Interaction between Intestinal Parasites and the Gut Microbiota: Implications for the Intestinal Immune Response and Host Defence
Abstract
:1. Introduction
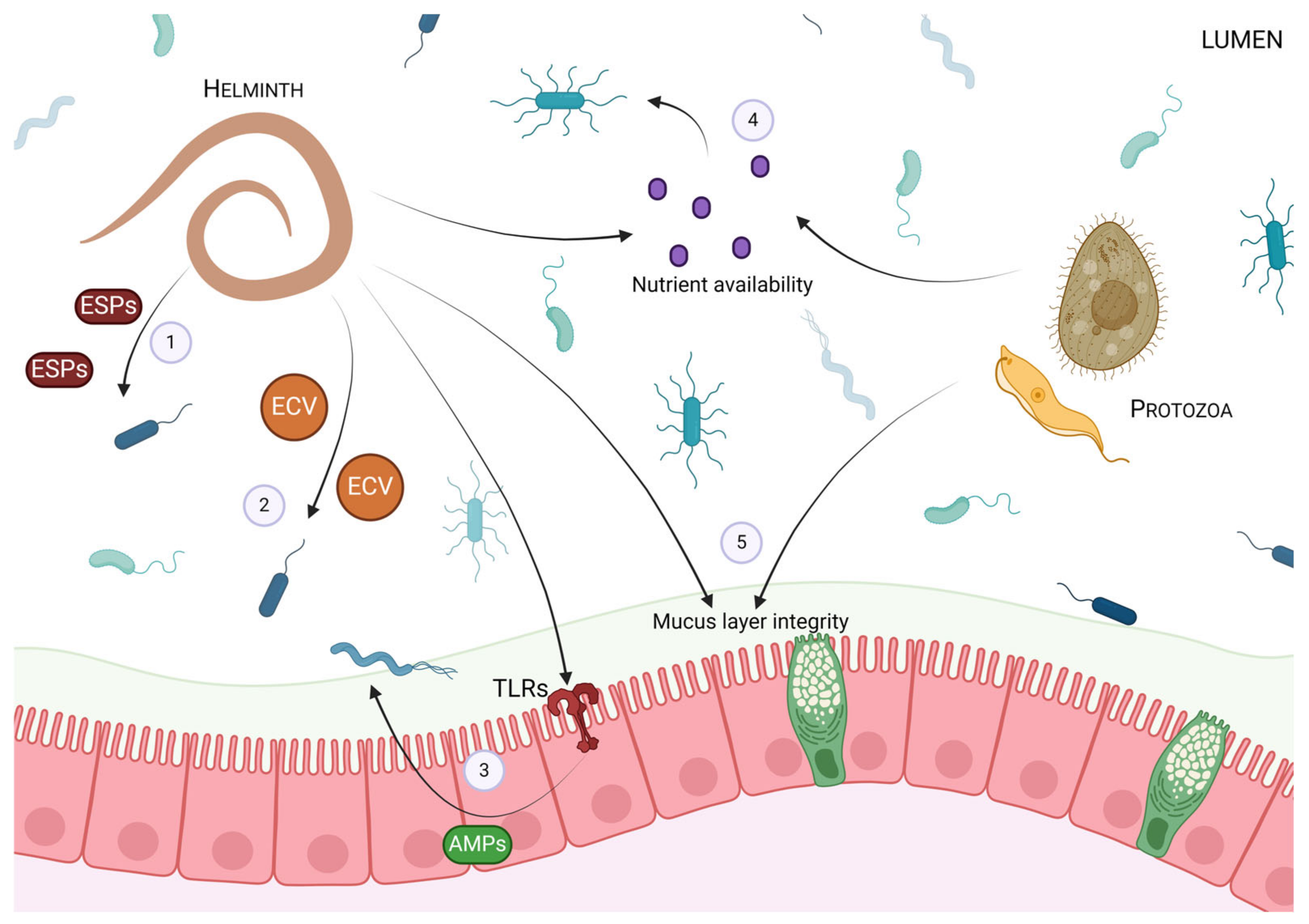
2. The Impact of Intestinal Parasites on the Gut Microbiota
