Understanding the Transmission Dynamics of the Chikungunya Virus in Africa
Abstract
:1. Introduction
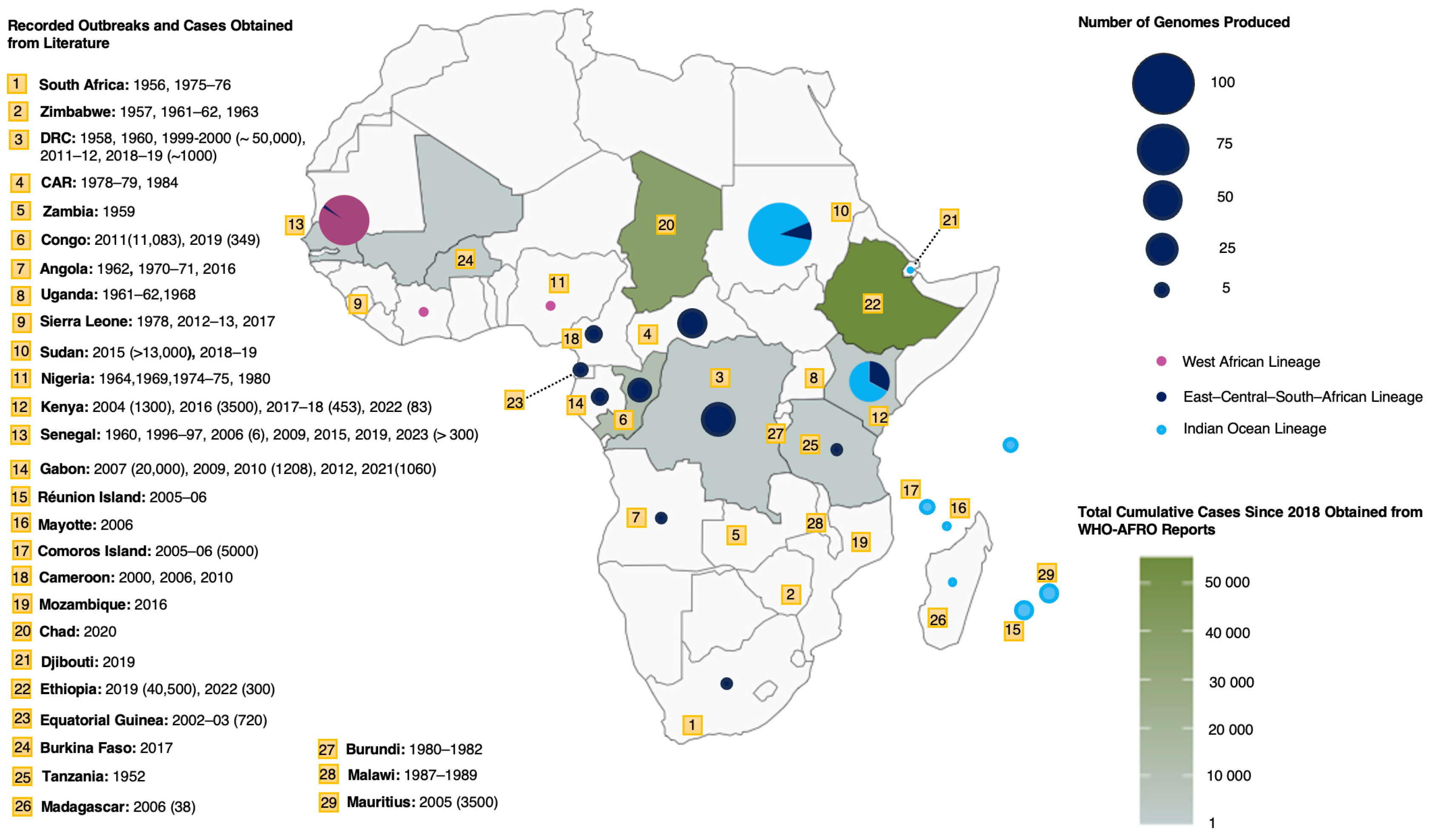
2. Cases, Outbreaks, and Current Burden in Africa
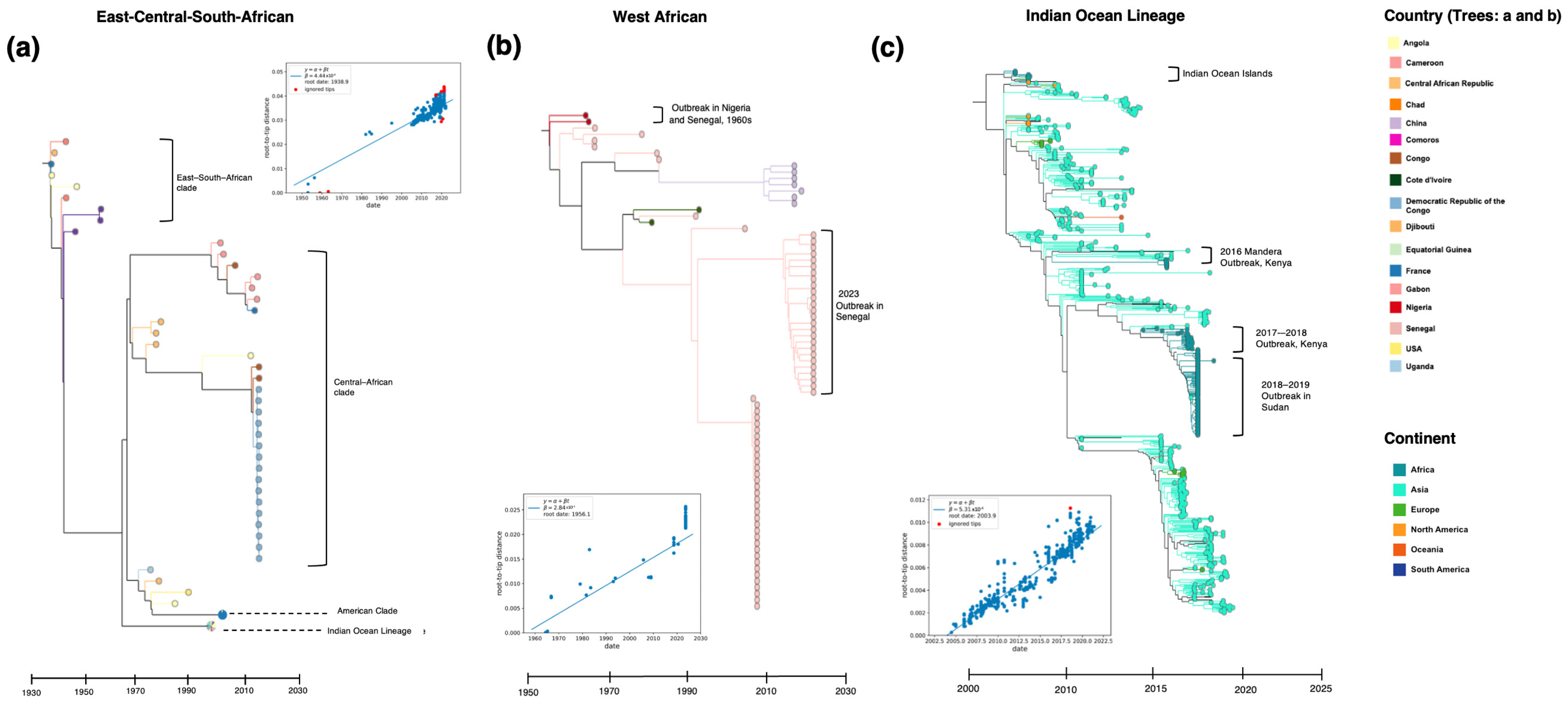
3. Genotype Distribution
4. Importance of Genomic and Epidemiological Surveillance
5. Genetic Diversity and Transmission Dynamics in Africa
6. Vectors and Transmission
7. Challenges and Gaps in CHIKV Surveillance in Africa
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phadungsombat, J.; Imad, H.; Rahman, M.; Nakayama, E.E.; Kludkleeb, S.; Ponam, T.; Rahim, R.; Hasan, A.; Poltep, K.; Yamanaka, A.; et al. A Novel Sub-Lineage of Chikungunya Virus East/Central/South African Genotype Indian Ocean Lineage Caused Sequential Outbreaks in Bangladesh and Thailand. Viruses 2020, 12, 1319. [Google Scholar] [CrossRef] [PubMed]
- Deeba, F.; Haider, M.S.H.; Ahmed, A.; Tazeen, A.; Faizan, M.I.; Salam, N.; Hussain, T.; Alamery, S.F.; Parveen, S. Global Transmission and Evolutionary Dynamics of the Chikungunya Virus. Epidemiol. Infect. 2020, 148, e63. [Google Scholar] [CrossRef]
- Schuffenecker, I.; Iteman, I.; Michault, A.; Murri, S.; Frangeul, L.; Vaney, M.-C.; Lavenir, R.; Pardigon, N.; Reynes, J.-M.; Pettinelli, F.; et al. Genome Microevolution of Chikungunya Viruses Causing the Indian Ocean Outbreak. PLoS Med. 2006, 3, e263. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.V.J.; Ludwig-Begall, L.F.; de Oliveira-Filho, E.F.; Oliveira, R.A.S.; Durães-Carvalho, R.; Lopes, T.R.R.; Silva, D.E.A.; Gil, L.H.V.G. A Scoping Review of Chikungunya Virus Infection: Epidemiology, Clinical Characteristics, Viral Co-Circulation Complications, and Control. Acta Trop. 2018, 188, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Bartholomeeusen, K.; Daniel, M.; LaBeaud, D.A.; Gasque, P.; Peeling, R.W.; Stephenson, K.E.; Ng, L.F.P.; Ariën, K.K. Chikungunya Fever. Nat. Rev. Dis. Primers 2023, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Subissi, L.; Rezza, G. Chikungunya Fever in Africa: A Systematic Review. Pathog. Glob. Health 2020, 114, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Mcintosh, B.M.; Harwin, R.M.; Paterson, H.E.; Westwatert, M.L. An Epidemic of Chikungunya in South-Eastern Southern Rhodesia. Cent. Afr. J. Med. 1963, 9, 351–359. [Google Scholar] [PubMed]
- Lumsden, W.H.R. An Epidemic of Virus Disease in Southern Province, Tanganyika Territory, in 1952–1953 II. General Description and Epidemiology. Trans. R. Soc. Trop. Med. Hyg. 1955, 49, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Reappearance of Chikungunya, Formerly Called Dengue, in the Americas. Emerg. Infect. Dis. 2015, 21, 557–561. [Google Scholar] [CrossRef]
- Carey, D.E. Chikungunya and Dengue: A Case of Mistaken Identity? J. Hist. Med. Allied Sci. 1971, 26, 243–262. [Google Scholar] [CrossRef]
