The Impact of Altitude on Tick-Borne Pathogens at Two Mountain Ranges in Central Slovakia
Abstract
1. Introduction
2. Materials and Methods
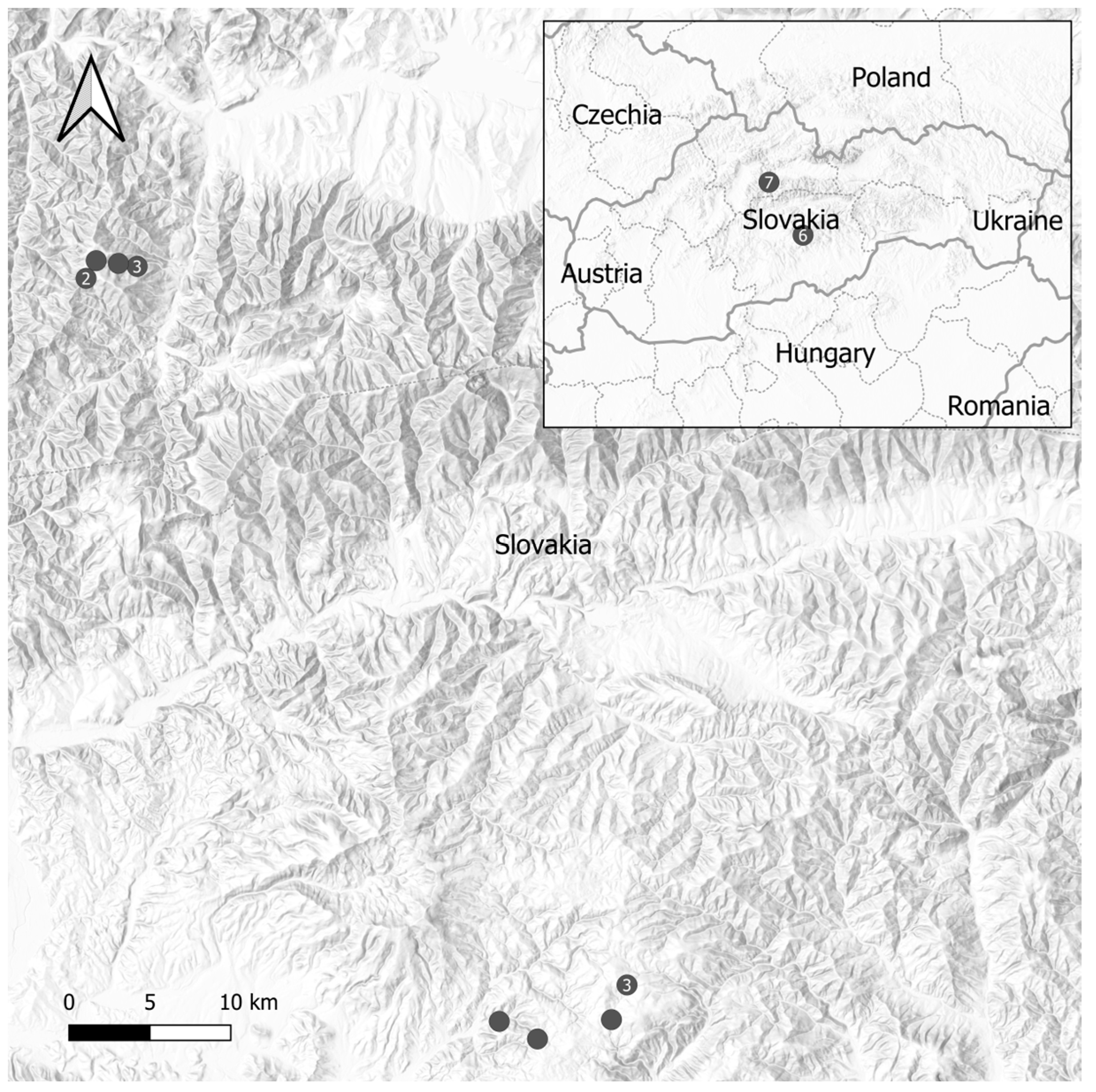
2.1. Characterization of Sampling Sites
2.2. Soil Sampling and Tick Collection
2.3. Laboratory Examination of the Soil
2.4. RNA Extraction and cDNA Production
2.5. Detection of Tick-Borne Pathogens in I. ricinus Ticks
2.6. Statistical Analysis
2.7. Regression Analysis
3. Results
3.1. Tick-Borne Pathogen Infection in Questing I. ricinus Ticks
3.2. Impact of Altitude and Soil pH on the Occurrence of Tick-Borne Pathogens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gern, L.; Humair, P.-F. Ecology of Borrelia burgdorferi Sensu Lato in Europe. Lyme Borreliosis Biol. Epidemiol. Control 2002, 6, 149–174. [Google Scholar] [CrossRef]
- Kahl, O.; Gray, J.S. The Biology of Ixodes ricinus with Emphasis on Its Ecology. Ticks Tick-Borne Dis. 2023, 14, 102114. [Google Scholar] [CrossRef] [PubMed]
- LoGiudice, K.; Duerr, S.T.K.; Newhouse, M.J.; Schmidt, K.A.; Killilea, M.E.; Ostfeld, R.S. Impact of Host Community Composition on Lyme Disease Risk. Ecology 2008, 89, 2841–2849. [Google Scholar] [CrossRef] [PubMed]
- Takumi, K.; Sprong, H.; Hofmeester, T.R. Impact of Vertebrate Communities on Ixodes ricinus-Borne Disease Risk in Forest Areas. Parasit. Vectors 2019, 12, 434. [Google Scholar] [CrossRef] [PubMed]
- Londo, A.J.; Kushla, J.D.; Carter, R.C. Soil pH and Tree Species Suitability in the South. 2006, Publication ID: SREF-FM-002. Available online: https://americaslongleaf.org/media/iqmc4tyw/soil-ph-tree-suitability-in-the-south-_sref_.pdf (accessed on 15 February 2024).
- Aponte, C.; García, L.V.; Marañón, T. Tree Species Effects on Nutrient Cycling and Soil Biota: A Feedback Mechanism Favouring Species Coexistence. For. Ecol. Manag. 2013, 309, 36–46. [Google Scholar] [CrossRef]
- Čerevková, A.; Renčo, M.; Miklisová, D.; Gömöryová, E. Soil Nematode Communities in Managed and Natural Temperate Forest. Diversity 2021, 13, 327. [Google Scholar] [CrossRef]
- Macko, J.; Machava, J.; Bublinec, E.; Hrkľová, G. Soil Reaction and Tick Abundance Ixodes Ricinus. Foelia Oecol. 2016, 43, 176–182. [Google Scholar]