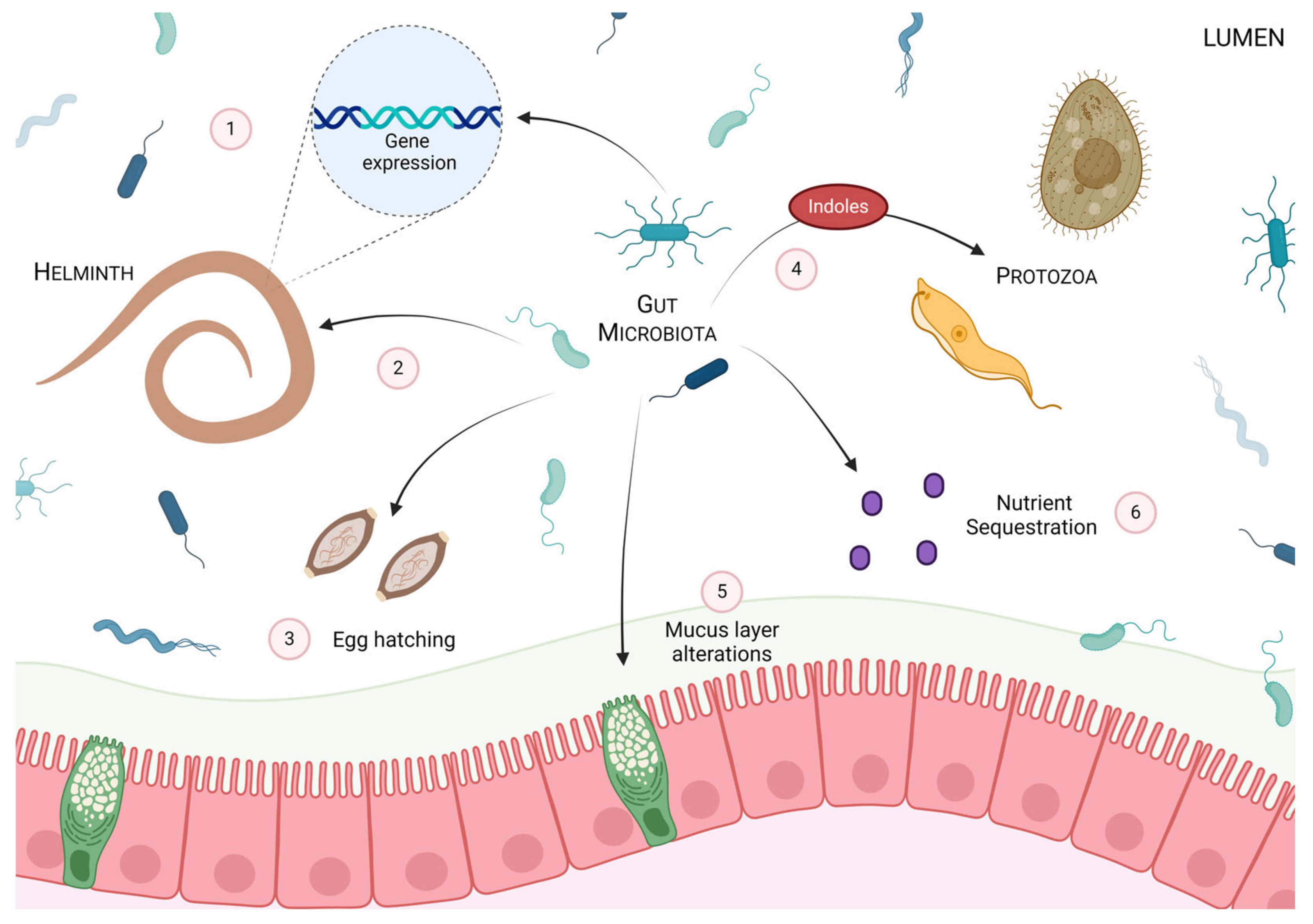
3. The Impact of the Gut Microbiota on Intestinal Parasites
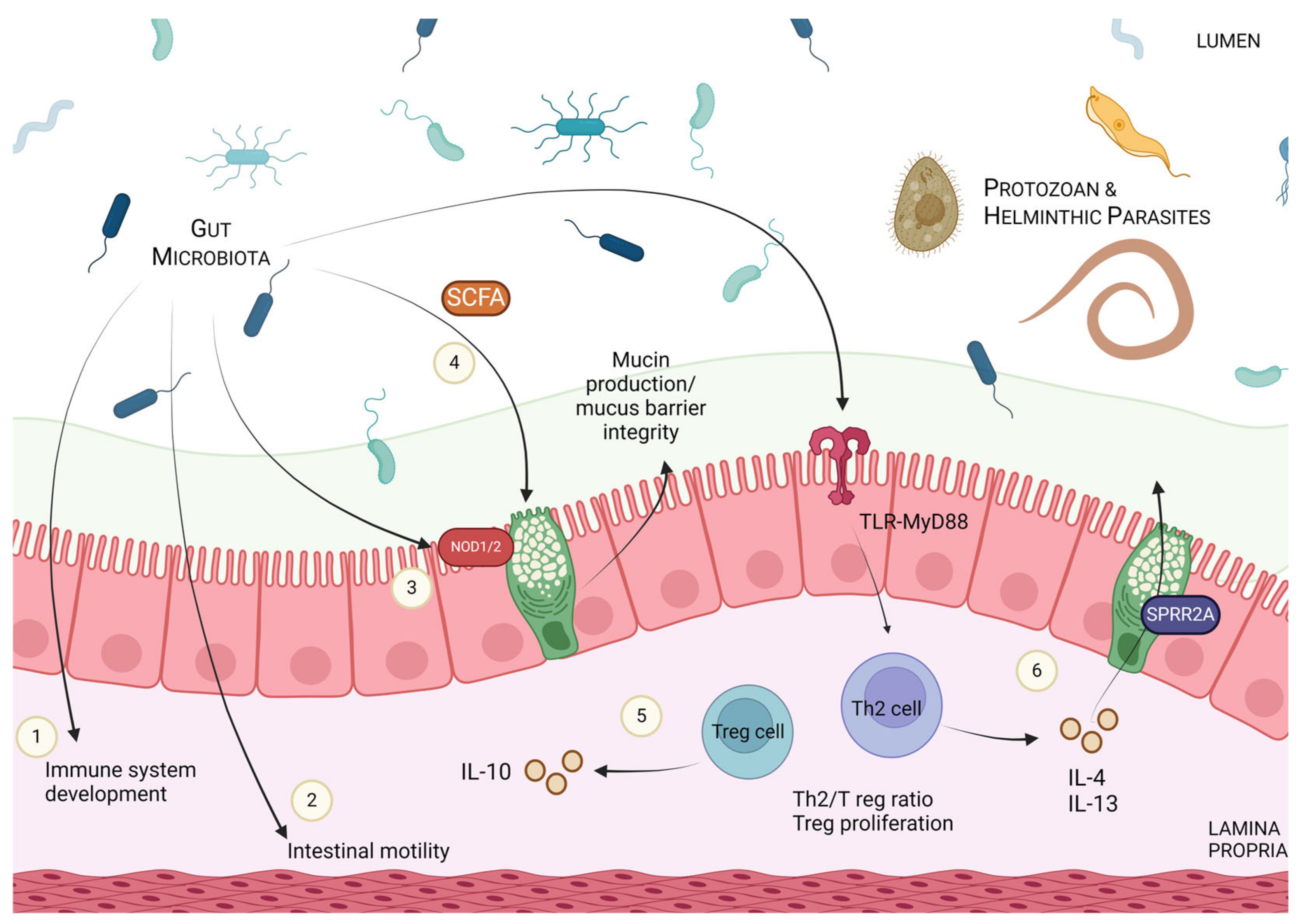
4. Modulation of Host Immune Responses and Parasitic Defence via Microbial Manipulation
5. Clinical Implications of the Host–Parasite–Microbiota Axis
5.1. Microbial Manipulation in Parasitic Infection
5.2. Utilization of Intestinal Parasites in Immunoregulatory Conditions
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Soil-Transmitted Helminth Infections. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 23 June 2024).
