Grade C Molar-Incisor Pattern Periodontitis in Young Adults: What Have We Learned So Far?
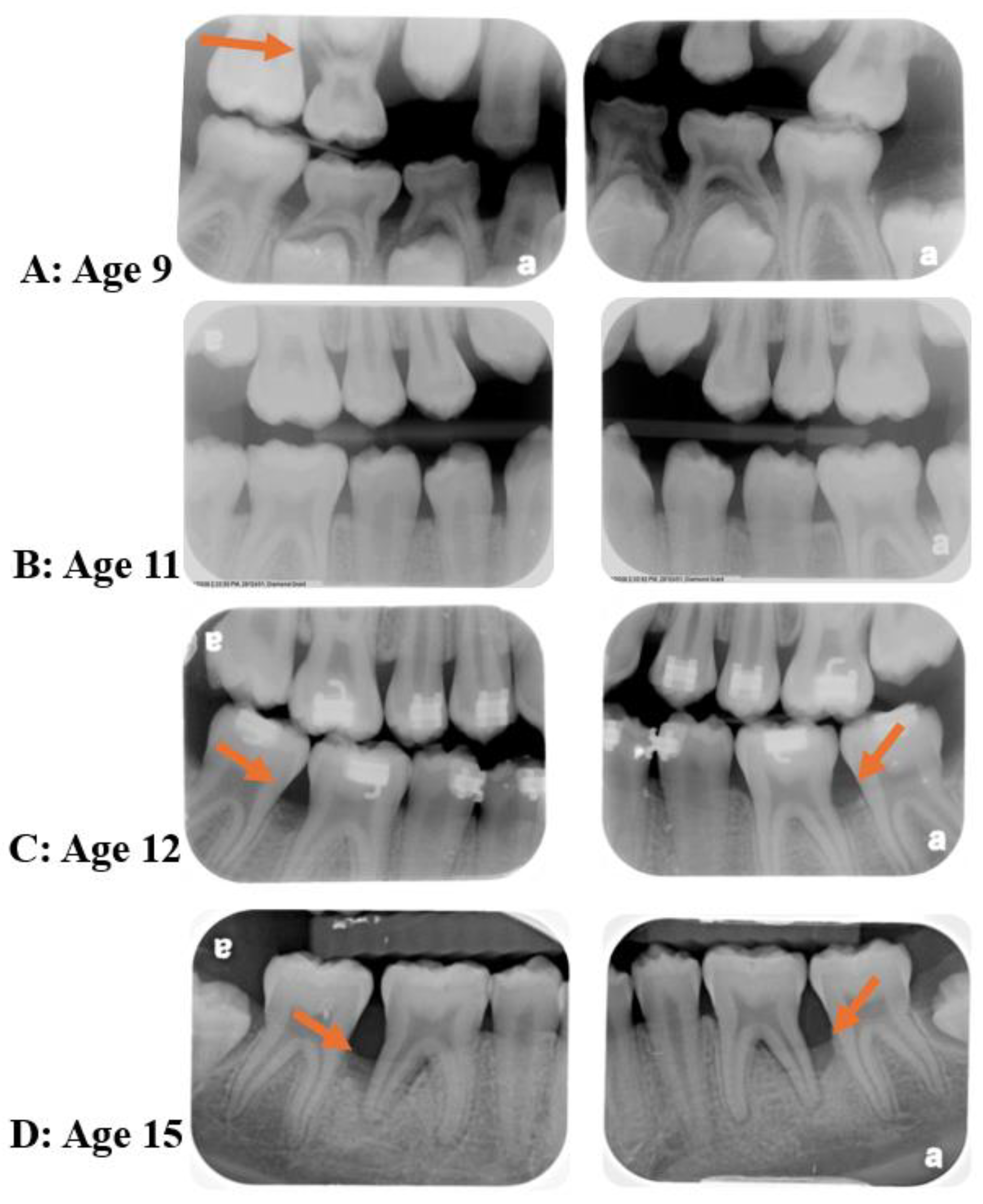
Abstract
:1. Clinical Overview
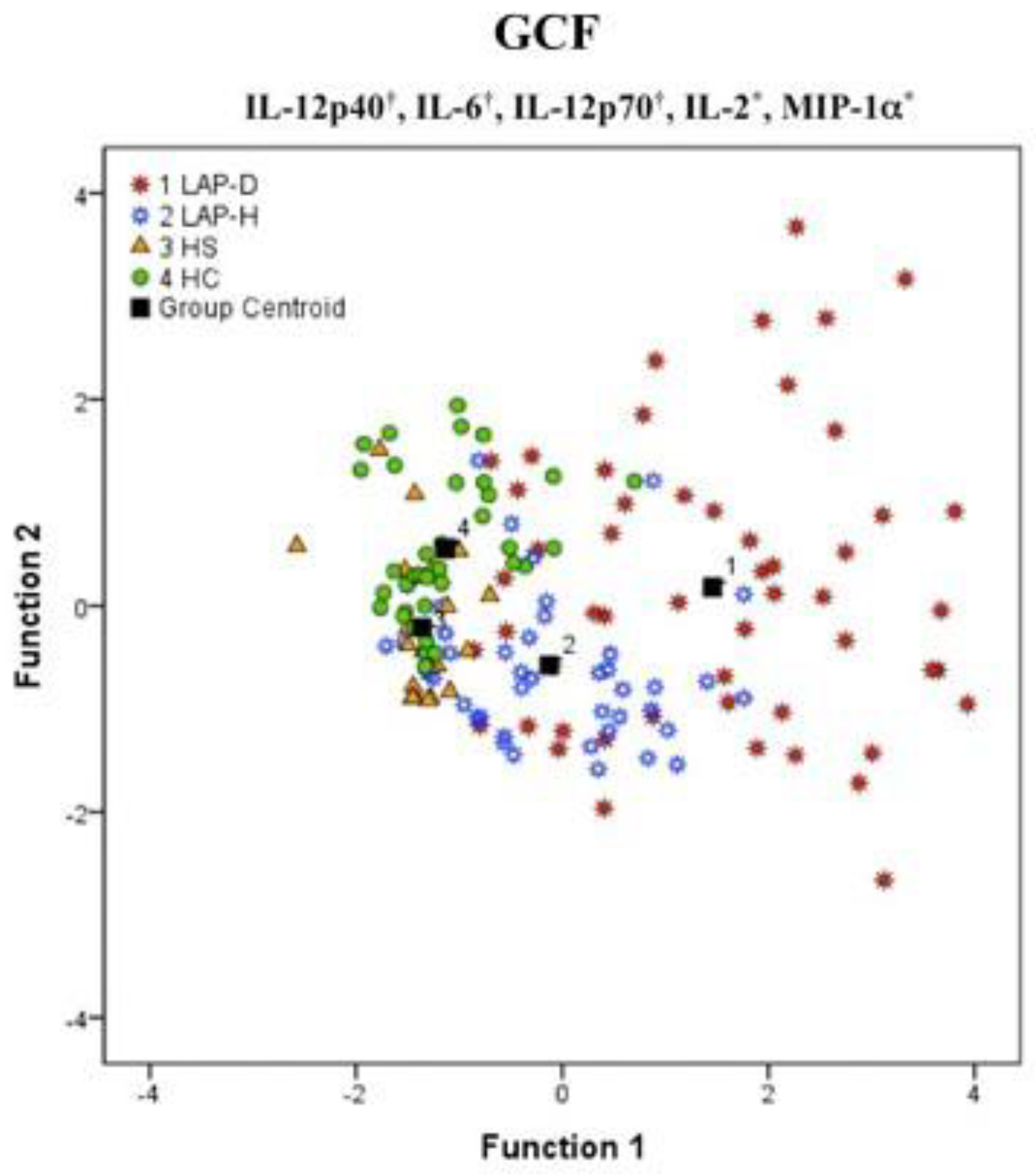
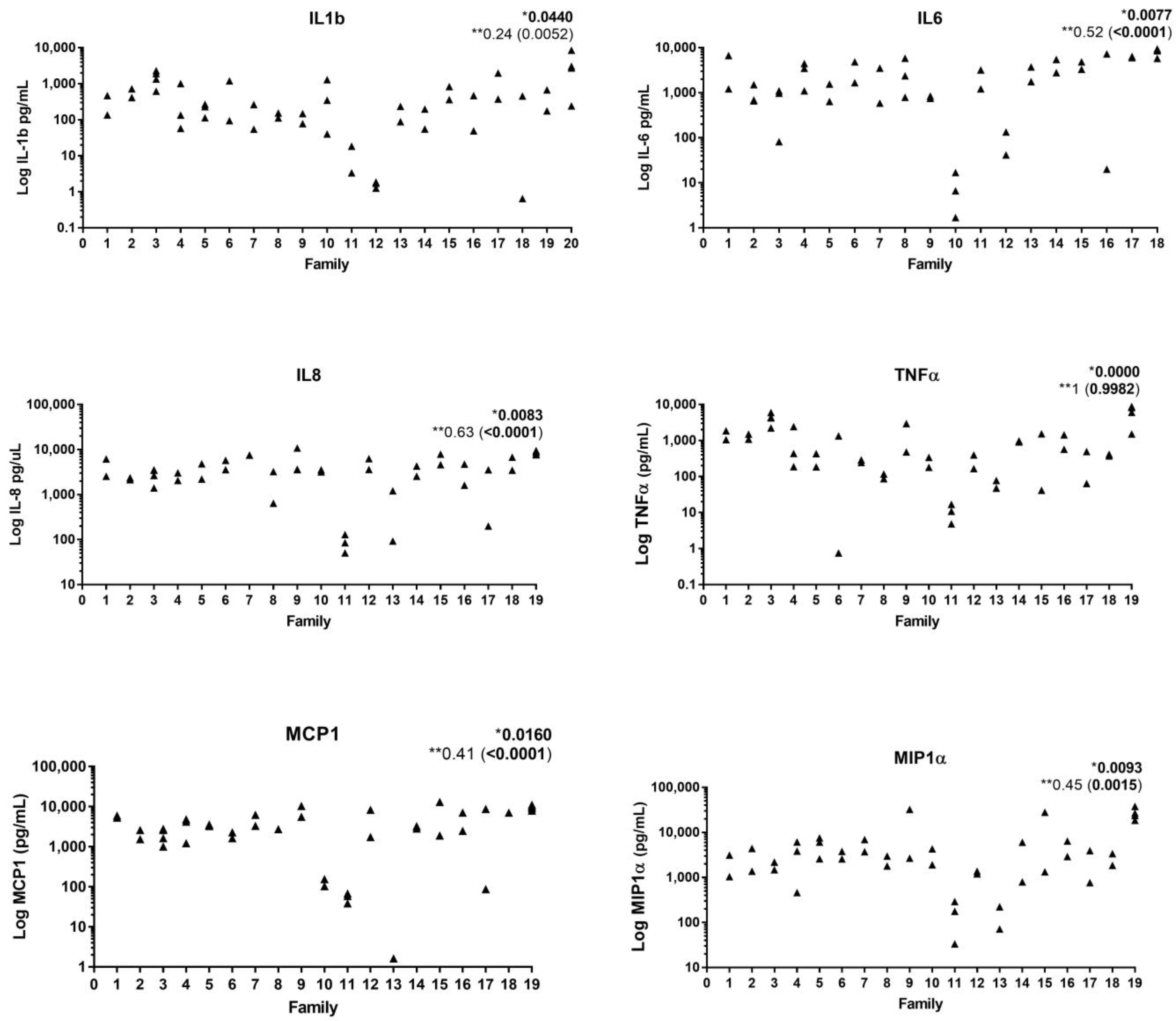
2. Immunological Aspects
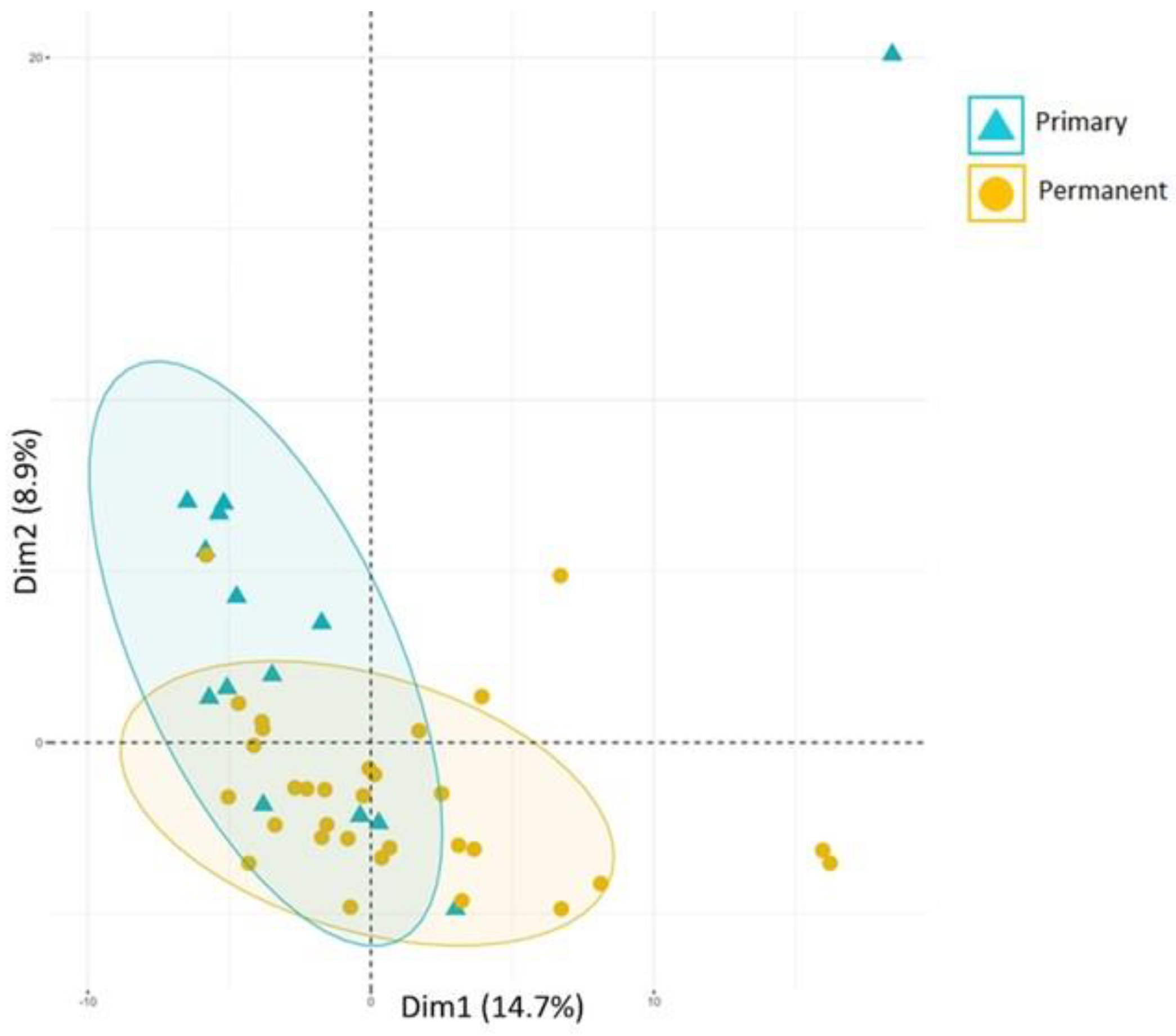
3. Microbiological Aspects
4. Diseases Initial Time-Point
5. Treatment Aspects
6. Conclusions
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scannapieco, F.A.; Dongari-Bagtzoglou, A. Dysbiosis Revisited: Understanding the Role of the Oral Microbiome in the Pathogenesis of Gingivitis and Periodontitis: A Critical Assessment. J. Periodontol. 2021, 92, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.L.; Dawson, D.R.; Morford, L.A.; Peyyala, R.; Miller, C.S.; Gonzaléz, O.A. Periodontal Disease Immunology: “double Indemnity” in Protecting the Host. Periodontology 2000 2013, 62, 163–202. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Development of a Classification System for Periodontal Diseases and Conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus Report of Workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Susin, C.; Haas, A.N.; Albandar, J.M. Epidemiology and Demographics of Aggressive Periodontitis. Periodontology 2000 2014, 65, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Albandar, J.M.; Tinoco, E.M.B. Global Epidemiology of Periodontal Diseases in Children and Young Persons. Periodontology 2000 2002, 29, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Bouziane, A.; Hamdoun, R.; Abouqal, R.; Ennibi, O. Global Prevalence of Aggressive Periodontitis: A Systematic Review and Meta-analysis. J. Clin. Periodontol. 2020, 47, 406–428. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.; Bartold, P.M.; Cullinan, M.; Jeffcoat, M.; Mombelli, A.; Murakami, S.; Page, R.; Papapanou, P.; Tonetti, M.; Van Dyke, T. Consensus Report: Aggressive Periodontitis. Ann. Periodontol. 1999, 4, 53. [Google Scholar] [CrossRef]
- Merchant, S.N.; Vovk, A.; Kalash, D.; Hovencamp, N.; Aukhil, I.; Harrison, P.; Zapert, E.; Bidwell, J.; Varnado, P.; Shaddox, L.M. Localized Aggressive Periodontitis Treatment Response in Primary and Permanent Dentitions. J. Periodontol. 2014, 85, 1722–1729. [Google Scholar] [CrossRef]
- Miller, K.; Treloar, T.; Guelmann, M.; Rody, W.J.; Shaddox, L.M. Clinical Characteristics of Localized Aggressive Periodontitis in Primary Dentition. J. Clin. Pediatr. Dent. 2018, 42, 95–102. [Google Scholar] [CrossRef]
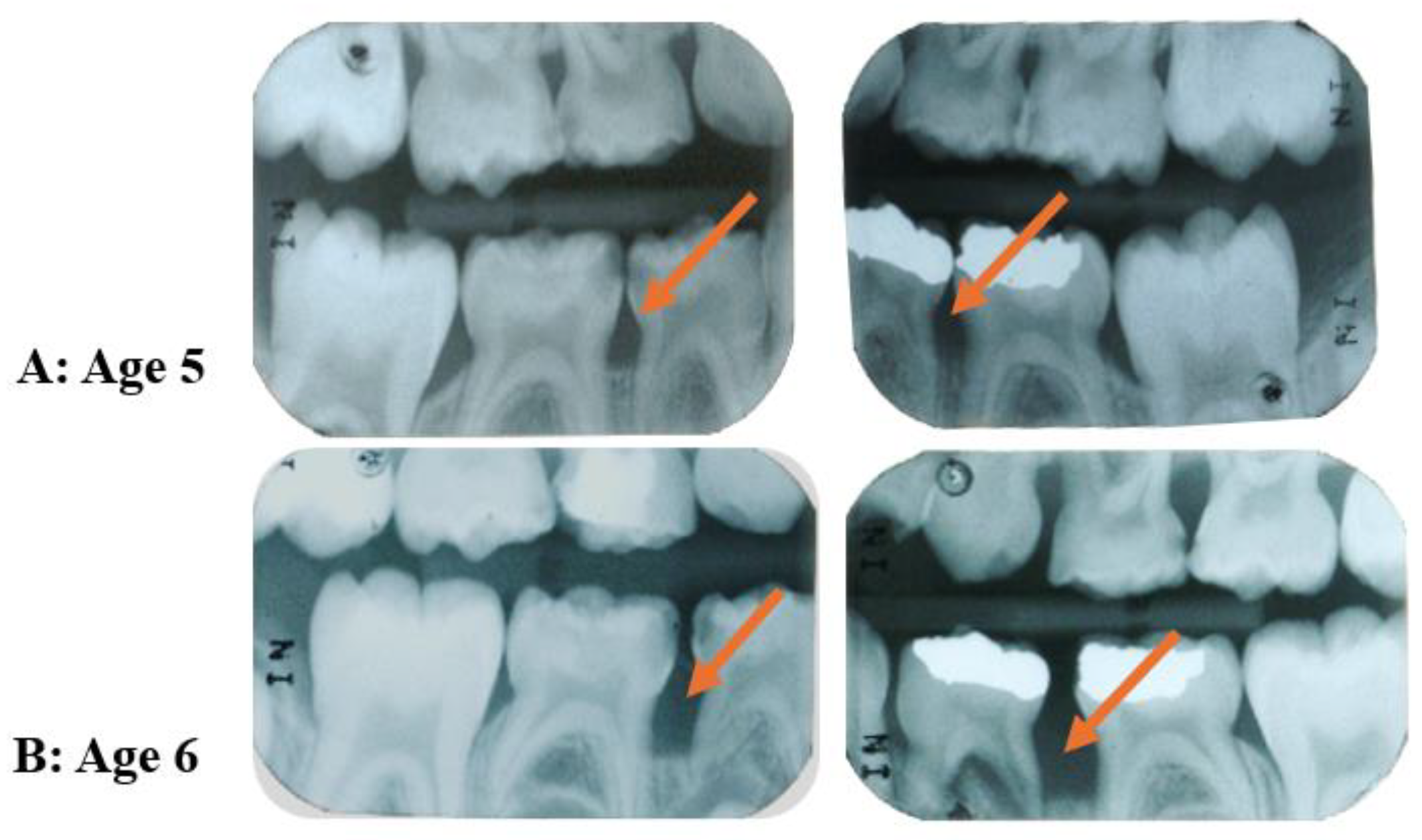
- Bimstein, E. Radiographic Description of the Distribution of Aggressive Periodontitis in Primary Teeth. J. Clin. Pediatr. Dent. 2018, 42, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.B.; Ennibi, O.K.; Ismaili, Z.; Poulsen, K.; Haubek, D. The JP2 Genotype of Aggregatibacter Actinomycetemcomitans and Marginal Periodontitis in the Mixed Dentition. J. Clin. Periodontol. 2016, 43, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Cogen, R.B.; Wright, J.T.; Tate, A.L. Destructive Periodontal Disease in Healthy Children. J. Periodontol. 1992, 63, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Mandell, R.L.; Siegal, M.D.; Umland, E. Localized Juvenile Periodontitis of the Primary Dentition. ASDC J. Dent. Child. 1986, 53, 193–196. [Google Scholar] [PubMed]
- Mros, S.T.; Berglundh, T. Aggressive Periodontitis in Children: A 14–19-Year Follow-Up. J. Clin. Periodontol. 2010, 37, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.A.F.S.; Branco-de-Almeida, L.S.; Wolf, S.; Hovencamp, N.; Treloar, T.; Harrison, P.; Aukhil, I.; Gong, Y.; Shaddox, L.M. Long-Term Clinical Response to Treatment and Maintenance of Localized Aggressive Periodontitis: A Cohort Study. J. Clin. Periodontol. 2017, 44, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Bimstein, E. Seven-Year Follow-up of 10 Children with Periodontitis. Pediatr. Dent. 2003, 25, 389–396. [Google Scholar]