- Burtis, J.C.; Yavitt, J.B.; Fahey, T.J.; Ostfeld, R.S. Ticks as Soil-Dwelling Arthropods: An Intersection between Disease and Soil Ecology. J. Med. Entomol. 2019, 56, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, N.; Aran, D.; Maul, A.; Camara, B.I.; Barthel, C.; Zaffino, M.; Lett, M.-C.; Schnitzler, A.; Bauda, P. Multiple Factors Affecting Ixodes ricinus Ticks and Associated Pathogens in European Temperate Ecosystems (Northeastern France). Sci. Rep. 2024, 14, 9391. [Google Scholar] [CrossRef]
- Pollet, T.; Sprong, H.; Lejal, E.; Krawczyk, A.I.; Moutailler, S.; Cosson, J.-F.; Vayssier-Taussat, M.; Estrada-Peña, A. The Scale Affects Our View on the Identification and Distribution of Microbial Communities in Ticks. Parasit. Vectors 2020, 13, 36. [Google Scholar] [CrossRef]
- Jouda, F.; Perret, J.-L.; Gern, L. Ixodes Ricinus Density, and Distribution and Prevalence of Borrelia burgdorferi Sensu Lato Infection Along an Altitudinal Gradient. J. Med. Entomol. 2004, 41, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Stünzner, D.; Hubálek, Z.; Halouzka, J.; Wendelin, I.; Sixl, W.; Marth, E. Prevalence of Borrelia burgdorferi Sensu Lato in the Tick Ixodes ricinus in the Styrian Mountains of Austria. Wien. Klin. Wochenschr. 2006, 118, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Burri, C.; Moran Cadenas, F.; Douet, V.; Moret, J.; Gern, L. Ixodes ricinus Density and Infection Prevalence of Borrelia burgdorferi Sensu Lato along a North-Facing Altitudinal Gradient in the Rhône Valley (Switzerland). Vector Borne Zoonotic Dis. Larchmt. N 2007, 7, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Prusinski, M.A.; White, J.L.; Falco, R.C.; Kokas, J.; Vinci, V.; Gall, W.K.; Tober, K.J.; Haight, J.; Oliver, J.; et al. Predicting Spatio-Temporal Population Patterns of Borrelia burgdorferi, the Lyme Disease Pathogen. J. Appl. Ecol. 2022, 59, 2779–2789. [Google Scholar] [CrossRef] [PubMed]
- Máliš, F.; Ujházy, K.; Hederová, L.; Ujházyová, M.; Csölleová, L.; Coomes, D.A.; Zellweger, F. Microclimate Variation and Recovery Time in Managed and Old-Growth Temperate Forests. Agric. For. Meteorol. 2023, 342, 109722. [Google Scholar] [CrossRef]
- Synek, M.; Janda, P.; Mikoláš, M.; Nagel, T.A.; Schurman, J.S.; Pettit, J.L.; Trotsiuk, V.; Morrissey, R.C.; Bače, R.; Čada, V.; et al. Contrasting Patterns of Natural Mortality in Primary Picea Forests of the Carpathian Mountains. For. Ecol. Manag. 2020, 457, 117734. [Google Scholar] [CrossRef]