- Sitotaw, B.; Mekuriaw, H.; Damtie, D. Prevalence of Intestinal Parasitic Infections and Associated Risk Factors among Jawi Primary School Children, Jawi Town, North-West Ethiopia. BMC Infect. Dis. 2019, 19, 341. [Google Scholar] [CrossRef] [PubMed]
- Alum, A.; Rubino, J.R.; Ijaz, M.K. The Global War against Intestinal Parasites—Should We Use a Holistic Approach? Int. J. Infect. Dis. 2010, 14, e732–e738. [Google Scholar] [CrossRef] [PubMed]
- WHO. Expert Committee on Prevention and Control of Intestinal Parasitic Infections. In Prevention and Control of Intestinal Parasitic Infections: Report of a WHO Expert Committee; WHO: Geneva, Switzerland, 1987; Volume 86. [Google Scholar]
- Borges, R.M. Co-Niche Construction between Hosts and Symbionts: Ideas and Evidence. J. Genet. 2017, 96, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Bakker, M.; Helm, B. The Influence of Biological Rhythms on Host–Parasite Interactions. Trends Ecol. Evol. 2015, 30, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Sorci, G.; Møller, A.P.; Boulinier, T. Genetics of Host-Parasite Interactions. Trends Ecol. Evol. 1997, 12, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Méthot, P.O.; Alizon, S. What Is a Pathogen? Toward a Process View of Host-Parasite Interactions. Virulence 2014, 5, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Mideo, N.; Reece, S.E. Plasticity in Parasite Phenotypes: Evolutionary and Ecological Implications for Disease. Future Microbiol. 2012, 7, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Drew, G.C.; Stevens, E.J.; King, K.C. Microbial Evolution and Transitions along the Parasite–Mutualist Continuum. Nat. Rev. Microbiol. 2021, 19, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Wolinska, J.; King, K.C. Environment Can Alter Selection in Host-Parasite Interactions. Trends Parasitol. 2009, 25, 236–244. [Google Scholar] [CrossRef]
- Laine, A.L. Evolution of Host Resistance: Looking for Coevolutionary Hotspots at Small Spatial Scales. Proc. R. Soc. B Biol. Sci. 2005, 273, 267–273. [Google Scholar] [CrossRef]
- Gandon, S.; van Zandt, P.A. Local Adaptation and Host–Parasite Interactions. Trends Ecol. Evol. 1998, 13, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Penczykowski, R.M.; Laine, A.L.; Koskella, B. Understanding the Ecology and Evolution of Host–Parasite Interactions across Scales. Evol. Appl. 2016, 9, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Rafaluk-Mohr, C.; Gerth, M.; Sealey, J.E.; Ekroth, A.K.; Aboobaker, A.A.; Kloock, A.; King, K.C. Microbial Protection Favors Parasite Tolerance and Alters Host-Parasite Coevolutionary Dynamics. Curr. Biol. 2022, 32, 1593–1598.e3. [Google Scholar] [CrossRef] [PubMed]
- McCullers, J.A. The Co-Pathogenesis of Influenza Viruses with Bacteria in the Lung. Nat. Rev. Microbiol. 2014, 12, 252–262. [Google Scholar] [CrossRef]
- Rook, G.A.W. Hygiene Hypothesis and Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2012, 42, 5–15. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning from Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef] [PubMed]
- Sansonetti, P.J. To Be or Not to Be a Pathogen: That Is the Mucosally Relevant Question. Mucosal Immunol. 2011, 4, 8–14. [Google Scholar] [CrossRef]