- de Lima Cavalcanti, T.Y.V.; Pereira, M.R.; de Paula, S.O.; Franca, R.F.D.O. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses 2022, 14, 969. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.M.; Nkoghe, D.; Ollomo, B.; Nze-Nkogue, C.; Becquart, P.; Grard, G.; Pourrut, X.; Charrel, R.; Moureau, G.; Ndjoyi-Mbiguino, A.; et al. Concurrent Chikungunya and Dengue Virus Infections during Simultaneous Outbreaks, Gabon, 2007. Emerg. Infect. Dis. 2009, 15, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Thiberville, S.D.; Boisson, V.; Gaudart, J.; Simon, F.; Flahault, A.; de Lamballerie, X. Chikungunya Fever: A Clinical and Virological Investigation of Outpatients on Reunion Island, South-West Indian Ocean. PLoS Negl. Trop. Dis. 2013, 7, e2004. [Google Scholar] [CrossRef] [PubMed]
- Sagay, A.S.; Hsieh, S.-C.; Dai, Y.-C.; Chang, C.A.; Ogwuche, J.; Ige, O.O.; Kahansim, M.L.; Chaplin, B.; Imade, G.; Elujoba, M.; et al. Chikungunya Virus Antepartum Transmission and Abnormal Infant Outcomes in a Cohort of Pregnant Women in Nigeria. Int. J. Infect. Dis. 2024, 139, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Contopoulos-Ioannidis, D.; Newman-Lindsay, S.; Chow, C.; LaBeaud, A.D. Mother-to-Child Transmission of Chikungunya Virus: A Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2018, 12, e0006510. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.B.; Barreto, F.K.D.A.; Barreto, M.C.A.; Santos, T.H.P.D.; Andrade, M.D.M.O.D.; Farias, L.A.B.G.; Freitas, A.R.R.D.; Martinez, M.J.; Cavalcanti, L.P.D.G. Epidemiology and Economic Burden of Chikungunya: A Systematic Literature Review. Trop. Med. Infect. Dis. 2023, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Soumahoro, M.K.; Boelle, P.Y.; Gaüzere, B.A.; Atsou, K.; Pelat, C.; Lambert, B.; La Ruche, G.; Gastellu-Etchegorry, M.; Renault, P.; Sarazin, M.; et al. The Chikungunya Epidemic on La Réunion Island in 2005–2006: A Cost-of-Illness Study. PLoS Negl. Trop. Dis. 2011, 5, e1197. [Google Scholar] [CrossRef]
- Paixão, E.S.; Rodrigues, L.C.; Costa, M.d.C.N.; Itaparica, M.; Barreto, F.; Gérardin, P.; Teixeira, M.G. Chikungunya Chronic Disease: A Systematic Review and Meta-Analysis. Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Tam, P.Y.I.; Obaro, S.K.; Storch, G. Challenges in the Etiology and Diagnosis of Acute Febrile Illness in Children in Low- and Middle- Income Countries. J. Pediatr. Infect. Dis. Soc. 2016, 5, 190–205. [Google Scholar] [CrossRef]
- Fenollar, F.; Mediannikov, O. Emerging Infectious Diseases in Africa in the 21st Century. New Microbes New Infect. 2018, 26, S10–S18. [Google Scholar] [CrossRef]
- Maze, M.J.; Bassat, Q.; Feasey, N.A.; Mandomando, I.; Musicha, P.; Crump, J.A. The Epidemiology of Febrile Illness in Sub-Saharan Africa: Implications for Diagnosis and Management. Clin. Microbiol. Infect. 2018, 24, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Nooh, F.; Chernet, A.; Reither, K.; Okuma, J.; Brattig, N.W.; Utzinger, J.; Probst-Hensch, N.; Paris, D.H.; Dreyfus, A. Prevalence of Fever of Unidentified Aetiology in East African Adolescents and Adults: A Systematic Review and Meta-Analysis. Infect. Dis. Poverty 2023, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- CLIMADE. Available online: https://climade.health/africa (accessed on 5 June 2024).