- Rigg, R.; Adamec, S. Status, Ecology and Management of the Brown Bear (Ursus Arctos) in Slovakia; Slovak Wildlife Society: Liptovský Hrádok, Slovakia, 2007; 128p. [Google Scholar]
- Derdáková, M.; Beati, L.; Pet’ko, B.; Stanko, M.; Fish, D. Genetic Variability within Borrelia burgdorferi Sensu Lato Genospecies Established by PCR-Single-Strand Conformation Polymorphism Analysis of the rrfA-rrlB Intergenic Spacer in Ixodes ricinus Ticks from the Czech Republic. Appl. Environ. Microbiol. 2003, 69, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Casati, S.; Sager, H.; Gern, L.; Piffaretti, J.-C. Presence of Potentially Pathogenic Babesia sp. for Human in Ixodes ricinus in Switzerland. Ann. Agric. Environ. Med. 2006, 13, 65–70. [Google Scholar] [PubMed]
- Massung, R.F.; Slater, K.G. Comparison of PCR Assays for Detection of the Agent of Human Granulocytic Ehrlichiosis, Anaplasma phagocytophilum. J. Clin. Microbiol. 2003, 41, 717–722. [Google Scholar] [CrossRef]
- Roux, V.; Rydkina, E.; Eremeeva, M.; Raoult, D. Citrate Synthase Gene Comparison, a New Tool for Phylogenetic Analysis, and Its Application for the Rickettsiae. Int. J. Syst. Bacteriol. 1997, 47, 252–261. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Jang, W.-J.; Kim, J.-H.; Ryu, J.-S.; Lee, S.-H.; Park, K.-H.; Paik, H.-S.; Koh, Y.-S.; Choi, M.-S.; Kim, I.-S. Spotted Fever Group and Typhus Group Rickettsioses in Humans, South Korea. Emerg. Infect. Dis. 2005, 11, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, M.; Cassinotti, P. Development of a Quantitative Real-Time RT-PCR Assay with Internal Control for the Laboratory Detection of Tick Borne Encephalitis Virus (TBEV) RNA. J. Clin. Virol. 2003, 27, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, E.S.G. Epitools Epidemiological Calculators. Ausvet. 2018. Available online: http://epitools.ausvet.com.au (accessed on 15 April 2023).
- Williams, C.; Moffitt, C. A Critique of Methods of Sampling and Reporting Pathogens in Populations of Fish. J. Aquat. Anim. Health 2001, 13, 300–309. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Lüdecke, D. sjPlot: Data Visualization for Statistics in Social Science (R Package Version 2.8.14). 2023. Available online: https://cran.r-project.org/web/packages/sjPlot/index.html (accessed on 1 August 2023).
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference (R Package Version 1.47.5). 2023. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 1 August 2023).