- Koskella, B.; Giraud, T.; Hood, M.E. Pathogen Relatedness Affects the Prevalence of Within-Host Competition. Am. Nat. 2006, 168, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Susi, H.; Vale, P.F.; Laine, A.L. Host Genotype and Coinfection Modify the Relationship of within and between Host Transmission. Am. Nat. 2015, 186, 252–263. [Google Scholar] [CrossRef]
- Susi, H.; Barrès, B.; Vale, P.F.; Laine, A.L. Co-Infection Alters Population Dynamics of Infectious Disease. Nat. Commun. 2015, 6, 5975. [Google Scholar] [CrossRef]
- Brosschot, T.P.; Reynolds, L.A. The Impact of a Helminth-Modified Microbiome on Host Immunity. Mucosal Immunol. 2018, 11, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Rausch, S.; Midha, A.; Kuhring, M.; Affinass, N.; Radonic, A.; Kühl, A.A.; Bleich, A.; Renard, B.Y.; Hartmann, S. Parasitic Nematodes Exert Antimicrobial Activity and Benefit from Microbiota-Driven Support for Host Immune Regulation. Front. Immunol. 2018, 9, 408870. [Google Scholar] [CrossRef] [PubMed]
- Midha, A.; Janek, K.; Niewienda, A.; Henklein, P.; Guenther, S.; Serra, D.O.; Schlosser, J.; Hengge, R.; Hartmann, S. The Intestinal Roundworm Ascaris Suum Releases Antimicrobial Factors Which Interfere with Bacterial Growth and Biofilm Formation. Front. Cell. Infect. Microbiol. 2018, 8, 392728. [Google Scholar] [CrossRef]
- Kato, Y.; Komatsu, S. ASABF, a Novel Cysteine-Rich Antibacterial Peptide Isolated from the Nematode Ascaris Suum: Purification, primary structure, and molecular cloning of CDNA. J. Biol. Chem. 1996, 271, 30493–30498. [Google Scholar] [CrossRef]
- Abner, S.R.; Parthasarathy, G.; Hill, D.E.; Mansfield, L.S. Trichuris Suis: Detection of Antibacterial Activity in Excretory-Secretory Products from Adults. Exp. Parasitol. 2001, 99, 26–36. [Google Scholar] [CrossRef]
- Rooney, J.; Williams, T.L.; Northcote, H.M.; Frankl, F.E.K.; Price, D.R.G.; Nisbet, A.J.; Morphew, R.M.; Cantacessi, C. Excretory-Secretory Products from the Brown Stomach Worm, Teladorsagia circumcincta, Exert Antimicrobial Activity in in Vitro Growth Assays. Parasites Vectors 2022, 15, 354. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.; Rivera-de-Torre, E.; Li, R.; Mclean, K.; Price, D.R.G.; Nisbet, A.J.; Laustsen, A.H.; Jenkins, T.P.; Hofmann, A.; Bakshi, S.; et al. Structural and Functional Analyses of Nematode-Derived Antimicrobial Peptides Support the Occurrence of Direct Mechanisms of Worm-Microbiota Interactions. Comput. Struct. Biotechnol. J. 2024, 23, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the Microbiota and the Immune System. Science 2012, 336, 1268. [Google Scholar] [CrossRef]
- Ubeda, C.; Djukovic, A.; Isaac, S. Roles of the Intestinal Microbiota in Pathogen Protection. Clin. Transl. Immunol. 2017, 6, e128. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.U.; Chen, G.Y.; Núñez, G. Role of the Gut Microbiota in Immunity and Inflammatory Disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Houlden, A.; Hayes, K.S.; Bancroft, A.J.; Worthington, J.J.; Wang, P.; Grencis, R.K.; Roberts, I.S. Chronic Trichuris Muris Infection in C57BL/6 Mice Causes Significant Changes in Host Microbiota and Metabolome: Effects Reversed by Pathogen Clearance. PLoS ONE 2015, 10, e0125945. [Google Scholar] [CrossRef]
