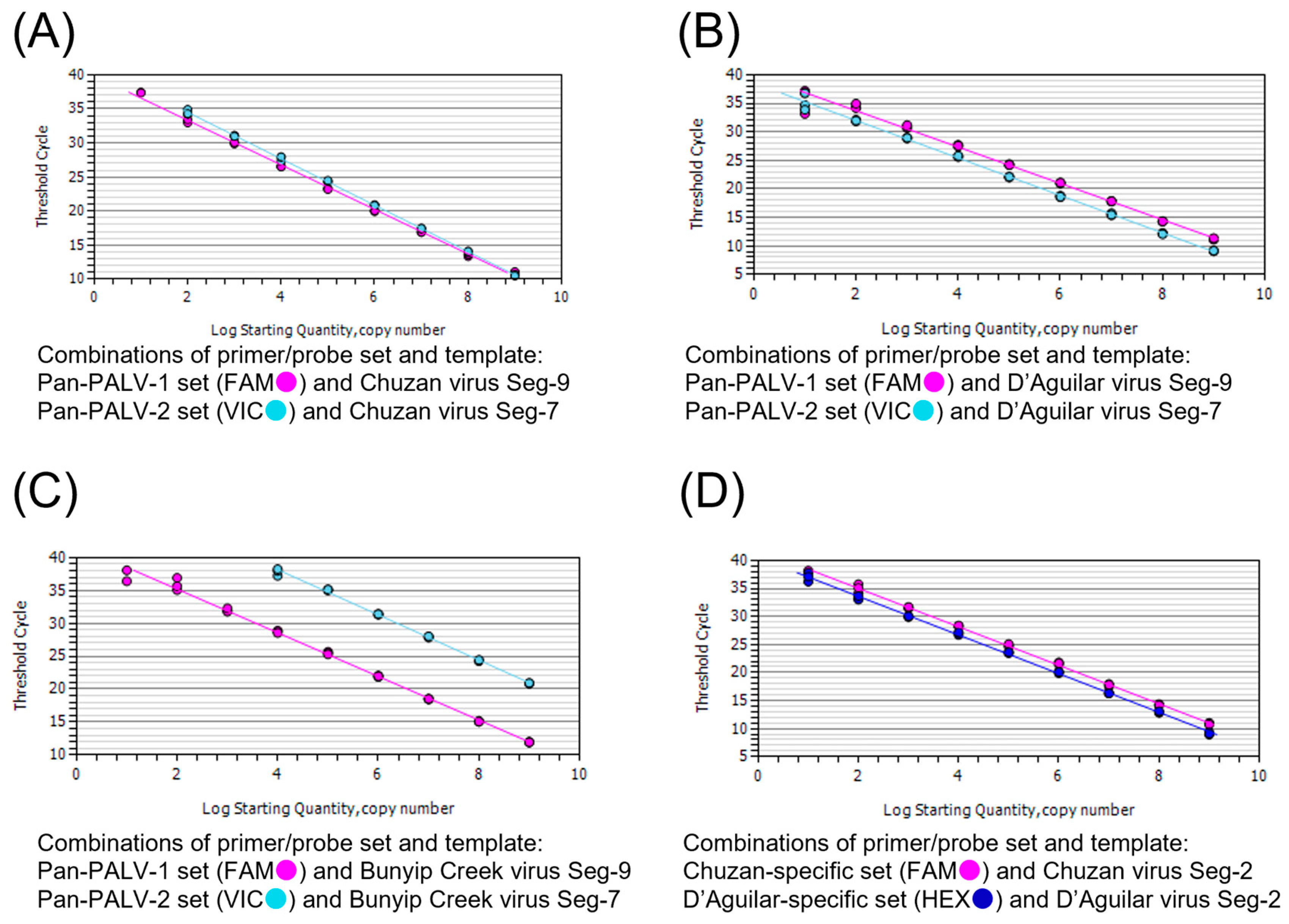
3.3. Analytical Specificity and Sensitivity of the Real-Time RT-PCR Assay
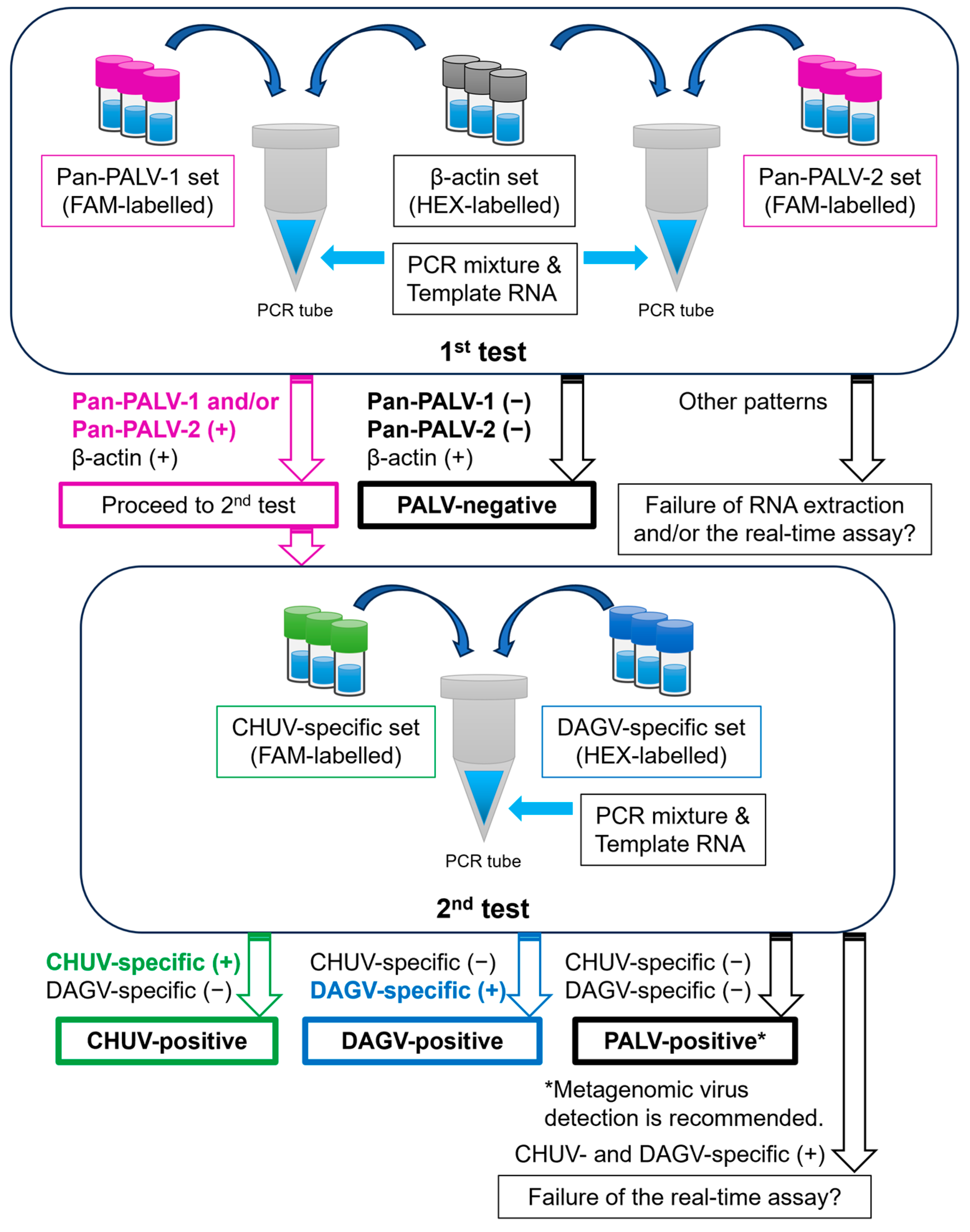
3.4. Diagnostic Specificity and Sensitivity of the Real-Time RT-PCR Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quan, M. Palyam serogroup orbivirus infections. In Infectious Diseases of Livestock, 3rd ed.; Coetzer, J.A.W., Penrith, M.L., Maclachlan, N.J., Thomson, G.R., Eds.; Oxford University Press: Cape Town, South Africa, 2020. [Google Scholar]
- Miura, Y.; Goto, Y.; Kubo, M.; Kono, Y. Isolation of Chuzan virus, a new member of the Palyam subgroup of the genus Orbivirus, from cattle and Culicoides oxystoma in Japan. Am. J. Vet. Res. 1988, 49, 2022–2025. [Google Scholar] [PubMed]
- Jusa, E.R.; Inaba, Y.; Kadoi, K.; Kurogi, H.; Fonseca, E.; Shope, R.E. Identification of Kagoshima and Chuzan viruses of Japan as Kasba virus, an orbivirus of the Palyam serogroup. Aust. Vet. J. 1994, 71, 57. [Google Scholar] [CrossRef]
- Miura, Y.; Kubo, M.; Goto, Y.; Kono, Y. Hydranencephaly-cerebellar hypoplasia in a newborn calf after infection of its dam with Chuzan virus. Nihon Juigaku Zasshi 1990, 52, 689–694. [Google Scholar] [CrossRef]
- Goto, Y.; Miura, Y.; Kono, Y. Serologic evidence for the etiologic role of Chuzan virus in an epizootic of congenital abnormalities with hydranencephaly-cerebellar hypoplasia syndrome of calves in Japan. Am. J. Vet. Res. 1988, 49, 2026–2029. [Google Scholar]