- Egid, B.R.; Coulibaly, M.; Dadzie, S.K.; Kamgang, B.; McCall, P.J.; Sedda, L.; Toe, K.H.; Wilson, A.L. Review of the Ecology and Behaviour of Aedes Aegypti and Aedes Albopictus in Western Africa and Implications for Vector Control. Curr. Res. Parasitol. Vector-Borne Dis. 2022, 2, 100074. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Technical Guidelines for Integrated Disease Surveillance and Response in the African Region: Third Edition. Available online: https://www.afro.who.int/publications/technical-guidelines-integrated-disease-surveillance-and-response-african-region-third (accessed on 15 May 2024).
- Mascarenhas, M.; Garasia, S.; Berthiaume, P.; Corrin, T.; Greig, J.; Ng, V.; Young, I.; Waddell, L. A Scoping Review of Published Literature on Chikungunya Virus. PLoS ONE 2018, 13, e0207554. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. African Region Outbreaks and Emergencies Bulletin. Available online: https://www.afro.who.int/health-topics/disease-outbreaks/outbreaks-and-other-emergencies-updates (accessed on 28 April 2024).
- Edwards, C.J.; Welch, S.R.; Chamberlain, J.; Hewson, R.; Tolley, H.; Cane, P.A.; Lloyd, G. Molecular Diagnosis and Analysis of Chikungunya Virus. J. Clin. Virol. 2007, 39, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Fourié, T.; Dia, A.; Savreux, Q.; Pommier de Santi, V.; de Lamballerie, X.; Leparc-Goffart, I.; Simon, F. Emergence of Indian Lineage of ECSA Chikungunya Virus in Djibouti, 2019. Int. J. Infect. Dis. 2021, 108, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Agbodzi, B.; Yousseu, F.B.S.; Simo, F.B.N.; Kumordjie, S.; Yeboah, C.; Mosore, M.T.; Bentil, R.E.; Prieto, K.; Colston, S.M.; Attram, N.; et al. Chikungunya Viruses Containing the A226V Mutation Detected Retrospectively in Cameroon Form a New Geographical Subclade. Int. J. Infect. Dis. 2021, 113, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, A.; Ciccozzi, M.; Cella, E.; Lai, A.; Simonetti, F.R.; Galli, M.; Zehender, G.; Rezza, G. Origin, Evolution, and Phylogeography of Recent Epidemic CHIKV Strains. Infect. Genet. Evol. 2012, 12, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Mudurangaplar, B. Molecular Characterisation of Clinical Isolates of Chikungunya Virus: A Study from Tertiary Care Hospitals in Southern India. J. Clin. Diagn. Res. 2016, 10, DC14. [Google Scholar] [CrossRef]
- Peyrefitte, C.N.; Rousset, D.; Pastorino, B.A.M.; Pouillot, R.; Bessaud, M.; Tock, F.; Mansaray, H.; Merle, O.L.; Pascual, A.M.; Paupy, C.; et al. Chikungunya Virus, Cameroon, 2006. Emerg. Infect. Dis. 2007, 13, 768–771. [Google Scholar] [CrossRef]
- Takaya, S.; Kutsuna, S.; Nakayama, E.; Taniguchi, S.; Tajima, S.; Katanami, Y.; Yamamoto, K.; Takeshita, N.; Hayakawa, K.; Kato, Y.; et al. Chikungunya Fever in Traveler from Angola to Japan, 2016. Emerg. Infect. Dis. 2017, 23, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Maljkovic Berry, I.; Eyase, F.; Pollett, S.; Konongoi, S.L.; Joyce, M.G.; Figueroa, K.; Ofula, V.; Koka, H.; Koskei, E.; Nyunja, A.; et al. Global Outbreaks and Origins of a Chikungunya Virus Variant Carrying Mutations Which May Increase Fitness for Aedes Aegypti: Revelations from the 2016 Mandera, Kenya Outbreak. Am. J. Trop. Med. Hyg. 2019, 100, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Bower, H.; el Karsany, M.; Adam, A.A.A.H.; Idriss, M.I.; Alzain, M.A.; Alfakiyousif, M.E.A.; Mohamed, R.; Mahmoud, I.; Albadri, O.; Mahmoud, S.A.A.; et al. “Kankasha” in Kassala: A Prospective Observational Cohort Study of the Clinical Characteristics, Epidemiology, Genetic Origin, and Chronic Impact of the 2018 Epidemic of Chikungunya Virus Infection in Kassala, Sudan. PLoS Negl. Trop. Dis. 2021, 15, e0009387. [Google Scholar] [CrossRef] [PubMed]
- Eyase, F.; Langat, S.; Berry, I.M.; Mulwa, F.; Nyunja, A.; Mutisya, J.; Owaka, S.; Limbaso, S.; Ofula, V.; Koka, H.; et al. Emergence of a Novel Chikungunya Virus Strain Bearing the E1:V80A Substitution, out of the Mombasa, Kenya 2017–2018 Outbreak. PLoS ONE 2020, 15, e0241754. [Google Scholar] [CrossRef] [PubMed]
- Moyen, N.; Thiberville, S.-D.; Pastorino, B.; Nougairede, A.; Thirion, L.; Mombouli, J.-V.; Dimi, Y.; Leparc-Goffart, I.; Capobianchi, M.R.; Lepfoundzou, A.D.; et al. First Reported Chikungunya Fever Outbreak in the Republic of Congo, 2011. PLoS ONE 2014, 9, e115938. [Google Scholar] [CrossRef] [PubMed]