- Tavakoli, T.T.; Gholami, F.; Huang, H.; Gonçalves, P.F.; Villasante-Tezanos, A.; Aukhil, I.; de Oliveira, R.C.G.; Hovencamp, N.; Wallet, S.; Ioannidou, E.; et al. Gender Differences in Immunological Response of African-American Juveniles with Grade C Molar Incisor Pattern Periodontitis. J. Periodontol. 2022, 93, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Melvin, W.L.; Sandifer, J.B.; Gray, J.L. The Prevalence and Sex Ratio of Juvenile Periodontitis in a Young Racially Mixed Population. J. Periodontol. 1991, 62, 330–334. [Google Scholar] [CrossRef]
- Nassar, M.M.; Afifi, O.; Deprez, R.D. The Prevalence of Localized Juvenile Periodontitis in Saudi Subjects. J. Periodontol. 1994, 65, 698–701. [Google Scholar] [CrossRef]
- Branco-De-Almeida, L.S.; Cruz-Almeida, Y.; Gonzalez-Marrero, Y.; Huang, H.; Aukhil, I.; Harrison, P.; Wallet, S.M.; Shaddox, L.M. Local and Plasma Biomarker Profiles in Localized Aggressive Periodontitis. JDR Clin. Trans. Res. 2017, 2, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, L.M.; Wiedey, J.; Calderon, N.L.; Magnusson, I.; Bimstein, E.; Bidwell, J.A.; Zapert, E.F.; Aukhil, I.; Wallet, S.M. Local Inflammatory Markers and Systemic Endotoxin in Aggressive Periodontitis. J. Dent. Res. 2011, 90, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.S.; César-Neto, J.B.; Albuquerque-Souza, E.; Rebeis, E.S.; Holzhausen, M.; Pannuti, C.M.; Mayer, M.P.A.; Saraiva, L. One-Year Follow-up of the Immune Profile in Serum and Selected Sites of Generalized and Localized Aggressive Periodontitis. Cytokine 2019, 116, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Alamri, M.M.; Antonoglou, G.N.; Proctor, G.; Balsa-Castro, C.; Tomás, I.; Nibali, L. Biomarkers for Diagnosis of Stage III, Grade C with Molar Incisor Pattern Periodontitis in Children and Young Adults: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2023, 27, 4929–4955. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, L.; Wiedey, J.; Bimstein, E.; Magnuson, I.; Clare-Salzler, M.; Aukhil, I.; Wallet, S.M. Hyper-Responsive Phenotype in Localized Aggressive Periodontitis. J. Dent. Res. 2010, 89, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, L.M.; Spencer, W.P.; Velsko, I.M.; Al-Kassab, H.; Huang, H.; Calderon, N.; Aukhil, I.; Wallet, S.M. Localized Aggressive Periodontitis Immune Response to Healthy and Diseased Subgingival Plaque. J. Clin. Periodontol. 2016, 43, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Tabaa, M.; Adatowovor, R.; Shabila, A.; Morford, L.; Dawson, D.; Harrison, P.; Aukhil, I.; Huang, H.; Stromberg, A.; Goncalves, J.; et al. Pattern of Grade C Molar-Incisor Pattern Periodontitis in Families. J. Periodontol. 2023, 94, 811–822. [Google Scholar] [CrossRef]
- Kantarci, A.; Oyaizu, K.; Van Dyke, T.E. Neutrophil-Mediated Tissue Injury in Periodontal Disease Pathogenesis: Findings from Localized Aggressive Periodontitis. J. Periodontol. 2003, 74, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E.; Horoszewicz, H.U.; Cianciola, L.J.; Genco, R.J. Neutrophil Chemotaxis Dysfunction in Human Periodontitis. Infect. Immun. 1980, 27, 124–132. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Schweinebraten, M.; Cianciola, L.J.; Offenbacher, S.; Genco, R.J. Neutrophil Chemotaxis in Families with Localized Juvenile Periodontitis. J. Periodontal Res. 1985, 20, 503–514. [Google Scholar] [CrossRef]