- Goto, Y.; Miura, Y.; Kono, Y. Epidemiological survey of an epidemic of congenital abnormalities with hydranencephaly-cerebellar hypoplasia syndrome of calves occurring in 1985/86 and seroepidemiological investigations on Chuzan virus, a putative causal agent of the disease, in Japan. Nihon Juigaku Zasshi 1988, 50, 405–413. [Google Scholar] [CrossRef]
- Ohashi, S.; Matsumori, Y.; Yanase, T.; Yamakawa, M.; Kato, T.; Tsuda, T. Evidence of an antigenic shift among Palyam serogroup orbiviruses. J. Clin. Microbiol. 2004, 42, 4610–4614. [Google Scholar] [CrossRef] [PubMed]
- Whistler, T.; Swanepoel, R. Characterization of potentially foetotropic Palyam serogroup orbiviruses isolated in Zimbabwe. J. Gen. Virol. 1988, 69, 2221–2227. [Google Scholar] [CrossRef]
- Yamakawa, M.; Kubo, M.; Furuuchi, S. Molecular analysis of the genome of Chuzan virus, a member of the Palyam serogroup viruses, and its phylogenetic relationships to other orbiviruses. J. Gen. Virol. 1999, 80, 937–941. [Google Scholar] [CrossRef]
- Harasawa, R.; Yoshida, T.; Iwashita, O.; Goto, Y.; Miura, Y. Biochemical characteristics of Chuzan virus, a new serotype of Palyam serogroup. Nihon Juigaku Zasshi 1988, 50, 777–782. [Google Scholar] [CrossRef]
- Ebersohn, K.; Coetzee, P.; Snyman, L.P.; Swanepoel, R.; Venter, E.H. Phylogenetic Characterization of the Palyam Serogroup Orbiviruses. Viruses 2019, 11, 446. [Google Scholar] [CrossRef]
- Yamakawa, M.; Ohashi, S.; Kanno, T.; Yamazoe, R.; Yoshida, K.; Tsuda, T.; Sakamoto, K. Genetic diversity of RNA segments 5, 7 and 9 of the Palyam serogroup orbiviruses from Japan, Australia and Zimbabwe. Virus Res. 2000, 68, 145–153. [Google Scholar] [CrossRef]