- Jackman, S.; Tahk, A.; Zeileis, A.; Maimone, C.; Fearon, J. Pscl: Classes and Methods for R Developed in the Political Science Computational Laboratory; United States Studies Centre, University of Sydney: Sydney, Australia, 2023; (R Package Version 1.5.5.1); Available online: https://github.com/Atahk/Pscl/ (accessed on 1 August 2023).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Blaňarová, L.; Stanko, M.; Carpi, G.; Miklisová, D.; Víchová, B.; Mošanský, L.; Bona, M.; Derdáková, M. Distinct Anaplasma phagocytophilum Genotypes Associated with Ixodes trianguliceps Ticks and Rodents in Central Europe. Ticks Tick-Borne Dis. 2014, 5, 928–938. [Google Scholar] [CrossRef]
- Víchová, B.; Majláthová, V.; Nováková, M.; Stanko, M.; Hviščová, I.; Pangrácová, L.; Chrudimský, T.; Čurlík, J.; Petko, B. Anaplasma Infections in Ticks and Reservoir Host from Slovakia. Infect. Genet. Evol. 2014, 22, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Bona, M.; Blaňárová, L.; Stanko, M.; Mošanský, L.; Čepčeková, E.; Víchová, B. Impact of Climate Factors on the Seasonal Activity of Ticks and Temporal Dynamics of Tick-Borne Pathogens in an Area with a Large Tick Species Diversity in Slovakia, Central Europe. Biologia 2022, 77, 1619–1631. [Google Scholar] [CrossRef]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum—A Widespread Multi-Host Pathogen with Highly Adaptive Strategies. Front. Cell. Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef]
- Jahfari, S.; Coipan, E.C.; Fonville, M.; van Leeuwen, A.D.; Hengeveld, P.; Heylen, D.; Heyman, P.; van Maanen, C.; Butler, C.M.; Földvári, G.; et al. Circulation of Four Anaplasma phagocytophilum Ecotypes in Europe. Parasit. Vectors 2014, 7, 365. [Google Scholar] [CrossRef] [PubMed]
- Štefanidesová, K.; Špitalská, E.; Krkoš, I.; Smetanová, E.; Kocianová, E. Anaplasma phagocytophilum and Other Tick-Borne Bacteria in Wild Animals in Western Slovakia. Biologia 2011, 66, 1087–1090. [Google Scholar] [CrossRef]
- Kazimírová, M.; Hamšíková, Z.; Špitalská, E.; Minichová, L.; Mahríková, L.; Caban, R.; Sprong, H.; Fonville, M.; Schnittger, L.; Kocianová, E. Diverse Tick-Borne Microorganisms Identified in Free-Living Ungulates in Slovakia. Parasit. Vectors 2018, 11, 495. [Google Scholar] [CrossRef]
- Michalik, J.; Stańczak, J.; Cieniuch, S.; Racewicz, M.; Sikora, B.; Dabert, M. Wild Boars as Hosts of Human-Pathogenic Anaplasma phagocytophilum Variants. Emerg. Infect. Dis. 2012, 18, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Dugat, T.; Zanella, G.; Véran, L.; Lesage, C.; Girault, G.; Durand, B.; Lagrée, A.-C.; Boulouis, H.-J.; Haddad, N. Multiple-Locus Variable-Number Tandem Repeat Analysis Potentially Reveals the Existence of Two Groups of Anaplasma phagocytophilum Circulating in Cattle in France with Different Wild Reservoirs. Parasit. Vectors 2016, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Fabri, N.D.; Sprong, H.; Hofmeester, T.R.; Heesterbeek, H.; Donnars, B.F.; Widemo, F.; Ecke, F.; Cromsigt, J.P.G.M. Wild Ungulate Species Differ in Their Contribution to the Transmission of Ixodes ricinus-Borne Pathogens. Parasit. Vectors 2021, 14, 360. [Google Scholar] [CrossRef]