- Schenkein, H.A.; Barbour, S.E.; Tew, J.G. Cytokines and Inflammatory Factors Regulating Immunoglobulin Production in Aggressive Periodontitis. Periodontology 2000 2007, 45, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.E.; Hamilton, R.G. Immunoglobulin G Subclass Response of Localized Juvenile Periodontitis Patients to Actinobacillus Actinomycetemcomitans Y4 Lipopolysaccharide. Infect. Immun. 1992, 60, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.Y.; Sims, T.J.; Chen, H.A.; Whitney, C.W.; Moncla, B.J.; Engel, L.D.; Page, R.C. Titer and Subclass Distribution of Serum IgG Antibody Reactive with Actinobacillus Actinomycetemcomitans in Localized Juvenile Periodontitis. J. Clin. Immunol. 1993, 13, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, M.; Gunsolley, J.C.; Schenkein, H.A.; Tew, J.G. Serum Immunoglobulin G Subclass Concentrations in Periodontally Healthy and Diseased Individuals. Infect. Immun. 1994, 62, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Steffens, J.P.; Wang, X.; Starr, J.R.; Spolidorio, L.C.; Van Dyke, T.E.; Kantarci, A. Associations Between Sex Hormone Levels and Periodontitis in Men: Results From NHANES III. J. Periodontol. 2015, 86, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Boyapati, R.; Cherukuri, S.A.; Bodduru, R.; Kiranmaye, A. Influence of Female Sex Hormones in Different Stages of Women on Periodontium. J. Midlife Health 2021, 12, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, L.M.; Morford, L.A.; Nibali, L. Periodontal Health and Disease: The Contribution of Genetics. Periodontology 2000 2021, 85, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.F.; Harris, T.H.; Elmariah, T.; Aukhil, I.; Wallace, M.R.; Shaddox, L.M. Genetic Polymorphisms and Periodontal Disease in Populations of African Descent: A Review. J. Periodontal Res. 2018, 53, 164–173. [Google Scholar] [CrossRef]
- Harris, T.H.; Wallace, M.R.; Huang, H.; Li, H.; Mohiuddeen, A.; Gong, Y.; Kompotiati, T.; Harrison, P.; Aukhil, I.; Shaddox, L.M. Association of P2RX7 Functional Variants with Localized Aggressive Periodontitis. J. Periodontal Res. 2020, 55, 32–40. [Google Scholar] [CrossRef]
- Gonçalves Fernandes, J.; Morford, L.A.; Harrison, P.L.; Kompotiati, T.; Huang, H.; Aukhil, I.; Wallet, S.M.; Macchion Shaddox, L. Dysregulation of Genes and MicroRNAs in Localized Aggressive Periodontitis. J. Clin. Periodontol. 2020, 47, 1317–1325. [Google Scholar] [CrossRef]
- Vollmer, S.; Strickson, S.; Zhang, T.; Gray, N.; Lee, K.L.; Rao, V.R.; Cohen, P. The Mechanism of Activation of IRAK1 and IRAK4 by Interleukin-1 and Toll-like Receptor Agonists. Biochem. J. 2017, 474, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, L.M.; Mullersman, A.F.; Huang, H.; Wallet, S.M.; Langaee, T.; Aukhil, I. Epigenetic Regulation of Inflammation in Localized Aggressive Periodontitis. Clin. Epigenetics 2017, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Diehl, S.R.; Wang, Y.; Brooks, C.N.; Burmeister, J.A.; Califano, J.V.; Wang, S.; Schenkein, H.A. Linkage Disequilibrium of Interleukin-1 Genetic Polymorphisms with Early-Onset Periodontitis. J. Periodontol. 1999, 70, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Jordan, W.J.; Eskdale, J.; Lennon, G.P.; Pestoff, R.; Wu, L.; Fine, D.H.; Gallagher, G. A Non-Conservative, Coding Single-Nucleotide Polymorphism in the N-Terminal Region of Lactoferrin Is Associated with Aggressive Periodontitis in an African-American, but Not a Caucasian Population. Genes Immun. 2005, 6, 632–635. [Google Scholar] [CrossRef]
- Wu, Y.M.; Juo, S.H.; Ho, Y.P.; Ho, K.Y.; Yang, Y.H.; Tsai, C.C. Association between Lactoferrin Gene Polymorphisms and Aggressive Periodontitis among Taiwanese Patients. J. Periodontal Res. 2009, 44, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Noack, B.; Görgens, H.; Hempel, U.; Fanghänel, J.; Hoffmann, T.; Ziegler, A.; Schackert, H.K. Cathepsin C Gene Variants in Aggressive Periodontitis. J. Dent. Res. 2008, 87, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.R.; Albandar, J.M. Role of Genetic Factors in the Pathogenesis of Aggressive Periodontitis. Periodontology 2000 2014, 65, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Hart, T.C. Genes and Gene Polymorphisms Associated with Periodontal Disease. Crit. Rev. Oral Biol. Med. 2003, 14, 430–449. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Xu, L.; Li, Q.; Han, J.; Zhao, Y. Determinants of Host Susceptibility in Aggressive Periodontitis. Periodontology 2000 2007, 43, 133–159. [Google Scholar] [CrossRef]