- Selhorst, P.; Makiala-Mandanda, S.; De Smet, B.; Mariën, J.; Anthony, C.; Binene-Mbuka, G.; De Weggheleire, A.; Ilombe, G.; Kinganda-Lusamaki, E.; Pukuta-Simbu, E.; et al. Molecular Characterization of Chikungunya Virus during the 2019 Outbreak in the Democratic Republic of the Congo. Emerg. Microbes Infect. 2020, 9, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Konongoi, S.L.; Nyunja, A.; Ofula, V.; Owaka, S.; Koka, H.; Koskei, E.; Eyase, F.; Langat, D.; Mancuso, J.; Lutomiah, J.; et al. Human and Entomologic Investigations of Chikungunya Outbreak in Mandera, Northeastern Kenya, 2016. PLoS ONE 2018, 13, e0205058. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; de Lamballerie, X.; Jourdan, J.; Rovery, C.; Vaillant, V.; Minodier, P.; Brouqui, P.; Flahault, A.; Raoult, D.; Charrel, R. Novel Chikungunya Virus Variant in Travelers Returning from Indian Ocean Islands. Emerg. Infect. Dis. 2006, 12, 1493–1499. [Google Scholar] [CrossRef]
- Caron, M.; Paupy, C.; Grard, G.; Becquart, P.; Mombo, I.; Nso, B.B.B.; Kassa Kassa, F.; Nkoghe, D.; Leroy, E.M. Recent Introduction and Rapid Dissemination of Chikungunya Virus and Dengue Virus Serotype 2 Associated with Human and Mosquito Coinfections in Gabon, Central Africa. Clin. Infect. Dis. 2012, 55, e45–e53. [Google Scholar] [CrossRef]
- Phadungsombat, J.; Imad, H.A.; Nakayama, E.E.; Leaungwutiwong, P.; Ramasoota, P.; Nguitragool, W.; Matsee, W.; Piyaphanee, W.; Shioda, T. Spread of a Novel Indian Ocean Lineage Carrying E1-K211E/E2-V264A of Chikungunya Virus East/Central/South African Genotype across the Indian Subcontinent, Southeast Asia, and Eastern Africa. Microorganisms 2022, 10, 354. [Google Scholar] [CrossRef]
- Simo Tchetgna, H.; Sem Ouilibona, R.; Nkili-Meyong, A.A.; Caron, M.; Labouba, I.; Selekon, B.; Njouom, R.; Leroy, E.M.; Nakoune, E.; Berthet, N. Viral Exploration of Negative Acute Febrile Cases Observed during Chikungunya Outbreaks in Gabon. Intervirology 2018, 61, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Vairo, F.; Aimè Coussoud-Mavoungou, M.; Ntoumi, F.; Castilletti, C.; Kitembo, L.; Haider, N.; Carletti, F.; Colavita, F.; Gruber, C.; Iannetta, M.; et al. Chikungunya Outbreak in the Republic of the Congo, 2019—Epidemiological, Virological and Entomological Findings of a South-North Multidisciplinary Taskforce Investigation. Viruses 2020, 12, 1020. [Google Scholar] [CrossRef]
- Onoja, A.B.; Omatola, A.C.; Maiga, M.; Gadzama, I.S. Recurrent Episodes of Some Mosquito-Borne Viral Diseases in Nigeria: A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 1162. [Google Scholar] [CrossRef]
- Delisle, E.; Rousseau, C.; Broche, B.; Leparc-Goffart, I.; L’Ambert, G.; Cochet, A.; Prat, C.; Foulongne, V.; Ferré, J.B.; Catelinois, O.; et al. Chikungunya Outbreak in Montpellier, France, September to October 2014. Eurosurveillance 2015, 20, 21108. [Google Scholar] [CrossRef] [PubMed]
- Chinedu Eneh, S.; Uwishema, O.; Nazir, A.; El Jurdi, E.; Faith Olanrewaju, O.; Abbass, Z.; Mustapha Jolayemi, M.; Mina, N.; kseiry, lea; Onyeaka, H. Chikungunya Outbreak in Africa: A Review of the Literature. Ann. Med. Surg. 2023, 85, 3545–3552. [Google Scholar] [CrossRef]
- Wahid, B.; Ali, A.; Rafique, S.; Idrees, M. Global Expansion of Chikungunya Virus: Mapping the 64-Year History. Int. J. Infect. Dis. 2017, 58, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Collao, X.; Negredo, A.I.; Cano, J.; Tenorio, A.; De Ory, F.; Benito, A.; Masia, M.; Sánchez-Seco, M.P. Different Lineages of Chikungunya Virus in Equatorial Guinea in 2002 and 2006. Am. J. Trop. Med. Hyg. 2010, 82, 505–507. [Google Scholar] [CrossRef]
- Bettis, A.A.; L’Azou Jackson, M.; Yoon, I.K.; Breugelmans, J.G.; Goios, A.; Gubler, D.J.; Powers, A.M. The Global Epidemiology of Chikungunya from 1999 to 2020: A Systematic Literature Review to Inform the Development and Introduction of Vaccines. PLoS Negl. Trop. Dis. 2022, 16, e0010069. [Google Scholar] [CrossRef]
- Desdouits, M.; Kamgang, B.; Berthet, N.; Tricou, V.; Ngoagouni, C.; Gessain, A.; Manuguerra, J.C.; Nakouné, E.; Kazanji, M. Genetic Characterization of Chikungunya Virus in the Central African Republic. Infect. Genet. Evol. 2015, 33, 25–31. [Google Scholar] [CrossRef]
- Ratsitorahina, M.; Harisoa, J.; Ratovonjato, J.; Biacabe, S.; Reynes, J.M.; Zeller, H.; Raoelina, Y.; Talarmin, A.; Richard, V.; Soares, J.L. Outbreak of Dengue and Chikungunya Fevers, Toamasina, Madagascar, 2006. Emerg. Infect. Dis. 2008, 14, 1135–1137. [Google Scholar] [CrossRef]
- Beesoon, S.; Funkhouser, E.; Kotea, N.; Spielman, A.; Robich, R.M. Chikungunya Fever, Mauritius, 2006. Emerg. Infect. Dis. 2008, 14, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Sergon, K.; Njuguna, C.; Kalani, R.; Ofula, V.; Onyango, C.; Konongoi, L.S.; Bedno, S.; Burke, H.; Dumilla, A.M.; Konde, J.; et al. Seroprevalence of Chikungunya Virus (CHIKV) Infection on Lamu Island, Kenya, October 2004. Am. J. Trop. Med. Hyg. 2008, 78, 333–337. [Google Scholar] [CrossRef]