- Kauffmann, M.; Rehbein, S.; Hamel, D.; Lutz, W.; Heddergott, M.; Pfister, K.; Silaghi, C. Anaplasma phagocytophilum and Babesia Spp. in Roe Deer (Capreolus capreolus), Fallow Deer (Dama Dama) and Mouflon (Ovis Musimon) in Germany. Mol. Cell. Probes 2017, 31, 46–54. [Google Scholar] [CrossRef]
- Razanske, I.; Rosef, O.; Radzijevskaja, J.; Bratchikov, M.; Griciuviene, L.; Paulauskas, A. Prevalence and Co-Infection with Tick-Borne Anaplasma phagocytophilum and Babesia Spp. in Red Deer (Cervus elaphus) and Roe Deer (Capreolus capreolus) in Southern Norway. Int. J. Parasitol. Parasites Wildl. 2019, 8, 127–134. [Google Scholar] [CrossRef]
- Rosef, O.; Radzijevskaja, J.; Paulauskas, A.; Haslekås, C. The Prevalence of Anaplasma phagocytophilum in Host-Seeking Ixodes ricinus Ticks in Norway. Clin. Microbiol. Infect. 2009, 15, 43–45. [Google Scholar] [CrossRef]
- Mysterud, A.; Langvatn, R.; Yoccoz, N.; Stenseth, N.C. Plant Phenology, Migration and Geographical Variation in Body Weight of a Large Herbivore: The Effect of a Variable Topography. J. Anim. Ecol. 2001, 70, 915–923. [Google Scholar] [CrossRef]
- Kropil, R.; Smolko, P.; Garaj, P. Home Range and Migration Patterns of Male Red Deer Cervus elaphus in Western Carpathians. Eur. J. Wildl. Res. 2015, 61, 63–72. [Google Scholar] [CrossRef]
- Stanko, M.; Derdáková, M.; Špitalská, E.; Kazimírová, M. Ticks and Their Epidemiological Role in Slovakia: From the Past till Present. Biologia 2022, 77, 1575–1610. [Google Scholar] [CrossRef] [PubMed]
- Bhide, M.R.; Travnicek, M.; Levkutova, M.; Curlik, J.; Revajova, V.; Levkut, M. Sensitivity of Borrelia Genospecies to Serum Complement from Different Animals and Human: A Host—Pathogen Relationship. FEMS Immunol. Med. Microbiol. 2005, 43, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ostfeld, R.; Keesing, F. Biodiversity and Disease Risk: The Case of Lyme Disease. Conserv. Biol. 2000, 14, 722–728. [Google Scholar] [CrossRef]
- Hamšíková, Z.; Kazimírová, M.; Haruštiaková, D.; Mahríková, L.; Slovák, M.; Berthová, L.; Kocianová, E.; Schnittger, L. Babesia Spp. in Ticks and Wildlife in Different Habitat Types of Slovakia. Parasit. Vectors 2016, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Blaňarová, L.; Stanko, M.; Miklisová, D.; Víchová, B.; Mošanský, L.; Kraljik, J.; Bona, M.; Derdáková, M. Presence of Candidatus Neoehrlichia Mikurensis and Babesia microti in Rodents and Two Tick Species (Ixodes ricinus and Ixodes trianguliceps) in Slovakia. Ticks Tick-Borne Dis. 2016, 7, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Onyiche, T.E.; Răileanu, C.; Fischer, S.; Silaghi, C. Global Distribution of Babesia Species in Questing Ticks: A Systematic Review and Meta-Analysis Based on Published Literature. Pathogens 2021, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, A.; Fritzsch, J.; Franke, J.; Sachse, S.; Dorn, W.; Straube, E. Co-Circulation of Emerging Tick-Borne Pathogens in Middle Germany. Vector-Borne Zoonotic Dis. 2011, 11, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Carpi, G.; Cagnacci, F.; Wittekindt, N.E.; Zhao, F.; Qi, J.; Tomsho, L.P.; Drautz, D.I.; Rizzoli, A.; Schuster, S.C. Metagenomic Profile of the Bacterial Communities Associated with Ixodes ricinus Ticks. PLoS ONE 2011, 6, e25604. [Google Scholar] [CrossRef]
- Kmeť, V.; Čaplová, Z. An Update on the Ixodes ricinus Microbiome. JMBFS 2019, 8, 1340. [Google Scholar] [CrossRef]