- de Carvalho, F.M.; Tinoco, E.M.B.; Govil, M.; Marazita, M.L.; Vieira, A.R. Aggressive Periodontitis Is Likely Influenced by a Few Small Effect Genes. J. Clin. Periodontol. 2009, 36, 468–473. [Google Scholar] [CrossRef]
- Nibali, L. Aggressive Periodontitis: Microbes and Host Response, Who to Blame? Virulence 2015, 6, 223–228. [Google Scholar] [CrossRef]
- Nibali, L.; Henderson, B.; Tariq Sadiq, S.; Donos, N. Genetic Dysbiosis: The Role of Microbial Insults in Chronic Inflammatory Diseases. J. Oral Microbiol. 2014, 6, 22962. [Google Scholar] [CrossRef]
- Zoheir, N.; Kurushima, Y.; Lin, G.-H.; Nibali, L. Periodontal Infectogenomics: A Systematic Review Update of Associations between Host Genetic Variants and Subgingival Microbial Detection. Clin. Oral Investig. 2022, 26, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Na, H.S.; Jeong, S.Y.; Jeong, S.H.; Park, H.R.; Chung, J. Comparison of Inflammatory MicroRNA Expression in Healthy and Periodontitis Tissues. Biocell 2011, 35, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Du, A.; Zhu, X.; Yu, B. MiR-203 Accelerates Apoptosis and Inflammation Induced by LPS via Targeting NFIL3 in Cardiomyocytes. J. Cell. Biochem. 2019, 120, 6605–6613. [Google Scholar] [CrossRef]
- Luan, X.; Zhou, X.; Naqvi, A.; Francis, M.; Foyle, D.; Nares, S.; Diekwisch, T.G.H. MicroRNAs and Immunity in Periodontal Health and Disease. Int. J. Oral Sci. 2018, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, F.; Rossato, M.; Fabbri, M.; Gaudiosi, D.; Mirolo, M.; Mori, L.; Tamassia, N.; Mantovani, A.; Cassatella, M.A.; Locati, M. Induction and Regulatory Function of MiR-9 in Human Monocytes and Neutrophils Exposed to Proinflammatory Signals. Proc. Natl. Acad. Sci. USA 2009, 106, 5282–5287. [Google Scholar] [CrossRef]
- Tang, B.; Xiao, B.; Liu, Z.; Li, N.; Zhu, E.-D.; Li, B.-S.; Xie, Q.-H.; Zhuang, Y.; Zou, Q.-M.; Mao, X.-H. Identification of MyD88 as a Novel Target of MiR-155, Involved in Negative Regulation of Helicobacter Pylori-Induced Inflammation. FEBS Lett. 2010, 584, 1481–1486. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.-J.; Cimino, A.; Costinean, S.; Dumitru, C.D.; Adair, B.; Fabbri, M.; Alder, H.; Liu, C.G.; Calin, G.A.; et al. Modulation of MiR-155 and MiR-125b Levels Following Lipopolysaccharide/TNF-Alpha Stimulation and Their Possible Roles in Regulating the Response to Endotoxin Shock. J. Immunol. 2007, 179, 5082–5089. [Google Scholar] [CrossRef]
- Liu, G.; Friggeri, A.; Yang, Y.; Park, Y.-J.; Tsuruta, Y.; Abraham, E. MiR-147, a MicroRNA That Is Induced upon Toll-like Receptor Stimulation, Regulates Murine Macrophage Inflammatory Responses. Proc. Natl. Acad. Sci. USA 2009, 106, 15819–15824. [Google Scholar] [CrossRef]
- Amado, P.P.P.; Kawamoto, D.; Albuquerque-Souza, E.; Franco, D.C.; Saraiva, L.; Casarin, R.C.V.; Horliana, A.C.R.T.; Mayer, M.P.A. Oral and Fecal Microbiome in Molar-Incisor Pattern Periodontitis. Front. Cell. Infect. Microbiol. 2020, 10, 583761. [Google Scholar] [CrossRef] [PubMed]
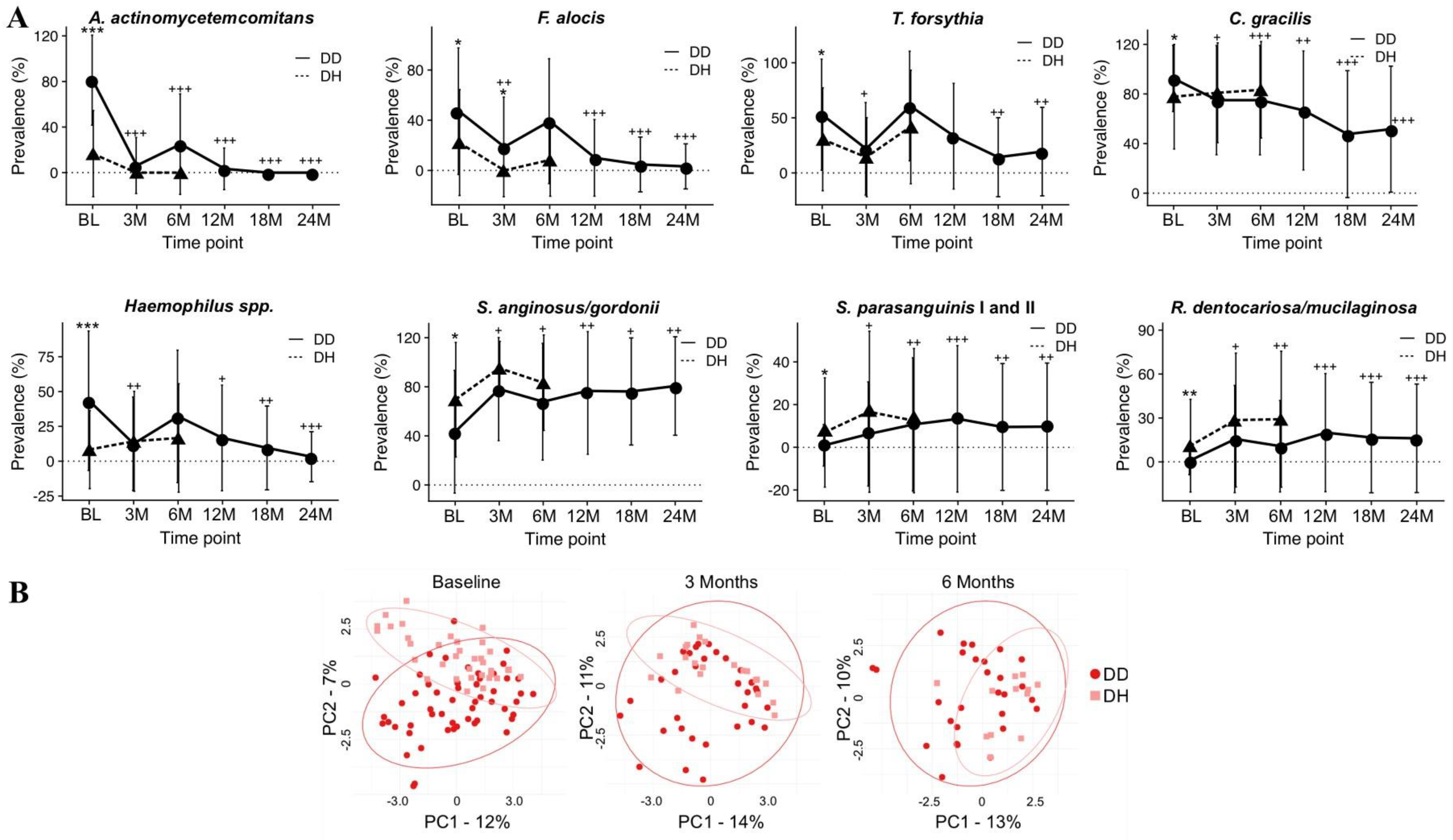
- Velsko, I.M.; Harrison, P.; Chalmers, N.; Barb, J.; Huang, H.; Aukhil, I.; Shaddox, L. Grade C Molar-Incisor Pattern Periodontitis Subgingival Microbial Profile before and after Treatment. J. Oral Microbiol. 2020, 12, 1814674. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.; Curtis, M. Microbiology of Periodontal Disease in Children and Young Adults. Periodontology 2000 2001, 26, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, L.M.; Huang, H.; Lin, T.; Hou, W.; Harrison, P.L.; Aukhil, I.; Walker, C.B.; Klepac-Ceraj, V.; Paster, B.J. Microbiological Characterization in Children with Aggressive Periodontitis. J. Dent. Res. 2012, 91, 927–933. [Google Scholar] [CrossRef]