- Yang, H.; Xiao, L.; Meng, J.; Xiong, H.; Gao, L.; Liao, D.; Li, H. Complete genome sequence of a Chuzan virus strain isolated for the first time in mainland China. Arch. Virol. 2016, 161, 1073–1077. [Google Scholar] [CrossRef]
- Wang, F.; Lin, J.; Chang, J.; Cao, Y.; Qin, S.; Wu, J.; Yu, L. Isolation, complete genome sequencing, and phylogenetic analysis of the first Chuzan virus in China. Virus Genes. 2016, 52, 138–141. [Google Scholar] [CrossRef]
- Guggemos, H.D.; Fendt, M.; Hermanns, K.; Hieke, C.; Heyde, V.; Mfune, J.K.E.; Borgemeister, C.; Junglen, S. Orbiviruses in biting midges and mosquitoes from the Zambezi region, Namibia. J. Gen. Virol. 2021, 102, 001662. [Google Scholar] [CrossRef]
- Cybinski, D.H.; St George, T.D. Preliminary characterization of D’Aguilar virus and three Palyam group viruses new to Australia. Aust. J. Biol. Sci. 1982, 35, 343–351. [Google Scholar] [CrossRef]
- Ohashi, S.; Yoshida, K.; Yanase, T.; Kato, T.; Tsuda, T. Simultaneous detection of bovine arboviruses using single-tube multiplex reverse transcription-polymerase chain reaction. J. Virol. Methods 2004, 120, 79–85. [Google Scholar] [CrossRef]
- Ebersohn, K. Phylogenetic Characterisation of the Palyam Serogroup Orbiviruses and Development of a Real-Time RT-PCR. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2018. [Google Scholar]
- Yang, H.; Liao, D.; Li, Z.; Li, Z.; Yang, Z.; Xiao, L.; Li, H. Real-Time Fluorescent Quantitative RT-PCR Detection Primers for Serotype of Palyam Serogroup Virus (PALV), Probes and Detection Kit. 2019. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=CN282352475&docAn=201910979854.1 (accessed on 8 May 2024).