- Tóth, A.G.; Farkas, R.; Papp, M.; Kilim, O.; Yun, H.; Makrai, L.; Maróti, G.; Gyurkovszky, M.; Krikó, E.; Solymosi, N. Ixodes ricinus Tick Bacteriome Alterations Based on a Climatically Representative Survey in Hungary. Microbiol. Spectr. 2023, 11, e0124323. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.-E.; et al. Update on Tick-Borne Rickettsioses around the World: A Geographic Approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed]
- Špitalská, E.; Boldiš, V.; Derdáková, M.; Selyemová, D.; Rusňáková Tarageľová, V. Rickettsial Infection in Ixodes ricinus Ticks in Urban and Natural Habitats of Slovakia. Ticks Tick-Borne Dis. 2014, 5, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Minichová, L.; Hamšíková, Z.; Mahríková, L.; Slovák, M.; Kocianová, E.; Kazimírová, M.; Škultéty, Ľ.; Štefanidesová, K.; Špitalská, E. Molecular Evidence of Rickettsia Spp. in Ixodid Ticks and Rodents in Suburban, Natural and Rural Habitats in Slovakia. Parasit. Vectors 2017, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Chvostáč, M.; Špitalská, E.; Václav, R.; Vaculová, T.; Minichová, L.; Derdáková, M. Seasonal Patterns in the Prevalence and Diversity of Tick-Borne Borrelia burgdorferi Sensu Lato, Anaplasma phagocytophilum and Rickettsia spp. in an Urban Temperate Forest in South Western Slovakia. Int. J. Environ. Res. Public Health 2018, 15, 994. [Google Scholar] [CrossRef]
- Blažeková, V.; Stanko, M.; Sprong, H.; Kohl, R.; Zubriková, D.; Vargová, L.; Bona, M.; Miklisová, D.; Víchová, B. Ixodiphagus hookeri (Hymenoptera: Encyrtidae) and Tick-Borne Pathogens in Ticks with Sympatric Occurrence (and Different Activities) in the Slovak Karst National Park (Slovakia), Central Europe. Pathogens 2024, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Berthová, L.; Slobodník, V.; Slobodník, R.; Olekšák, M.; Sekeyová, Z.; Svitálková, Z.; Kazimírová, M.; Špitalská, E. The Natural Infection of Birds and Ticks Feeding on Birds with Rickettsia spp. and Coxiella burnetii in Slovakia. Exp. Appl. Acarol. 2016, 68, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Lieskovská, N.; Minichová, L.; Šorf, R.; Gacíková, E.; Vrbová, E.; Kazimírová, M.; Sekeyová, Z. Dogs as Sentinels for Distribution of Spotted-Fever Group Rickettsiae in Slovakia. Travel Med. Infect. Dis. 2018, 26, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Kupča, A.M.; Essbauer, S.; Zoeller, G.; de Mendonça, P.G.; Brey, R.; Rinder, M.; Pfister, K.; Spiegel, M.; Doerrbecker, B.; Pfeffer, M.; et al. Isolation and Molecular Characterization of a Tick-Borne Encephalitis Virus Strain from a New Tick-Borne Encephalitis Focus with Severe Cases in Bavaria, Germany. Ticks Tick-Borne Dis. 2010, 1, 44–51. [Google Scholar] [CrossRef]
- Hönig, V.; Svec, P.; Halas, P.; Vavruskova, Z.; Tykalova, H.; Kilian, P.; Vetiskova, V.; Dornakova, V.; Sterbova, J.; Simonova, Z.; et al. Ticks and Tick-Borne Pathogens in South Bohemia (Czech Republic)—Spatial Variability in Ixodes ricinus Abundance, Borrelia burgdorferi and Tick-Borne Encephalitis Virus Prevalence. Ticks Tick-Borne Dis. 2015, 6, 559–567. [Google Scholar] [CrossRef]
- Zubriková, D.; Wittmann, M.; Hönig, V.; Švec, P.; Víchová, B.; Essbauer, S.; Dobler, G.; Grubhoffer, L.; Pfister, K. Prevalence of Tick-Borne Encephalitis Virus and Borrelia burgdorferi Sensu Lato in Ixodes ricinus Ticks in Lower Bavaria and Upper Palatinate, Germany. Ticks Tick-Borne Dis. 2020, 11, 101375. [Google Scholar] [CrossRef] [PubMed]