- Könönen, E.; Müller, H. Microbiology of Aggressive Periodontitis. Periodontology 2000 2014, 65, 46–78. [Google Scholar] [CrossRef] [PubMed]
- Faveri, M.; Figueiredo, L.C.; Duarte, P.M.; Mestnik, M.J.; Mayer, M.P.A.; Feres, M. Microbiological Profile of Untreated Subjects with Localized Aggressive Periodontitis. J. Clin. Periodontol. 2009, 36, 739–749. [Google Scholar] [CrossRef]
- Cortelli, J.R.; Cortelli, S.C.; Jordan, S.; Haraszthy, V.I.; Zambon, J.J. Prevalence of Periodontal Pathogens in Brazilians with Aggressive or Chronic Periodontitis. J. Clin. Periodontol. 2005, 32, 860–866. [Google Scholar] [CrossRef]
- Slots, J.; Ting, M. Actinobacillus Actinomycetemcomitans and Porphyromonas Gingivalis in Human Periodontal Disease: Occurrence and Treatment. Periodontology 2000 1999, 20, 82–121. [Google Scholar] [CrossRef]
- Åberg, C.H.; Kelk, P.; Johansson, A. Aggregatibacter Actinomycetemcomitans: Virulence of Its Leukotoxin and Association with Aggressive Periodontitis. Virulence 2015, 6, 188–195. [Google Scholar] [CrossRef]
- Fine, D.H.; Markowitz, K.; Furgang, D.; Fairlie, K.; Ferrandiz, J.; Nasri, C.; McKiernan, M.; Gunsolley, J. Aggregatibacter Actinomycetemcomitans and Its Relationship to Initiation of Localized Aggressive Periodontitis: Longitudinal Cohort Study of Initially Healthy Adolescents. J. Clin. Microbiol. 2007, 45, 3859–3869. [Google Scholar] [CrossRef]
- Fine, D.H.; Markowitz, K.; Fairlie, K.; Tischio-Bereski, D.; Ferrendiz, J.; Furgang, D.; Paster, B.J.; Dewhirst, F.E. A Consortium of Aggregatibacter Actinomycetemcomitans, Streptococcus Parasanguinis, and Filifactor Alocis Is Present in Sites Prior to Bone Loss in a Longitudinal Study of Localized Aggressive Periodontitis. J. Clin. Microbiol. 2013, 51, 2850–2861. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Patil, A.G.; Velusamy, S.K. Aggregatibacter Actinomycetemcomitans (Aa) Under the Radar: Myths and Misunderstandings of Aa and Its Role in Aggressive Periodontitis. Front. Immunol. 2019, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Herbert, B.A.; Novince, C.M.; Kirkwood, K.L. Aggregatibacter Actinomycetemcomitans, a Potent Immunoregulator of the Periodontal Host Defense System and Alveolar Bone Homeostasis. Mol. Oral Microbiol. 2016, 31, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Raja, M. Aggregatibacter Actinomycetemcomitans—A Tooth Killer? J. Clin. Diagn. Res. 2014, 8, ZE13–6. [Google Scholar] [CrossRef] [PubMed]
- Haubek, D.; Poulsen, K.; Kilian, M. Microevolution and Patterns of Dissemination of the JP2 Clone of Aggregatibacter (Actinobacillus) Actinomycetemcomitans. Infect. Immun. 2007, 75, 3080–3088. [Google Scholar] [CrossRef] [PubMed]
- Haubek, D.; Ennibi, O.-K.; Poulsen, K.; Vaeth, M.; Poulsen, S.; Kilian, M. Risk of Aggressive Periodontitis in Adolescent Carriers of the JP2 Clone of Aggregatibacter (Actinobacillus) Actinomycetemcomitans in Morocco: A Prospective Longitudinal Cohort Study. Lancet 2008, 371, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Höglund Åberg, C.; Kwamin, F.; Claesson, R.; Dahlén, G.; Johansson, A.; Haubek, D. Progression of Attachment Loss Is Strongly Associated with Presence of the JP2 Genotype of Aggregatibacter Actinomycetemcomitans: A Prospective Cohort Study of a Young Adolescent Population. J. Clin. Periodontol. 2014, 41, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Burgess, D.K.; Huang, H.; Harrison, P.; Kompotiati, T.; Aukhil, I.; Shaddox, L.M. Non-Surgical Therapy Reduces Presence of JP2 Clone in Localized Aggressive Periodontitis. J. Periodontol. 2017, 88, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Höglund Åberg, C.; Haubek, D.; Kwamin, F.; Johansson, A.; Claesson, R. Leukotoxic Activity of Aggregatibacter Actinomycetemcomitans and Periodontal Attachment Loss. PLoS ONE 2014, 9, e104095. [Google Scholar] [CrossRef]