- Nkoghe, D.; Kassa, R.F.; Caron, M.; Grard, G.; Mombo, I.; Bikié, B.; Paupy, C.; Becquart, P.; Bisvigou, U.; Leroy, E.M. Clinical Forms of Chikungunya in Gabon, 2010. PLoS Negl. Trop. Dis. 2012, 6, e1517. [Google Scholar] [CrossRef] [PubMed]
- Suhana, O.; Nazni, W.A.; Apandi, Y.; Farah, H.; Lee, H.L.; Sofian-Azirun, M. Insight into the Origin of Chikungunya Virus in Malaysian Non-Human Primates via Sequence Analysis. Heliyon 2019, 5, e02682. [Google Scholar] [CrossRef] [PubMed]
- Nyamwaya, D.K.; Otiende, M.; Omuoyo, D.O.; Githinji, G.; Karanja, H.K.; Gitonga, J.N.; de Laurent, Z.R.; Otieno, J.R.; Sang, R.; Kamau, E.; et al. Endemic Chikungunya Fever in Kenyan Children: A Prospective Cohort Study. BMC Infect. Dis. 2021, 21, 186. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, Y.; Abe, H.; Mbadinga, M.J.V.M.; Ondo, G.N.; Bikangui, R.; Agnandji, S.T.; Lell, B.; Yasuda, J. Re-Emergence of Dengue, Chikungunya, and Zika Viruses in 2021 after a 10-Year Gap in Gabon. IJID Reg. 2022, 5, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, B.; Muyembe-Tamfum, J.J.; Bessaud, M.; Tock, F.; Tolou, H.; Durand, J.P.; Peyrefitte, C.N. Epidemic Resurgence of Chikungunya Virus in Democratic Republic of the Congo: Identification of a New Central African Strain. J. Med. Virol. 2004, 74, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Bacterial and Viral Bioinformatics Resource Center (BV-BRC). Available online: https://www.bv-brc.org/ (accessed on 12 January 2024).
- Khare, S.; Gurry, C.; Freitas, L.; B Schultz, M.; Bach, G.; Diallo, A.; Akite, N.; Ho, J.; TC Lee, R.; Yeo, W.; et al. GISAID’s Role in Pandemic Response. China CDC Wkly 2021, 3, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- De Weggheleire, A.; Nkuba-Ndaye, A.; Mbala-Kingebeni, P.; Mariën, J.; Kindombe-Luzolo, E.; Ilombe, G.; Mangala-Sonzi, D.; Binene-Mbuka, G.; De Smet, B.; Vogt, F.; et al. A Multidisciplinary Investigation of the First Chikungunya Virus Outbreak in Matadi in the Democratic Republic of the Congo. Viruses 2021, 13, 1988. [Google Scholar] [CrossRef]
- Demanou, M.; Antonio-Nkondjio, C.; Ngapana, E.; Rousset, D.; Paupy, C.; Manuguerra, J.-C.; Zeller, H. Chikungunya Outbreak in a Rural Area of Western Cameroon in 2006: A Retrospective Serological and Entomological Survey. BMC Res. Notes 2010, 3, 128. [Google Scholar] [CrossRef]
- Buchwald, A.G.; Hayden, M.H.; Dadzie, S.K.; Paull, S.H.; Carlton, E.J. Aedes-Borne Disease Outbreaks in West Africa: A Call for Enhanced Surveillance. Acta Trop. 2020, 209, 105468. [Google Scholar] [CrossRef] [PubMed]
- Ansumana, R.; Jacobsen, K.H.; Leski, T.A.; Covington, A.L.; Bangura, U.; Hodges, M.H.; Lin, B.; Bockarie, A.S.; Lamin, J.M.; Bockarie, M.J.; et al. Reemergence of Chikungunya Virus in Bo, Sierra Leone. Emerg. Infect. Dis. 2013, 19, 1108–1110. [Google Scholar] [CrossRef]
- Jones, R.T.; Tytheridge, S.J.; Smith, S.J.; Levine, R.S.; Hodges, M.H.; Ansumana, R.; Wulff, S.; Whitworth, J.; Logan, J.G. The Threat of Vector-Borne Diseases in Sierra Leone. Am. J. Trop. Med. Hyg. 2023, 109, 10–21. [Google Scholar] [CrossRef]
- Moore, D.L.; Reddy, S.; Akinkugbe, F.M.; Lee, V.H.; David-West, T.S.; Causey, O.R.; Carey, D.E. An Epidemic of Chikungunya Fever at Ibadan, Nigeria, 1969. Ann. Trop. Med. Parasitol. 1974, 68, 59–68. [Google Scholar] [CrossRef]
- Powers, A.M.; Logue, C.H. Changing Patterns of Chikungunya Virus: Re-Emergence of a Zoonotic Arbovirus. J. Gen. Virol. 2007, 88, 2363–2377. [Google Scholar] [CrossRef] [PubMed]
- Bane, S.; Rosenke, K.; Feldmann, F.; Meade-White, K.; Diawara, S.; Keita, M.; Maiga, O.; Diakite, M.; Safronetz, D.; Doumbia, S.; et al. Seroprevalence of Arboviruses in a Malaria Hyperendemic Area in Southern Mali. Am. J. Trop. Med. Hyg. 2024, 111, 107–112. [Google Scholar] [CrossRef]
- Sow, A.; Faye, O.; Diallo, M.; Diallo, D.; Chen, R.; Faye, O.; Diagne, C.T.; Guerbois, M.; Weidmann, M.; Ndiaye, Y.; et al. Chikungunya Outbreak in Kedougou, Southeastern Senegal in 2009–2010. Open Forum Infect. Dis. 2018, 5, ofx259. [Google Scholar] [CrossRef] [PubMed]
- Njenga, M.K.; Nderitu, L.; Ledermann, J.P.; Ndirangu, A.; Logue, C.H.; Kelly, C.H.L.; Sang, R.; Sergon, K.; Breiman, R.; Powers, A.M. Tracking Epidemic Chikungunya Virus into the Indian Ocean from East Africa. J. Gen. Virol. 2008, 89, 2754–2760. [Google Scholar] [CrossRef]
- Gérardin, P.; Guernier, V.; Perrau, J.; Fianu, A.; Le Roux, K.; Grivard, P.; Michault, A.; de Lamballerie, X.; Flahault, A.; Favier, F. Estimating Chikungunya Prevalence in La Réunion Island Outbreak by Serosurveys: Two Methods for Two Critical Times of the Epidemic. BMC Infect. Dis. 2008, 8, 99. [Google Scholar] [CrossRef]