- Shirafuji, H.; Kato, T.; Yamakawa, M.; Tanaka, T.; Minemori, Y.; Yanase, T. Characterization of genome segments 2, 3 and 6 of epizootic hemorrhagic disease virus strains isolated in Japan in 1985–2013: Identification of their serotypes and geographical genetic types. Infect. Genet. Evol. 2017, 53, 38–46. [Google Scholar] [CrossRef]
- Maan, S.; Rao, S.; Maan, N.S.; Anthony, S.J.; Attoui, H.; Samuel, A.R.; Mertens, P.P. Rapid cDNA synthesis and sequencing techniques for the genetic study of bluetongue and other dsRNA viruses. J. Virol. Methods 2007, 143, 132–139. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Toussaint, J.F.; Sailleau, C.; Breard, E.; Zientara, S.; De Clercq, K. Bluetongue virus detection by two real-time RT-qPCRs targeting two different genomic segments. J. Virol. Methods 2007, 140, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Shirafuji, H.; Ikemiyagi, K.; Nitta, Y.; Suzuki, M.; Kato, T.; Yanase, T. Occurrence of bovine ephemeral fever in Okinawa Prefecture, Japan, in 2012 and development of a reverse-transcription polymerase chain reaction assay to detect bovine ephemeral fever virus gene. J. Vet. Med. Sci. 2015, 77, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yanase, T.; Suzuki, M.; Katagiri, Y.; Ikemiyagi, K.; Takayoshi, K.; Shirafuji, H.; Ohashi, S.; Yoshida, K.; Yamakawa, M.; et al. Monitoring for bovine arboviruses in the most southwestern islands in Japan between 1994 and 2014. BMC Vet. Res. 2016, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Standfast, H.A.; Dyce, A.L.; St George, T.D.; Muller, M.J.; Doherty, R.L.; Carley, J.G.; Filippich, C. Isolation of arboviruses from insects collected at Beatrice Hill, Northern Territory of Australia, 1974–1976. Aust. J. Biol. Sci. 1984, 37, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.J.; Maan, S.; Maan, N.; Kgosana, L.; Bachanek-Bankowska, K.; Batten, C.; Darpel, K.E.; Sutton, G.; Attoui, H.; Mertens, P.P. Genetic and phylogenetic analysis of the outer-coat proteins VP2 and VP5 of epizootic haemorrhagic disease virus (EHDV): Comparison of genetic and serological data to characterise the EHDV serogroup. Virus Res. 2009, 145, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Shirafuji, H.; Yanase, T.; Kato, T.; Yamakawa, M. Genetic and phylogenetic characterization of genome segments 2 and 6 of bluetongue virus isolates in Japan from 1985 to 2008. J. Gen. Virol. 2012, 93, 1465–1473. [Google Scholar] [CrossRef]
- Sakai, Y.; Suzuta, F. Occurrence of Congenital Abnormalities in Cattle Suspected to be Caused by D’Aguilar Virus Infection and Molecular Epidemiology of D’Aguilar Virus Isolates Obtained from Sentinel Cattle; Nagasaki Prefectural Government: Nagasaki, Japan, 2019; (Unpublished work). [Google Scholar]
| Strain | Year Collected | Geographical Origin (Prefecture) | Source | Serotype |