- Johansson, A.; Claesson, R.; Hänström, L.; Sandström, G.; Kalfas, S. Polymorphonuclear Leukocyte Degranulation Induced by Leukotoxin from Actinobacillus Actinomycetemcomitans. J. Periodontal Res. 2000, 35, 85–92. [Google Scholar] [CrossRef]
- Saglie, F.R.; Marfany, A.; Camargo, P. Intragingival Occurrence of Actinobacillus Actinomycetemcomitans and Bacteroides Gingivalis in Active Destructive Periodontal Lesions. J. Periodontol. 1988, 59, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Lamell, C.W.; Griffen, A.L.; McClellan, D.L.; Leys, E.J. Acquisition and Colonization Stability of Actinobacillus Actinomycetemcomitans and Porphyromonas Gingivalis in Children. J. Clin. Microbiol. 2000, 38, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Oscarsson, J.; Claesson, R.; Lindholm, M.; Åberg, C.H.; Johansson, A. Tools of Aggregatibacter Actinomycetemcomitans to Evade the Host Response. J. Clin. Med. 2019, 8, 1079. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, R.; Komatsuzawa, H.; Kawai, T.; Yamada, S.; Goncalves, R.B.; Izumi, S.; Fujiwara, T.; Nakano, Y.; Suzuki, N.; Uchida, Y.; et al. Outer Membrane Protein 100, a Versatile Virulence Factor of Actinobacillus Actinomycetemcomitans. Mol. Microbiol. 2003, 50, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, M.; Komatsuzawa, H.; Papantonakis, A.; Seki, M.; Makihira, S.; Ouhara, K.; Kusumoto, Y.; Murakami, S.; Taubman, M.A.; Kawai, T. Aggregatibacter Actinomycetemcomitans Omp29 Is Associated with Bacterial Entry to Gingival Epithelial Cells by F-Actin Rearrangement. PLoS ONE 2011, 6, e18287. [Google Scholar] [CrossRef] [PubMed]
- DiRienzo, J.M. Breaking the Gingival Epithelial Barrier: Role of the Aggregatibacter Actinomycetemcomitans Cytolethal Distending Toxin in Oral Infectious Disease. Cells 2014, 3, 476–499. [Google Scholar] [CrossRef] [PubMed]
- Alaluusua, S.; Saarela, M.; Jousimies-Somer, H.; Asikainen, S. Ribotyping Shows Intrafamilial Similarity in Actinobacillus Actinomycetemcomitans Isolates. Oral Microbiol. Immunol. 1993, 8, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.F.; Altabtbaei, K.; Kumar, P.S.; Casati, M.Z.; Ruiz, K.G.S.; Sallum, E.A.; Nociti-Junior, F.H.; Casarin, R.C.V. Parents with Periodontitis Impact the Subgingival Colonization of Their Offspring. Sci. Rep. 2021, 11, 1357. [Google Scholar] [CrossRef]
- Koo, S.S.; Fernandes, J.G.; Li, L.; Huang, H.; Aukhil, I.; Harrison, P.; Diaz, P.I.; Shaddox, L.M. Evaluation of Microbiome in Primary and Permanent Dentition in Grade C Periodontitis in Young Individuals. J. Periodontol. 2024; Epub ahead of print. [Google Scholar] [CrossRef]
- Dibart, S.; Chapple, I.L.; Skobe, Z.; Shusterman, S.; Nedleman, H.L. Microbiological Findings in Prepubertal Periodontitis. A Case Report. J. Periodontol. 1998, 69, 1172–1175. [Google Scholar] [CrossRef]
- Yoshida-Minami, I.; Kishimoto, K.; Suzuki, A.; Fujiwara, T.; Shintani, S.; Morisaki, I.; Sobue, S.; Miyamoto, M.; Nagai, A.; Kurihara, H. Clinical, Microbiological and Host Defense Parameters Associated with a Case of Localized Prepubertal Periodontitis. J. Clin. Periodontol. 1995, 22, 56–62. [Google Scholar] [CrossRef]
- Zambon, J.J.; Christersson, L.A.; Slots, J. Actinobacillus Actinomycetemcomitans in Human Periodontal Disease. Prevalence in Patient Groups and Distribution of Biotypes and Serotypes within Families. J. Periodontol. 1983, 54, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Christersson, L.A. Actinobacillus Actinomycetemcomitans and Localized Juvenile Periodontitis. Clinical, Microbiologic and Histologic Studies. Swed. Dent. J. Suppl. 1993, 90, 1–46. [Google Scholar] [PubMed]
- Haubek, D.; Poulsen, K.; Westergaard, J.; Dahlèn, G.; Kilian, M. Highly Toxic Clone of Actinobacillus Actinomycetemcomitans in Geographically Widespread Cases of Juvenile Periodontitis in Adolescents of African Origin. J. Clin. Microbiol. 1996, 34, 1576–1578. [Google Scholar] [CrossRef] [PubMed]