- Grešíková, M.; Nosek, J. Arboviruses Isolated from Ticks in Central Europe. Biologia 1982, 37, 755–763. [Google Scholar]
- Kožuch, O.; Nosek, J.; Lysý, J. Natural Focus of Tick-Borne Encephalitis in Southern Slovakia. Biológia 1982, 37, 321–325. [Google Scholar]
- Nosek, J.; Kožuch, O.; Grulich, I. The Structure of Tick-Borne Encephalitis (TBE) Foci in Central Europe. Oecologia 1970, 5, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Dobler, G.; Hufert, F.T.; Pfeffer, M.; Essbauer, S. Tick-Borne Encephalitis: From Microfocus to Human Disease. In Progress in Parasitology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 323–331. ISBN 978-3-642-21395-3. [Google Scholar]
- Korenberg, E.I. Natural Focality of Infections: Current Problems and Prospects of Research. Biol. Bull. 2010, 37, 665–676. [Google Scholar] [CrossRef]
- Cagnacci, F.; Bolzoni, L.; Rosà, R.; Carpi, G.; Hauffe, H.C.; Valent, M.; Tagliapietra, V.; Kazimirova, M.; Koci, J.; Stanko, M.; et al. Effects of Deer Density on Tick Infestation of Rodents and the Hazard of Tick-Borne Encephalitis. I: Empirical Assessment. Int. J. Parasitol. 2012, 42, 365–372. [Google Scholar] [CrossRef]

| No. of Ticks, Pools | Bbsl | Ba./Th. spp. | A. phag. | Rick. spp. | TBEV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altitude | Soil pH | A | N | Total | Pools | + | EPP [CI95%] | + | EPP [CI95%] | + | EPP [CI95%] | + | MIR | + | MIR |
| 600 | 4.45 | 146 | 364 | 510 | 68 | 22 | 5.13 [3.29–7.51] | 14 | 3.07 [1.74–4.93] | 4 | 0.81 [0.25–1.86] | 38 | 10.57 [7.6–14.18] | 3 | 0.6 [0.15–1.55] |
| 700 | 4.68 | 50 | 270 | 320 | 39 | 13 | 3.66 [1.85–6.36] | 10 | 3.59 [1.81–6.23] | 3 | 0.97 [0.24–2.5] | 29 | 16.7 [11.27–23.69] | 4 | 1.32 [0.41–3.03] |
| 800 | 4.63 | 76 | 116 | 192 | 29 | 8 | 4.74 [2.19–8.67] | 2 | 10.8 [0.18–3.28] | 2 | 10.7 [0.18–3.27] | 16 | 11.74 [6.96–18.17] | 1 | 0.52 [0.0003–2.29] |
| 900 | 4.04 | 96 | 166 | 262 | 38 | 3 | 1.18 [0.3–3.04] | 5 | 2.02 [0.73–4.3] | 8 | 3.38 [1.56–6.22] | 19 | 10.27 [6.37–15.43] | 1 | 0.39 [0.0002–1.69] |
| 1000 | 4.07 | 49 | 60 | 109 | 21 | 4 | 4.25 [1.34–9.67] | 3 | 2.9 [0.73–7.36] | 5 | 5.17 [1.88–10.83] | 12 | 17.06 [9.28–27.91] | 0 | 0 |
| 1050 | 4.02 | 93 | 143 | 236 | 38 | 7 | 3.31 [1.43–6.33] | 10 | 4.91 [2.48–8.48] | 11 | 5.29 [2.77–8.91] | 26 | 18.45 [12.31–26.23] | 0 | 0 |
| Total | 510 | 1119 | 1629 | 233 | 57 | 4.0 [3.06–5.11] | 44 | 2.98 [2.19–3.94] | 33 | 2.15 [1.50–2.96] | 140 | 13.07 [11.05–15.30] | 9 | 0.56 [0.27–1.01] | |
| No. of Ticks, Pooles | Bbsl | Ba./Th. spp. | A. phag. | Rick. spp. | TBEV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altitude | Soil pH | A | N | Total | Pools | + | EPP [CI95%] | + | EPP [CI95%] | + | EPP [CI95%] | + | EPP [CI95%] | + | EPP [CI95%] |
| 680 | 4.46 | 37 | 268 | 305 | 39 | 7 | 2.5 [1.08–4.79] | 1 | 0.33 [0.0002–1.44] | 1 | 0.33 [0.0002–1.44] | 24 | 12.9 [8.41–18.74] | 1 | 0.33 [0.0002–1.44] |
| 830 | 6.13 | 51 | 109 | 160 | 24 | 4 | 2.71 [0.85–6.2] | 1 | 0.64 [0.0004–2.8] | 2 | 1.28 [0.21–3.91] | 19 | 26.59 [15.99–41.03] | 2 | 1.3 [0.22–3.96] |
| 990 | 5.89 | 51 | 106 | 157 | 25 | 9 | 7.04 [3.43–12.46] | 2 | 1.32 [0.22–4.02] | 1 | 0.65 [0.0004–2.82] | 16 | 15.7 [9.3–24.3] | 1 | 0.66 [0.0004–2.89] |