|---|---|---|---|---|
| KC-05Y84 | 1984 | Kagoshima | Culicoides oxystoma | Chuzan |
| 31 | 1985 | Oita | bovine erythrocytes | Chuzan |
| KY-115 | 1987 | Okinawa | bovine erythrocytes | D’Aguilar |
| FO-88-2 | 1988 | Fukuoka | Culex spp. | Chuzan |
| FO-90-8 | 1990 | Fukuoka | Culex spp. | Chuzan |
| ON91-5 | 1991 | Okinawa | bovine erythrocytes | D’Aguilar |
| KSB-30/C/97 | 1997 | Kagoshima | Culicoides spp. | Marrakai a |
| MZ-16/E/97 | 1997 | Miyazaki | bovine erythrocytes | Marrakai a |
| ON-1/E/00 | 2000 | Okinawa | bovine erythrocytes | D’Aguilar |
| ON-1/E/02 | 2002 | Okinawa | bovine erythrocytes | Chuzan |
| ON-5/E/12 | 2012 | Okinawa | bovine erythrocytes | D’Aguilar |
| KSB-1/C/13 | 2013 | Kagoshima | Culicoides spp. | D’Aguilar |
| ON-3/E/17 | 2017 | Okinawa | bovine erythrocytes | D’Aguilar |
| ON-14/E/17 | 2017 | Okinawa | bovine erythrocytes | Bunyip Creek |
| ON-1/E/18 | 2018 | Okinawa | bovine erythrocytes | D’Aguilar |
| Set | Oligo Name | Sequence (5′-3′) | Target | Genome Position |
|---|---|---|---|---|
| Primers and probes for the real-time RT-PCR assay | ||||
| Pan-PALV-1 | PALV/S9/182F | GGTGGAGGCGGAGAGAATAAA | Palyam serogroup | 182–202 (Seg-9) |
| PALV/S9/246R | TTAGCCTCTGTTGTGCGATCTG | 225–246 | ||
| PALV/S9/204P a | AAAAGGGAGGAGATGAAAGT | 204–223 | ||
| Pan-PALV-2 | PALV/S7/1047F | GGCGCAAGCGTACAGATGA | Palyam serogroup | 1047–1065 (Seg-7) |
| PALV/S7/1109R | TCTAGTGTGACTGATGCATTGTGAA | 1085–1109 | ||
| PALV/S7/1067P a | CGGTGTTGCATGGCA | 1067–1081 | ||
| Chuzan- specific | CHUV/S2/316F | CTCGTAAAGGACCAAGAAATAAACCT | Chuzan | 316–341 (Seg-2) |
| CHUV/S2/443R | ACCGCACTTGTAGTCTCTAAACGAT | 419–443 | ||
| CHUV/S2/365P | TGAACATATACCAACGTCAATACAGGCGGA | 365–394 | ||
| D’Aguilar- specific | DAGV/S2/10F | TCGCAGGATGGACGAGTTTT | D’Aguilar | 10–29 (Seg-2) |
| DAGV/S2/99R | CTAACAACGATCTCATGTTGCAAA | 76–99 | ||
| DAGV/S2/49P | CTTCGATACTCAGCGATTGGCCCAAA | 49–74 | ||
| β-actin | ACT-1005-F | CAGCACAATGAAGATCAAGATCATC | β-actin (bovine) | |
| ACT-1135-R | CGGACTCATCGTACTCCTGCTT | |||
| ACT-1081-Probe | TCGCTGTCCACCTTCCAGCAGATGT | |||
| Primers for molecular cloning | ||||
| PALV/S9 | PALV/S9/7F | AAAGTTGTGGTTGATGACG | Palyam serogroup | 7–25 (Seg-9) |
| PALV/S9/352R | TCCAATCTCTGTTCCTGTTC | 333–352 | ||
| PALV/S7 | PALV/S7/1021F | GTTGCACCACAGAATAGAG | Palyam serogroup | 1021–1039 (Seg-7) |
| PALV/S7/1131R | CGTGCTAACACTAAATACC | 1113–1131 | ||
| CHUV/S2 | CHUV/S2/256F | GGGATGAAGGAAGGAGAAC | Chuzan | 256–274 (Seg-2) |
| CHUV/S2/470R | GCCTACATAAGGTTCAACGC | 451–470 | ||
| DAGV/S2 | DAGV/S2/1F | GTTAAATTTTCGCAGGATGG | D’Aguilar | 1–20 (Seg-2) |
| DAGV/S2/185R | GCTCCTCATTCCATACAACC | 166–185 | ||
| [Chuzan] ON-1/E/02 (Okinawa, Japan 2002) | [Marrakai] KSB-30/C/97 (Kagoshima, Japan 1997) | [Bunyip Creek] ON-14/E/17 (Okinawa, Japan 2017) | [D’Aguilar] ON-1/E/18 (Okinawa, Japan 2018) | |||||