- Von Huth, S.; Thingholm, L.B.; Kofoed, P.E.; Bang, C.; Rühlemann, M.C.; Franke, A.; Holmskov, U. Intestinal Protozoan Infections Shape Fecal Bacterial Microbiota in Children from Guinea-Bissau. PLoS Negl. Trop. Dis. 2021, 15, e0009232. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.B.; Sorobetea, D.; Kiilerich, P.; Ramayo-Caldas, Y.; Estellé, J.; Ma, T.; Madsen, L.; Kristiansen, K.; Svensson-Frej, M. Chronic Trichuris Muris Infection Decreases Diversity of the Intestinal Microbiota and Concomitantly Increases the Abundance of Lactobacilli. PLoS ONE 2015, 10, e0125495. [Google Scholar] [CrossRef] [PubMed]
- Walk, S.T.; Blum, A.M.; Ewing, S.A.S.; Weinstock, J.V.; Young, V.B. Alteration of the Murine Gut Microbiota during Infection with the Parasitic Helminth Heligmosomoides polygyrus. Inflamm. Bowel Dis. 2010, 16, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, Y.; Vallee, I.; Karadjian, G.; Liu, M.; Liu, X. Lentinan -Triggered Butyrate-Producing Bacteria Drive the Expulsion of the Intestinal Helminth Trichinella spiralis in Mice. Front. Immunol. 2022, 13, 926765. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, X.; Ding, J.; Zhang, L.; Yang, Y.; Wang, X.; Yang, Y.; Liu, M. Lentinan Improved the Efficacy of Vaccine against Trichinella spiralis in an NLRP3 Dependent Manner. PLoS Negl. Trop. Dis. 2020, 14, e0008632. [Google Scholar] [CrossRef]
- Barash, N.R.; Maloney, J.G.; Singer, S.M.; Dawson, S.C. Giardia Alters Commensal Microbial Diversity throughout the Murine Gut. Infect. Immun. 2017, 85, e00948-16. [Google Scholar] [CrossRef]
- Lee, S.C.; Tang, M.S.; Lim, Y.A.L.; Choy, S.H.; Kurtz, Z.D.; Cox, L.M.; Gundra, U.M.; Cho, I.; Bonneau, R.; Blaser, M.J.; et al. Helminth Colonization Is Associated with Increased Diversity of the Gut Microbiota. PLoS Negl. Trop. Dis. 2014, 8, e2880. [Google Scholar] [CrossRef]
- Jenkins, T.P.; Rathnayaka, Y.; Perera, P.K.; Peachey, L.E.; Nolan, M.J.; Krause, L.; Rajakaruna, R.S.; Cantacessi, C. Infections by Human Gastrointestinal Helminths Are Associated with Changes in Faecal Microbiota Diversity and Composition. PLoS ONE 2017, 12, e0184719. [Google Scholar] [CrossRef]
- Morton, E.R.; Lynch, J.; Froment, A.; Lafosse, S.; Heyer, E.; Przeworski, M.; Blekhman, R.; Ségurel, L. Variation in Rural African Gut Microbiota Is Strongly Correlated with Colonization by Entamoeba and Subsistence. PLOS Genet. 2015, 11, e1005658. [Google Scholar] [CrossRef]
- Manivel, G.; Meyyazhagan, A.; Durairaj, D.R.; Piramanayagam, S. Genome-Wide Analysis of Excretory/Secretory Proteins in Trypanosoma brucei brucei: Insights into Functional Characteristics and Identification of Potential Targets by Immunoinformatics Approach. Genomics 2019, 111, 1124–1133. [Google Scholar] [CrossRef]
- Lightowlers, M.W.; Rickard, M.D. Excretory-Secretory Products of Helminth Parasites: Effects on Host Immune Responses. Parasitology 1988, 96, S123–S166. [Google Scholar] [CrossRef] [PubMed]
- Harnett, W. Secretory Products of Helminth Parasites as Immunomodulators. Mol. Biochem. Parasitol. 2014, 195, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Maruszewska-Cheruiyot, M.; Szewczak, L.; Krawczak-Wójcik, K.; Głaczyńska, M.; Donskow-Łysoniewska, K. The Production of Excretory-Secretory Molecules from Heligmosomoides polygyrus bakeri Fourth Stage Larvae Varies between Mixed and Single Sex Cultures. Parasit. Vectors 2021, 14, 1–10. [Google Scholar] [CrossRef]