- van Steenbergen, T.J.; van der Velden, U.; Abbas, F.; de Graaff, J. Microbiological and Clinical Monitoring of Non-Localized Juvenile Periodontitis in Young Adults: A Report of 11 Cases. J. Periodontol. 1993, 64, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Sixou, M.; Duffaut-Lagarrigue, D.; Lodter, J.P. [The Distribution and Prevalence of Haemophilus Actinomycetemcomitans in the Oral Cavity]. J. Biol. Buccale 1991, 19, 221–228. [Google Scholar] [PubMed]
- Saarela, M.; Mättö, J.; Asikainen, S.; Jousimies-Somer, H.; Torkko, H.; Pyhälä, L.; Stucki, A.M.; Hannula, J.; Hölttä, P.; Alaluusua, S. Clonal Diversity OfActinobacillus Actinomycetemcomitans, Porphyromonas GingivalisandPrevotella Intermedia/Nigrescensin Two Families: CLINICAL MICROBIOLOGY. Anaerobe 1996, 2, 19–27. [Google Scholar] [CrossRef]
- Petit, M.D.; van Steenbergen, T.J.; Scholte, L.M.; van der Velden, U.; de Graaff, J. Epidemiology and Transmission of Porphyromonas Gingivalis and Actinobacillus Actinomycetemcomitans among Children and Their Family Members. A Report of 4 Surveys. J. Clin. Periodontol. 1993, 20, 641–650. [Google Scholar] [CrossRef] [PubMed]
- DiRienzo, J.M.; Slots, J.; Sixou, M.; Sol, M.A.; Harmon, R.; McKay, T.L. Specific Genetic Variants of Actinobacillus Actinomycetemcomitans Correlate with Disease and Health in a Regional Population of Families with Localized Juvenile Periodontitis. Infect. Immun. 1994, 62, 3058–3065. [Google Scholar] [CrossRef] [PubMed]
- Hodge, P.; Michalowicz, B. Genetic Predisposition to Periodontitis in Children and Young Adults. Periodontology 2000 2001, 26, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.E.; Burmeister, J.A.; Brooks, C.N.; Ranney, R.R.; Hinkelmann, K.H.; Schieken, R.M.; Moore, L. V Investigation of the Influences of Puberty, Genetics, and Environment on the Composition of Subgingival Periodontal Floras. Infect. Immun. 1993, 61, 2891–2898. [Google Scholar] [CrossRef]
- Wennström, J.L.; Dahlén, G.; Svensson, J.; Nyman, S. Actinobacillus Actinomycetemcomitans, Bacteroides Gingivalis and Bacteroides Intermedius: Predictors of Attachment Loss? Oral Microbiol. Immunol. 1987, 2, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Christersson, L.A.; Albini, B.; Zambon, J.J.; Wikesjö, U.M.E.; Genco, R.J. Tissue Localization of Actinobacillus Actinomycetemcomitans in Human Periodontitis. J. Periodontol. 1987, 58, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Clerehugh, V.; Tugnait, A. Diagnosis and Management of Periodontal Diseases in Children and Adolescents. Periodontology 2000 2001, 26, 146–168. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatric Dentistry. Periodontal Diseases of Children and Adolescents. J. Periodontol. 2003, 74, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Rebeis, E.S.; Albuquerque-Souza, E.; Paulino da Silva, M.; Giudicissi, M.; Mayer, M.P.A.; Saraiva, L. Effect of Periodontal Treatment on Aggregatibacter Actinomycetemcomitans Colonization and Serum IgG Levels against A. Actinomycetemcomitans Serotypes and Omp29 of Aggressive Periodontitis Patients. Oral Dis. 2019, 25, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, C.C.; Feres, M.; Gonçalves, C.; Figueiredo, L.C.; Faveri, M.; Tu, Y.-K.; Chambrone, L. Systemic Antibiotics in the Treatment of Aggressive Periodontitis. A Systematic Review and a Bayesian Network Meta-Analysis. J. Clin. Periodontol. 2015, 42, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Keestra, J.A.J.; Grosjean, I.; Coucke, W.; Quirynen, M.; Teughels, W. Non-Surgical Periodontal Therapy with Systemic Antibiotics in Patients with Untreated Chronic Periodontitis: A Systematic Review and Meta-Analysis. J. Periodontal Res. 2015, 50, 294–314. [Google Scholar] [CrossRef] [PubMed]