- Kang, H.; Auzenbergs, M.; Clapham, H.; Maure, C.; Kim, J.-H.; Salje, H.; Taylor, C.G.; Lim, A.; Clark, A.; Edmunds, W.J.; et al. Chikungunya Seroprevalence, Force of Infection, and Prevalence of Chronic Disability after Infection in Endemic and Epidemic Settings: A Systematic Review, Meta-Analysis, and Modelling Study. Lancet Infect. Dis. 2024, 24, 488–503. [Google Scholar] [CrossRef]
- Volk, S.M.; Chen, R.; Tsetsarkin, K.A.; Adams, A.P.; Garcia, T.I.; Sall, A.A.; Nasar, F.; Schuh, A.J.; Holmes, E.C.; Higgs, S.; et al. Genome-Scale Phylogenetic Analyses of Chikungunya Virus Reveal Independent Emergences of Recent Epidemics and Various Evolutionary Rates. J. Virol. 2010, 84, 6497–6504. [Google Scholar] [CrossRef] [PubMed]
- Cottis, S.; Blisnick, A.A.; Failloux, A.-B.; Vernick, K.D. Determinants of Chikungunya and O’nyong-Nyong Virus Specificity for Infection of Aedes and Anopheles Mosquito Vectors. Viruses 2023, 15, 589. [Google Scholar] [CrossRef]
- Chen, R.; Puri, V.; Fedorova, N.; Lin, D.; Hari, K.L.; Jain, R.; Rodas, J.D.; Das, S.R.; Shabman, R.S.; Weaver, S.C. Comprehensive Genome Scale Phylogenetic Study Provides New Insights on the Global Expansion of Chikungunya Virus. J. Virol. 2016, 90, 10600–10611. [Google Scholar] [CrossRef] [PubMed]
- Galán-Huerta, K.A.; Rivas-Estilla, A.M.; Fernández-Salas, I.; Farfan-Ale, J.A.; Ramos-Jiménez, J. Chikungunya Virus: A General Overview. Med. Univ. 2015, 17, 175–183. [Google Scholar] [CrossRef]
- de Souza, W.M.; Ribeiro, G.S.; de Lima, S.T.; de Jesus, R.; Moreira, F.R.; Whittaker, C.; Sallum, M.A.M.; Carrington, C.V.; Sabino, E.C.; Kitron, U.; et al. Chikungunya: A Decade of Burden in the Americas. Lancet Reg. Health-Am. 2024, 30, 100673. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; Chen, R.; Sherman, M.B.; Weaver, S.C. Chikungunya Virus: Evolution and Genetic Determinants of Emergence. Curr. Opin. Virol. 2011, 1, 310–317. [Google Scholar] [CrossRef]
- Tsetsarkin, K.A.; Chen, R.; Weaver, S.C. Interspecies Transmission and Chikungunya Virus Emergence. Curr. Opin. Virol. 2016, 16, 143–150. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Di Maio Ferreira, F.C.P.; Da Silva, A.S.V.; Recht, J.; Guaraldo, L.; Moreira, M.E.L.; Siqueira, A.M.D.; Gerardin, P.; Brasil, P. Vertical Transmission of Chikungunya Virus: A Systematic Review. PLoS ONE 2021, 16, e0249166. [Google Scholar]
- Faria, N.R.; Lourenço, J.; Marques de Cerqueira, E.; Maia de Lima, M.; Carlos Junior Alcantara, L. Epidemiology of Chikungunya Virus in Bahia, Brazil, 2014–2015. PLoS Curr. 2016, 8, 1–9. [Google Scholar] [CrossRef]
- Grubaugh, N.D.; Ladner, J.T.; Lemey, P.; Pybus, O.G.; Rambaut, A.; Holmes, E.C.; Andersen, K.G. Tracking Virus Outbreaks in the Twenty-First Century. Nat. Microbiol. 2019, 4, 10–19. [Google Scholar] [CrossRef]
- Wallau, G.L.; Abanda, N.N.; Abbud, A.; Abdella, S.; Abera, A.; Ahuka-Mundeke, S.; Falconi-Agapito, F.; Alagarasu, K.; Ariën, K.K.; Ayres, C.F.J.; et al. Arbovirus Researchers Unite: Expanding Genomic Surveillance for an Urgent Global Need. Lancet Glob. Health 2023, 11, e1501–e1502. [Google Scholar] [CrossRef] [PubMed]
- Zeghbib, S.; Kemenesi, G.; Jakab, F. The Importance of Equally Accessible Genomic Surveillance in the Age of Pandemics. Biol. Futur. 2023, 74, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Naveca, F.G.; Claro, I.; Giovanetti, M.; de Jesus, J.G.; Xavier, J.; Iani, F.C.D.M.; Do Nascimento, V.A.; de Souza, V.C.; Silveira, P.P.; Lourenco, J.; et al. Genomic, Epidemiological and Digital Surveillance of Chikungunya Virus in the Brazilian Amazon. PLoS Negl. Trop. Dis. 2018, 13, e0007065. [Google Scholar] [CrossRef] [PubMed]
- Burgueño, A.; Giovanetti, M.; Fonseca, V.; Morel, N.; Lima, M.; Castro, E.; Guimarães, N.R.; Iani, F.C.M.; Bormida, V.; Cortinas, M.N.; et al. Genomic and Eco-Epidemiological Investigations in Uruguay Reveal Local Chikungunya Virus Transmission Dynamics during Its Expansion across the Americas in 2023. Emerg. Microbes Infect. 2024, 13, 2332672. [Google Scholar] [CrossRef] [PubMed]
- Giovanetti, M.; Vazquez, C.; Lima, M.; Castro, E.; Rojas, A.; Gomez de la Fuente, A.; Aquino, C.; Cantero, C.; Fleitas, F.; Torales, J.; et al. Rapid Epidemic Expansion of Chikungunya Virus East/Central/South African Lineage, Paraguay. Emerg. Infect. Dis. 2023, 9, 1859–1863. [Google Scholar] [CrossRef]
- Huber, J.H.; Childs, M.L.; Caldwell, J.M.; Mordecai, E.A. Seasonal Temperature Variation Influences Climate Suitability for Dengue, Chikungunya, and Zika Transmission. PLoS Negl. Trop. Dis. 2018, 12, e0006451. [Google Scholar] [CrossRef] [PubMed]
- Vazeille, M.; Moutailler, S.; Coudrier, D.; Rousseaux, C.; Khun, H.; Huerre, M.; Thiria, J.; Dehecq, J.S.; Fontenille, D.; Schuffenecker, I.; et al. Two Chikungunya Isolates from the Outbreak of La Reunion (Indian Ocean) Exhibit Different Patterns of Infection in the Mosquito, Aedes Albopictus. PLoS ONE 2007, 2, e1168. [Google Scholar] [CrossRef] [PubMed]