- McSorley, H.J.; Hewitson, J.P.; Maizels, R.M. Immunomodulation by Helminth Parasites: Defining Mechanisms and Mediators. Int. J. Parasitol. 2013, 43, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.H.; Coakley, G.; Simbari, F.; McSorley, H.J.; Quintana, J.F.; Le Bihan, T.; Kumar, S.; Abreu-Goodger, C.; Lear, M.; Harcus, Y.; et al. Exosomes Secreted by Nematode Parasites Transfer Small RNAs to Mammalian Cells and Modulate Innate Immunity. Nat. Commun. 2014, 5, 5488. [Google Scholar] [CrossRef]
- Hewitson, J.P.; Harcus, Y.; Murray, J.; van Agtmaal, M.; Filbey, K.J.; Grainger, J.R.; Bridgett, S.; Blaxter, M.L.; Ashton, P.D.; Ashford, D.A.; et al. Proteomic Analysis of Secretory Products from the Model Gastrointestinal Nematode Heligmosomoides polygyrus Reveals Dominance of Venom Allergen-Like (VAL) Proteins. J. Proteom. 2011, 74, 1573. [Google Scholar] [CrossRef]
- Harris, J.B.; Podolsky, M.J.; Bhuiyan, T.R.; Chowdhury, F.; Khan, A.I.; LaRocque, R.C.; Logvinenko, T.; Kendall, J.; Faruque, A.S.G.; Nagler, C.R.; et al. Immunologic Responses to Vibrio cholerae in Patients Co-Infected with Intestinal Parasites in Bangladesh. PLoS Negl. Trop. Dis. 2009, 3, e403. [Google Scholar] [CrossRef]
- Venugopal, P.G.; Nutman, T.B.; Semnani, R.T. Activation and Regulation of Toll-Like Receptors (TLRs) by Helminth Parasites. Immunol. Res. 2009, 43, 252. [Google Scholar] [CrossRef]
- Sun, S.; Wang, X.; Wu, X.; Zhao, Y.; Wang, F.; Liu, X.; Song, Y.; Wu, Z.; Liu, M. Toll-like Receptor Activation by Helminths or Helminth Products to Alleviate Inflammatory Bowel Disease. Parasit. Vectors 2011, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Kosik-Bogacka, D.I.; Wojtkowiak-Giera, A.; Kolasa, A.; Salamatin, R.; Jagodzinski, P.P.; Wandurska-Nowak, E. Hymenolepis diminuta: Analysis of the Expression of Toll-like Receptor Genes (TLR2 and TLR4) in the Small and Large Intestines of Rats. Exp. Parasitol. 2012, 130, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, A.; Borji, H.; Haghparast, A. Interaction Between Helminths and Toll-Like Receptors: Possibilities and Potentials for Asthma Therapy. Int. Rev. Immunol. 2016, 35, 219–248. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.; Northcote, H.M.; Williams, T.L.; Cortés, A.; Cantacessi, C.; Morphew, R.M. Parasitic Helminths and the Host Microbiome—A Missing ‘Extracellular Vesicle-Sized’ Link? Trends Parasitol. 2022, 38, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.J.; Theodoropoulos, G.; Carrington, S.D.; Corfield, A.P. The Role of Mucins in Host-Parasite Interactions. Part I-Protozoan Parasites. Parasitol. Today 2000, 16, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Khan, W.; Kim, J.J.; Khan, W.I. Goblet Cells and Mucins: Role in Innate Defense in Enteric Infections. Pathogens 2013, 2, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.S.; Bancroft, A.J.; Goldrick, M.; Portsmouth, C.; Roberts, I.S.; Grencis, R.K. Exploitation of the Intestinal Microflora by the Parasitic Nematode Trichuris muris. Science 2010, 328, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Funkhouser-Jones, L.J.; Xu, R.; Wilke, G.; Stappenbeck, T.S.; Baldridge, M.T.; David, L.; Correspondence, S.; Fu, Y.; Schriefer, L.A.; Makimaa, H.; et al. Microbiota-Produced Indole Metabolites Disrupt Mitochondrial Function and Inhibit Cryptosporidium parvum Growth. Cell Rep. 2023, 42, 112680. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, Y.; Wang, J.; Wang, X.; Tang, B.; Liu, M.; Liu, X. β-Glucan-Triggered Akkermansia muciniphila Expansion Facilitates the Expulsion of Intestinal Helminth via TLR2 in Mice. Carbohydr. Polym. 2022, 275, 118719. [Google Scholar] [CrossRef]