|---|---|---|---|---|---|---|---|---|
| NT | AA | NT | AA | NT | AA | NT | AA | |
| [Chuzan] K-47 (Kagoshima, Japan 1985) | 98.07 | 98.70 | 55.29 | 44.53 | 53.96 | 41.00 | 52.49 | 40.35 |
| [Kasba] GG15534 (India 1957) | 90.16 | 95.50 | 55.61 | 43.94 | 53.83 | 41.20 | 52.26 | 40.55 |
| [Vellore] 6888 (India 1956) | 89.10 | 93.61 | 55.05 | 43.25 | 53.33 | 40.71 | 52.31 | 40.01 |
| [Abadina] Ib Ar 22388 (Nigeria 1967) | 80.29 | 86.52 | 55.82 | 43.34 | 53.50 | 39.52 | 52.62 | 39.00 |
| [CSIRO Village] CSIRO 11 (Australia 1974) | 59.44 | 53.00 | 56.08 | 44.87 | 53.41 | 39.13 | 53.06 | 37.19 |
| [Gweru] VRL1726/76 (Zimbabwe 1976) | 59.44 | 53.00 | 56.11 | 44.87 | 53.57 | 39.13 | 53.00 | 37.19 |
| [Marrakai] CSIRO 82 (Australia 1975) | 55.29 | 44.22 | 87.34 | 89.92 | 54.43 | 39.92 | 53.13 | 38.38 |
| [Petevo] Ar TB 2032 (Central African Rep. 1978) | 54.34 | 44.95 | 58.62 | 51.72 | 52.90 | 39.60 | 52.37 | 37.08 |
| [Bunyip Creek] CSIRO 87 (Australia 1976) | 54.46 | 42.19 | 53.20 | 39.98 | 89.97 | 94.23 | 56.76 | 47.32 |
| [Marondera] VRL1070/78 (Zimbabwe 1978) | 52.89 | 40.92 | 53.41 | 39.51 | 71.47 | 74.01 | 56.21 | 46.38 |
| [Apies River] O/4518 (South Africa 2005) | 52.60 | 41.11 | 53.37 | 39.31 | 71.24 | 73.91 | 56.69 | 46.68 |
| [D’Aguilar] B8112 (Australia 1968) | 52.73 | 40.35 | 52.95 | 38.34 | 56.58 | 47.42 | 91.60 | 94.45 |
| [Nyabira] VRL792/73 (Zimbabwe 1973) | 52.37 | 40.25 | 53.05 | 38.44 | 56.18 | 47.71 | 91.77 | 96.37 |
| [Palyam] G5287 (India 1956) | 52.57 | 39.46 | 52.65 | 37.62 | 56.22 | 46.23 | 89.64 | 91.23 |
| Serotype/Virus | Strain | Year Collected | Geographical Origin | Ct Value | |||
|---|---|---|---|---|---|---|---|
| Pan-PALV Set-1 | Pan-PALV Set-2 | CHUV- Specific Set | DAGV- Specific Set | ||||
| Chuzan (Kasba) | KC-05Y84 | 1984 | Kagoshima | 17.78 | 20.72 | 16.93 | – a |
| 31 | 1985 | Oita | 17.67 | 20.81 | 16.90 | – | |
| FO88-2 | 1988 | Fukuoka | 18.93 | 21.90 | 18.12 | – | |
| FO90-8 | 1990 | Fukuoka | 19.98 | 23.02 | 19.16 | – | |
| ON-1/E/02 | 2002 | Okinawa | 15.23 | 17.93 | 15.17 | – | |
| (Not typed) | DPP66 b | 1981 | Australia | 15.75 | 26.92 | 22.83 | – |
| D’Aguilar | KY-115 | 1987 | Okinawa | 19.97 | 18.05 | – | 20.73 |
| ON91-5 | 1991 | Okinawa | 19.42 | 18.81 | – | 21.22 | |
| ON-1/E/00 | 2000 | Okinawa | 18.94 | 21.86 | – | 21.17 | |
| KSB-29/E/01 c | 2001 | Kagoshima | 19.34 | 22.47 | – | 21.35 | |
| ON-5/E/12 | 2012 | Okinawa | 19.12 | 19.08 | – | 21.61 | |
| KSB-1/C/13 | 2013 | Kagoshima | 19.64 | 19.29 | – | 21.78 | |
| ON-3/E/17 | 2017 | Okinawa | 21.02 | 19.79 | – | 21.71 | |
| ON-1/E/18 | 2018 | Okinawa | 20.23 | 18.75 | – | 20.21 | |
| B8112 | 1968 | Australia | 19.48 | 18.28 | – | 35.88 | |
| Nyabira | 792/73 | 1973 | Zimbabwe | – | 21.74 | – | 22.24 |