- Yonga, M.G.; Yandai, F.H.; Sadeuh-Mba, S.; Abdallah, A.H.; Ouapi, D.; Gamougam, K.; Abanda, N.N.; Endengue-Zanga, M.C.; Demanou, M.; Njouom, R. Molecular Characterization of Chikungunya Virus from the First Cluster of Patients during the 2020 Outbreak in Chad. Arch Virol. 2022, 167, 1301–1305. [Google Scholar] [CrossRef]
- Tsetsarkin, K.A.; Chen, R.; Yun, R.; Rossi, S.L.; Plante, K.S.; Guerbois, M.; Forrester, N.; Perng, G.C.; Sreekumar, E.; Leal, G.; et al. Multi-Peaked Adaptive Landscape for Chikungunya Virus Evolution Predicts Continued Fitness Optimization in Aedes Albopictus Mosquitoes. Nat. Commun. 2014, 5, 4084. [Google Scholar] [CrossRef]
- Nunes, M.R.T.; Faria, N.R.; de Vasconcelos, J.M.; Golding, N.; Kraemer, M.U.G.; de Oliveira, L.F.; da Silva Azevedo, R.D.S.; da Silva, D.E.A.; da Silva, E.V.P.; da Silva, S.P.; et al. Emergence and Potential for Spread of Chikungunya Virus in Brazil. BMC Med. 2015, 13, 102. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Panella, A.J.; Velez, J.O.; Lambert, A.J.; Campbell, G.L. Chikungunya Virus in US Travelers Returning from India, 2006. Emerg. Infect. Dis. 2007, 13, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; Vanlandingham, D.L.; McGee, C.E.; Higgs, S. A Single Mutation in Chikungunya Virus Affects Vector Specificity and Epidemic Potential. PLoS Pathog. 2007, 3, 1895–1906. [Google Scholar] [CrossRef] [PubMed]
- Mourad, O.; Makhani, L.; Chen, L.H. Chikungunya: An Emerging Public Health Concern. Curr. Infect. Dis. Rep. 2022, 24, 217–228. [Google Scholar] [CrossRef]
- Yergolkar, P.N.; Tandale, B.V.; Arankalle, V.A.; Sathe, P.S.; Sudeep, A.B.; Gandhe, S.S.; Gokhle, M.D.; Jacob, G.P.; Hundekar, S.L.; Mishra, A.C. Chikungunya Outbreaks Caused by African Genotype, India. Emerg. Infect. Dis. 2006, 12, 1580–1583. [Google Scholar] [CrossRef]
- Powers, A.M.; Brault, A.C.; Tesh, R.B.; Weaver, S.C. Re-Emergence of Chikungunya and o’nyong-Nyong Viruses: Evidence for Distinct Geographical Lineages and Distant Evolutionary Relationships. Microbiology 2000, 81, 471–479. [Google Scholar] [CrossRef]
- Weaver, S.C.; Chen, R.; Diallo, M. Chikungunya Virus: Role of Vectors in Emergence from Enzootic Cycles. Annu. Rev. Entomol. 2020, 65, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Valentine, M.J.; Murdock, C.C.; Kelly, P.J. Sylvatic Cycles of Arboviruses in Non-Human Primates. Parasites Vectors 2019, 12, 463. [Google Scholar] [CrossRef]
- Diallo, M.; Thonnon, J.; Traore-Lamizana, M.; Fontenille, D. Vectors of Chikungunya Virus in Senegal: Current Data and Transmission Cycles. Am. J. Trop. Med. Hyg. 1999, 60, 281–286. [Google Scholar] [CrossRef]
- Diallo, D.; Sall, A.A.; Buenemann, M.; Chen, R.; Faye, O.; Diagne, C.T.; Faye, O.; Ba, Y.; Dia, I.; Watts, D.; et al. Landscape Ecology of Sylvatic Chikungunya Virus and Mosquito Vectors in Southeastern Senegal. PLoS Negl. Trop. Dis. 2012, 6, e1649. [Google Scholar] [CrossRef]
- Pialoux, G.; Gaüzère, B.-A.; Jauréguiberry, S.; Strobel, M. Chikungunya, an Epidemic Arbovirosis. Lancet Infect. Dis. 2007, 7, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Richman, R.; Diallo, D.; Diallo, M.; Sall, A.A.; Faye, O.; Diagne, C.T.; Dia, I.; Weaver, S.C.; Hanley, K.A.; Buenemann, M. Ecological Niche Modeling of Aedes Mosquito Vectors of Chikungunya Virus in Southeastern Senegal. Parasites Vectors 2018, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Lecuit, M. Chikungunya Virus and the Global Spread of a Mosquito-Borne Disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Mcintosh, B.M.; Jupp, P.G.; Dos Santos, I. Rural Epidemic of Chikungunya in South Africa with Involvement of Aedes (Diceromyia) Furcifer (Edwards) and Baboons. S. Afr. J. Sci. 1977, 73, 267. [Google Scholar]
- Powell, J.R.; Tabachnick, W.J. History of Domestication and Spread of Aedes Aegypti—A Review. Memórias Inst. Oswaldo Cruz 2013, 108, 11–17. [Google Scholar] [CrossRef]
- Laporta, G.Z.; Potter, A.M.; Oliveira, J.F.A.; Bourke, B.P.; Pecor, D.B.; Linton, Y.M. Global Distribution of Aedes Aegypti and Aedes Albopictus in a Climate Change Scenario of Regional Rivalry. Insects 2023, 14, 49. [Google Scholar] [CrossRef]
- Pfeffer, M.; Zöller, G.; Essbauer, S.; Tomaso, H.; Behrens-Riha, N.; Löscher, T.; Dobler, G. Clinical and Virological Characterization of Imported Cases of Chikungunya Fever. Wien. Klin. Wochenschr. 2008, 120, 95–100. [Google Scholar] [CrossRef]
- Longbottom, J.; Walekhwa, A.W.; Mwingira, V.; Kijanga, O.; Mramba, F.; Lord, J.S. Aedes Albopictus Invasion across Africa: The Time Is Now for Cross-Country Collaboration and Control. Lancet Glob. Health 2023, 11, e623–e628. [Google Scholar] [CrossRef]