| 1066 | 4.35 | 13 | 13 | 26 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 18.7 [6.15–38.89] | 0 | 0 |
| 1280 | 3.66 | 9 | 8 | 17 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 18.87 [5.05–42.14] | 0 | 0 |
| 1370 | 3.72 | 5 | 2 | 7 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 17.02 [1.05–57.62] | 0 | 0 |
| 1450 | 5.56 | 5 | 2 | 7 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 171 | 508 | 679 | 110 | 20 | 3.26 [2.04–4.87] | 4 | 0.60 [0.19–1.38] | 4 | 0.60 [0.19–1.38] | 67 | 16.19 [12.65–20.31] | 4 | 0.60 [0.19–1.39] | |
| A. phag. | Ba./Th. spp. | Bbsl | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fixed effects | |||||||||
| predictors | estimate | Se | p-value | estimate | Se | p-value | estimate | Se | p-value |
| (Intercept) | −2.72 | 0.74 | <0.001 | −2.56 | 0.92 | 0.006 | −2.12 | 1.02 | 0.038 |
| altitude | 0.60 | 0.24 | 0.014 | −0.03 | 0.20 | 0.880 | −0.43 | 0.17 | 0.009 |
| pH | 0.05 | 0.32 | 0.872 | 0.17 | 0.30 | 0.580 | 0.54 | 0.20 | 0.007 |
| Random Effects | |||||||||
| σ2 | 3.29 | 3.29 | 3.29 | ||||||
| τ00 | 0.04 year | 0.37 year | 1.54 year | ||||||
| 0.85 locality | 1.15 locality | 0.42 locality | |||||||
| ICC adjusted | 0.21 | 0.32 | 0.37 | ||||||
| N | 2 years | 2 years | 2 years | ||||||
| 2 localities | 2 localities | 2 localities | |||||||
| Performance | |||||||||
| AUC | 0.73 | 0.71 | 0.77 | ||||||
| R2m/R2c | 0.07/0.27 | 0.006/0.32 | 0.09/0.43 | ||||||
| Observations | 343 | 343 | 343 | ||||||
| Rick. spp. | TBEV | |||||
|---|---|---|---|---|---|---|
| Fixed effect | ||||||
| predictors | estimate | Se | p-value | estimate | Se | p-value |
| (Intercept) | 0.46 | 0.12 | <0.001 | −3.49 | 0.37 | <0.001 |
| altitude | 0.12 | 0.12 | 0.346 | −0.62 | 0.40 | 0.118 |
| pH | 0.23 | 0.12 | 0.056 | 0.49 | 0.29 | 0.085 |
| amount | 0.59 | 0.13 | <0.001 | |||
| Performance | ||||||
| AUC | 0.67 | 0.66 | ||||
| R2 | 0.06 | 0.05 | ||||
| Observations | 343 | 343 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubriková, D.; Blaňarová, L.; Hrkľová, G.; Syrota, Y.; Macko, J.; Blahútová, D.; Blažeková, V.; Stanko, M.; Švirlochová, K.; Víchová, B. The Impact of Altitude on Tick-Borne Pathogens at Two Mountain Ranges in Central Slovakia. Pathogens 2024, 13, 586. https://doi.org/10.3390/pathogens13070586
Zubriková D, Blaňarová L, Hrkľová G, Syrota Y, Macko J, Blahútová D, Blažeková V, Stanko M, Švirlochová K, Víchová B. The Impact of Altitude on Tick-Borne Pathogens at Two Mountain Ranges in Central Slovakia. Pathogens. 2024; 13(7):586. https://doi.org/10.3390/pathogens13070586
Chicago/Turabian StyleZubriková, Dana, Lucia Blaňarová, Gabriela Hrkľová, Yaroslav Syrota, Jozef Macko, Dana Blahútová, Veronika Blažeková, Michal Stanko, Klaudia Švirlochová, and Bronislava Víchová. 2024. "The Impact of Altitude on Tick-Borne Pathogens at Two Mountain Ranges in Central Slovakia" Pathogens 13, no. 7: 586. https://doi.org/10.3390/pathogens13070586
APA StyleZubriková, D., Blaňarová, L., Hrkľová, G., Syrota, Y., Macko, J., Blahútová, D., Blažeková, V., Stanko, M., Švirlochová, K., & Víchová, B. (2024). The Impact of Altitude on Tick-Borne Pathogens at Two Mountain Ranges in Central Slovakia. Pathogens, 13(7), 586. https://doi.org/10.3390/pathogens13070586