| Bunyip Creek | CSIRO 58 | 1976 | Australia | 17.45 | 16.47 | – | – |
| ON-1/E/08 d | 2008 | Okinawa | 19.76 | 27.05 | – | – | |
| ON-14/E/17 | 2017 | Okinawa | 18.98 | 30.08 | – | – | |
| CSIRO Village | CSIRO 11 | 1974 | Australia | 16.90 | – | – | – |
| Marrakai | CSIRO 82 | 1975 | Australia | 19.36 | 30.31 | – | – |
| KSB-30/C/97 | 1997 | Kagoshima | 15.35 | 14.83 | – | – | |
| MZ-16/E/97 | 1997 | Miyazaki | 20.12 | 19.91 | – | – | |
| Akabane | OBE-1 | 1974 | Okayama | – | – | – | – |
| Aino | JaNAr28 | 1964 | Nagasaki | – | – | – | – |
| Peaton | KSB-1/P/06 | 2006 | Kagoshima | – | – | – | – |
| Bluetongue | TO2-1 | 1994 | Tochigi | – | – | – | – |
| Epizootic hemorrhagic disease | Ibaraki No. 2 | 1959 | Ibaraki | – | – | – | – |
| Bovine ephemeral fever | YHL | 1966 | Yamaguchi | – | – | – | – |
| Sample | Ct Value | |||
|---|---|---|---|---|
| Pan-PALV Set-1 | Pan-PALV Set-2 | CHUV- Specific Set | DAGV- Specific Set | |
| Chuzan virus strain ON-1/E/02 | ||||
| Spiked (brain homogenate) | 17.18 | 22.68 | 18.93 | – a |
| Spiked (blood cells) | 17.30 | 22.47 | 18.75 | – |
| MEM | 17.28 | 23.80 | 18.46 | – |
| D’Aguilar virus strain ON-1/E/18 | ||||
| Spiked (brain homogenate) | 20.38 | 20.35 | – | 21.40 |
| Spiked (blood cells) | 21.20 | 20.73 | – | 20.71 |
| MEM | 20.26 | 20.32 | – | 20.03 |
| Bunyip Creek virus strain ON-14/E/17 | ||||
| Spiked (brain homogenate) | 21.46 | – | – | – |
| Spiked (blood cells) | 22.68 | – | – | – |
| MEM | 21.73 | – | – | – |
| Cattle No. | Sample | Ct Value a | ||
|---|---|---|---|---|
| Pan-PALV Set-1 | Pan-PALV Set-2 | DAGV- Specific Set | ||
| 1 | Cerebellum | – b | 37.03 | – |
| Thoracic spinal cord | – | 36.78 | – | |
| Lumbar spinal cord | – | 36.07 | – | |
| Blood cells | 34.48 | 33.65 | 33.94 | |
| Cervical spinal cord | – | – | – | |
| 2 | Cerebellum | 36.25 | 36.81 | 36.55 |
| Cervical spinal cord | – | 37.60 | – | |
| Blood cells | 33.90 | 33.99 | 33.62 | |
| Others (thoracic spinal cord, lumbar spinal cord) | – | – | – | |
| 3 | Cerebellum and brain stem | 36.09 | 35.18 | 34.89 |
| Blood cells | 30.74 | 31.04 | 29.97 | |
| Others (cervical spinal cord, thoracic spinal cord, lumbar spinal cord) | – | – | – | |
| 4 | Cerebellum and brain stem | 35.81 | 35.99 | – |
| Blood cells | 36.69 | 35.94 | – | |
| Others (cervical spinal cord, thoracic spinal cord, lumbar spinal cord) | – | – | – | |
| 5 | Cerebellum and brain stem | 32.21 | 33.07 | 33.83 |
| Others (cervical spinal cord, thoracic spinal cord) | – | – | – | |
| 6 | Blood cells | 32.34 | 32.65 | 31.94 |
| Lumbar spinal cord | – | – | – | |
| 7 | Cerebellum and brain stem | – | – | 38.11 |
| Cervical spinal cord | – | 36.62 | – | |
| Blood cells | – | 37.37 | 35.96 | |
| Others (thoracic spinal cord, lumbar spinal cord) | – | – | – | |