- Cornel, A.J.; Hunt, R.H. Aedes Albopictus in Africa? First Records of Live Specimens in Imported Tires in Capetown. J. Am. Mosq. Control Assoc. 1991, 7, 107–108. [Google Scholar]
- Bonizzoni, M.; Gasperi, G.; Chen, X.; James, A.A. The Invasive Mosquito Species Aedes Albopictus: Current Knowledge and Future Perspectives. Trends Parasitol. 2013, 29, 460–468. [Google Scholar] [CrossRef]
- Weetman, D.; Kamgang, B.; Badolo, A.; Moyes, C.L.; Shearer, F.M.; Coulibaly, M.; Pinto, J.; Lambrechts, L.; McCall, P.J. Aedes Mosquitoes and Aedes-Borne Arboviruses in Africa: Current and Future Threats. Int. J. Environ. Res. Public Health 2018, 15, 220. [Google Scholar] [CrossRef]
- Tajudeen, Y.A.; Oladunjoye, I.O.; Bajinka, O.; Oladipo, H.J. Zoonotic Spillover in an Era of Rapid Deforestation of Tropical Areas and Unprecedented Wildlife Trafficking: Into the Wild. Challenges 2022, 13, 41. [Google Scholar] [CrossRef]
- Pfeffer, M.; Dobler, G. Emergence of zoonotic arboviruses by animal trade and migration. Parasites Vectors 2010, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Tajudeen, Y.A.; Oladipo, H.J.; Oladunjoye, I.O.; Mustapha, M.O.; Mustapha, S.T.; Abdullahi, A.A.; Yusuf, R.O.; Abimbola, S.O.; Adebayo, A.O.; Ikebuaso, J.G.; et al. Preventing the Next Pandemic through a Planetary Health Approach: A Focus on Key Drivers of Zoonosis. Challenges 2022, 13, 50. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.; Shearer, F.M.; Brady, O.J.; Messina, J.P.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; et al. The Global Compendium of Aedes aegypti and Ae. albopictus Occurrence. Sci. Data 2015, 2, 150035. [Google Scholar] [CrossRef] [PubMed]
- Giovanetti, M.; Alcantara, L.C.J.; Dorea, A.S.; Ferreira, Q.R.; Marques, W.D.A.; Junior Franca de Barros, J.; Adelino, T.E.R.; Tosta, S.; Fritsch, H.; Iani, F.C.D.M.; et al. Promoting Responsible Research and Innovation (RRI) During Brazilian Activities of Genomic and Epidemiological Surveillance of Arboviruses. Front. Public Health 2021, 9, 693743. [Google Scholar] [CrossRef] [PubMed]
- Althouse, B.M.; Guerbois, M.; Cummings, D.A.T.; Diop, O.M.; Faye, O.; Faye, A.; Diallo, D.; Sadio, B.D.; Sow, A.; Faye, O.; et al. Role of Monkeys in the Sylvatic Cycle of Chikungunya Virus in Senegal. Nat. Commun. 2018, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Ly, H. Ixchiq (VLA1553): The First FDA-Approved Vaccine to Prevent Disease Caused by Chikungunya Virus Infection. Virulence 2024, 15, 2301573. [Google Scholar] [CrossRef]
- Braack, L.; Wulandhari, S.A.; Chanda, E.; Fouque, F.; Merle, C.S.; Nwangwu, U.; Velayudhan, R.; Venter, M.; Yahouedo, A.G.; Lines, J.; et al. Developing African Arbovirus Networks and Capacity Strengthening in Arbovirus Surveillance and Response: Findings from a Virtual Workshop. Parasits Vectors 2023, 16, 129. [Google Scholar] [CrossRef]
- Giovanetti, M.; Salgado, A.; De Souza Fonseca, V.; Tosta, F.D.O.; Xavier, J.; De Jesus, J.G.; Iani, F.C.M.; Adelino, T.E.R.; Barreto, F.K.; Faria, N.R.; et al. Pan-Genomics of Virus and Its Applications. Pan-Genom. Appl. Chall. Future Prospect. 2020, 237–250. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Padane, A.; Tegally, H.; Ramphal, Y.; Seyni, N.; Sarr, M.; Diop, M.M.; Diedhiou, C.K.; Mboup, A.; Diouf, N.D.; Souaré, A.; et al. An Emerging Clade of Chikungunya West African Genotype Discovered in Real-Time during 2023 Outbreak in Senegal. medRxiv 2023. [Google Scholar] [CrossRef]
- Wilkinson, E.; Giovanetti, M.; Tegally, H.; San, J.E.; Lessells, R.; Cuadros, D.; Martin, D.P.; Rasmussen, D.A.; Zekri, A.-R.N.; Sangare, A.K.; et al. A Year of Genomic Surveillance Reveals How the SARS-CoV-2 Pandemic Unfolded in Africa. Science 2021, 374, 423–431. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramphal, Y.; Tegally, H.; San, J.E.; Reichmuth, M.L.; Hofstra, M.; Wilkinson, E.; Baxter, C.; CLIMADE Consortium; de Oliveira, T.; Moir, M. Understanding the Transmission Dynamics of the Chikungunya Virus in Africa. Pathogens 2024, 13, 605. https://doi.org/10.3390/pathogens13070605
Ramphal Y, Tegally H, San JE, Reichmuth ML, Hofstra M, Wilkinson E, Baxter C, CLIMADE Consortium, de Oliveira T, Moir M. Understanding the Transmission Dynamics of the Chikungunya Virus in Africa. Pathogens. 2024; 13(7):605. https://doi.org/10.3390/pathogens13070605
Chicago/Turabian StyleRamphal, Yajna, Houriiyah Tegally, James Emmanuel San, Martina Larissa Reichmuth, Marije Hofstra, Eduan Wilkinson, Cheryl Baxter, CLIMADE Consortium, Tulio de Oliveira, and Monika Moir. 2024. "Understanding the Transmission Dynamics of the Chikungunya Virus in Africa" Pathogens 13, no. 7: 605. https://doi.org/10.3390/pathogens13070605