- Branco-de-Almeida, L.S.; Cruz-Almeida, Y.; Gonzalez-Marrero, Y.; Kudsi, R.; de Oliveira, I.C.V.; Dolia, B.; Huang, H.; Aukhil, I.; Harrison, P.; Shaddox, L.M. Treatment of Localized Aggressive Periodontitis Alters Local Host Immunoinflammatory Profiles: A Long-Term Evaluation. J. Clin. Periodontol. 2021, 48, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Allin, N.; Cruz-Almeida, Y.; Velsko, I.; Vovk, A.; Hovemcamp, N.; Harrison, P.; Huang, H.; Aukhil, I.; Wallet, S.M.; Shaddox, L.M. Inflammatory Response Influences Treatment of Localized Aggressive Periodontitis. J. Dent. Res. 2016, 95, 635–641. [Google Scholar] [CrossRef]
- Branco-de-Almeida, L.S.; Velsko, I.M.; de Oliveira, I.C.V.; de Oliveira, R.C.G.; Shaddox, L.M. Impact of Treatment on Host Responses in Young Individuals with Periodontitis. J. Dent. Res. 2023, 102, 473–488. [Google Scholar] [CrossRef]
- Shaddox, L.M.; Gonçalves, P.F.; Vovk, A.; Allin, N.; Huang, H.; Hou, W.; Aukhil, I.; Wallet, S.M. LPS-Induced Inflammatory Response after Therapy of Aggressive Periodontitis. J. Dent. Res. 2013, 92, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Cifcibasi, E.; Koyuncuoglu, C.; Ciblak, M.; Badur, S.; Kasali, K.; Firatli, E.; Cintan, S. Evaluation of Local and Systemic Levels of Interleukin-17, Interleukin-23, and Myeloperoxidase in Response to Periodontal Therapy in Patients with Generalized Aggressive Periodontitis. Inflammation 2015, 38, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, E.M.B.; Beldi, M.I.; Campedelli, F.; Lana, M.; Loureiro, C.A.; Bellini, H.T.; Rams, T.E.; Tinoco, N.M.B.; Gjermo, P.; Preus, H.R. Clinical and Microbiological Effects of Adjunctive Antibiotics in Treatment of Localized Juvenile Periodontitis. A Controlled Clinical Trial. J. Periodontol. 1998, 69, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, L.; Hu, J.; Vovk, A.; Huang, H.; Wallet, S.; Harrison, P.; Aukhil, I.L. Hyper-Inflammatory Response between Primary and Permanent Dentition with Aggressive Periodontitis. Int. Assoc. Dent. Res.-J. Den. Res. 2012, 91, 941. [Google Scholar]
- Suzuki, J.; Okada, M.; Wang, Y.; Nii, N.; Miura, K.; Kozai, K. Localized Aggressive Periodontitis in Primary Dentition: A Case Report. J. Periodontol. 2003, 74, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Castro dos Santos, N.C.; Furukawa, M.V.; Oliveira-Cardoso, I.; Cortelli, J.R.; Feres, M.; Van Dyke, T.; Rovai, E.S. Does the Use of Omega-3 Fatty Acids as an Adjunct to Non-surgical Periodontal Therapy Provide Additional Benefits in the Treatment of Periodontitis? A Systematic Review and Meta-analysis. J. Periodontal Res. 2022, 57, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Castro dos Santos, N.C.; Andere, N.M.R.B.; Araujo, C.F.; de Marco, A.C.; Kantarci, A.; Van Dyke, T.E.; Santamaria, M.P. Omega-3 PUFA and Aspirin as Adjuncts to Periodontal Debridement in Patients with Periodontitis and Type 2 Diabetes Mellitus: Randomized Clinical Trial. J. Periodontol. 2020, 91, 1318–1327. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Aboelsaad, N.; Eliwa, M.; Darweesh, M.; Alshahat, M.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Adjunctive Treatment of Chronic Periodontitis With Daily Dietary Supplementation With Omega-3 Fatty Acids and Low-Dose Aspirin. J. Periodontol. 2010, 81, 1635–1643. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguel, M.M.V.; Shaddox, L.M. Grade C Molar-Incisor Pattern Periodontitis in Young Adults: What Have We Learned So Far? Pathogens 2024, 13, 580. https://doi.org/10.3390/pathogens13070580
Miguel MMV, Shaddox LM. Grade C Molar-Incisor Pattern Periodontitis in Young Adults: What Have We Learned So Far? Pathogens. 2024; 13(7):580. https://doi.org/10.3390/pathogens13070580
Chicago/Turabian StyleMiguel, Manuela Maria Viana, and Luciana Macchion Shaddox. 2024. "Grade C Molar-Incisor Pattern Periodontitis in Young Adults: What Have We Learned So Far?" Pathogens 13, no. 7: 580. https://doi.org/10.3390/pathogens13070580