| 8 | Cervical spinal cord | – | 37.56 | – |
| Blood cells | 36.32 | 36.49 | 36.05 | |
| Others (thoracic spinal cord, lumbar spinal cord) | – | – | – | |
| 9 | Blood cells | – | 34.66 | 32.01 |
| Others (cerebellum, brain stem, cervical spinal cord, thoracic spinal cord, lumbar spinal cord) | – | – | – | |
| 10 | Cerebrum | 32.65 | 32.58 | 31.73 |
| Others (cerebellum, brain stem, spinal cord) | – | – | – | |
| 11 | Cerebellum | 36.38 | 35.92 | 34.57 |
| Blood cells | 29.81 | 30.33 | 28.94 | |
| Others (cerebrum, brain stem, cervical spinal cord, thoracic spinal cord, lumbar spinal cord) | – | – | – | |
| 12 | Blood cells | – | – | 38.43 |
| Others (cerebrum, cerebellum, brain stem, cervical spinal cord, thoracic spinal cord, lumbar spinal cord) | – | – | – | |
| 13 | Cerebrum | – | 36.73 | 36.18 |
| Medulla oblongata | 38.23 | 37.20 | – | |
| Heart | – | – | 37.50 | |
| Lung | 29.75 | 29.99 | 28.42 | |
| Liver | 36.39 | – | 35.93 | |
| Kidney | 37.48 | 37.75 | 36.41 | |
| Spleen | 32.49 | 33.00 | 31.33 | |
| Spinal cord | – | – | – | |
| 14 | Heart | – | 38.38 | – |
| Lung | 33.99 | 34.04 | 32.46 | |
| Liver | 37.26 | 36.71 | 36.70 | |
| Kidney | – | 38.06 | 38.17 | |
| Spleen | 32.38 | 33.03 | 30.80 | |
| Others (cerebrum, medulla oblongata) | – | – | – | |
| 15 | Cerebrum | 34.93 | 36.98 | 35.66 |
| Lung | – | – | – | |
| 16 | Cerebrospinal fluid | – | 37.73 | 37.13 |
| Spleen | – | – | 38.26 | |
| Placenta | – | 38.51 | 35.73 | |
| Others (cerebrum, cerebellum, medulla oblongata, spinal cord, heart, lung, liver, spleen, kidney) | – | – | – | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirafuji, H.; Kishida, N.; Murota, K.; Suda, Y.; Yanase, T. Genetic Characterization of Palyam Serogroup Viruses Isolated in Japan from 1984 to 2018 and Development of a Real-Time RT-PCR Assay for Broad Detection of Palyam Serogroup Viruses and Specific Detection of Chuzan (Kasba) and D’Aguilar Viruses. Pathogens 2024, 13, 550. https://doi.org/10.3390/pathogens13070550
Shirafuji H, Kishida N, Murota K, Suda Y, Yanase T. Genetic Characterization of Palyam Serogroup Viruses Isolated in Japan from 1984 to 2018 and Development of a Real-Time RT-PCR Assay for Broad Detection of Palyam Serogroup Viruses and Specific Detection of Chuzan (Kasba) and D’Aguilar Viruses. Pathogens. 2024; 13(7):550. https://doi.org/10.3390/pathogens13070550
Chicago/Turabian StyleShirafuji, Hiroaki, Natsumi Kishida, Katsunori Murota, Yuto Suda, and Tohru Yanase. 2024. "Genetic Characterization of Palyam Serogroup Viruses Isolated in Japan from 1984 to 2018 and Development of a Real-Time RT-PCR Assay for Broad Detection of Palyam Serogroup Viruses and Specific Detection of Chuzan (Kasba) and D’Aguilar Viruses" Pathogens 13, no. 7: 550. https://doi.org/10.3390/pathogens13070550