- Gaboriaud, P.; Sadrin, G.; Guitton, E.; Fort, G.; Niepceron, A.; Lallier, N.; Rossignol, C.; Larcher, T.; Sausset, A.; Guabiraba, R.; et al. The Absence of Gut Microbiota Alters the Development of the Apicomplexan Parasite Eimeria tenella. Front. Cell. Infect. Microbiol. 2020, 10, 632556. [Google Scholar] [CrossRef]
- Alam, M.Z.; Dey, A.R.; Rony, S.A.; Parvin, S.; Akter, S. Phylogenetic Analysis of Eimeria tenella Isolated from the Litter of Different Chicken Farms in Mymensingh, Bangladesh. Vet. Med. Sci. 2022, 8, 1563. [Google Scholar] [CrossRef] [PubMed]
- Beyhan, Y.E.; Yıldız, M.R. Microbiota and Parasite Relationship. Diagn. Microbiol. Infect. Dis. 2023, 106, 115954. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.L.; Gilchrist, C.A.; Lynn, T.C.; Petri, W.A. Parasitic Protozoa and Interactions with the Host Intestinal Microbiota. Infect. Immun. 2017, 85, e00101-17. [Google Scholar] [CrossRef] [PubMed]
- Rajamanikam, A.; Isa, M.N.M.; Samudi, C.; Devaraj, S.; Govind, S.K. Gut Bacteria Influence Blastocystis Sp. Phenotypes and May Trigger Pathogenicity. PLoS Negl. Trop. Dis. 2023, 17, e0011170. [Google Scholar] [CrossRef] [PubMed]
- Shute, A.; Wang, A.; Jayme, T.S.; Strous, M.; McCoy, K.D.; Buret, A.G.; McKay, D.M. Worm Expulsion Is Independent of Alterations in Composition of the Colonic Bacteria That Occur during Experimental Hymenolepis diminuta—Infection in Mice. Gut Microbes 2020, 11, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Sulima-Celińska, A.; Kalinowska, A.; Młocicki, D. The Tapeworm Hymenolepis diminuta as an Important Model Organism in the Experimental Parasitology of the 21st Century. Pathogens 2022, 11, 1439. [Google Scholar] [CrossRef] [PubMed]
- Mckay, D.M.; Khan, W.I. STAT-6 Is an Absolute Requirement for Murine Rejection of Hymenolepis diminuta. J. Parasitol. 2003, 89, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.A.; Smith, K.A.; Filbey, K.J.; Harcus, Y.; Hewitson, J.P.; Redpath, S.A.; Valdez, Y.; Yebra, M.J.; Brett Finlay, B.; Maizels, R.M. Commensal-Pathogen Interactions in the Intestinal Tract: Lactobacilli Promote Infection with, and Are Promoted by, Helminth Parasites. Gut Microbes 2014, 5, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Su, L.; Li, Y.; Long, S.R.; Chang, J.; Zhang, W.; Walker, W.A.; Xavier, R.J.; Cherayil, B.J.; Shi, H.N. Helminth-Induced Alterations of the Gut Microbiota Exacerbate Bacterial Colitis. Mucosal Immunol. 2017, 11, 144–157. [Google Scholar] [CrossRef]
- Moyat, M.; Lebon, L.; Perdijk, O.; Wickramasinghe, L.C.; Zaiss, M.M.; Mosconi, I.; Volpe, B.; Guenat, N.; Shah, K.; Coakley, G.; et al. Microbial Regulation of Intestinal Motility Provides Resistance against Helminth Infection. Mucosal Immunol. 2022, 15, 1283–1295. [Google Scholar] [CrossRef]
- Robertson, L.J. Parasites in Food: From a Neglected Position to an Emerging Issue. Adv. Food Nutr. Res. 2018, 86, 71–113. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sharma, S. Trichinella spiralis Infection. In Textbook of Medical Parasitology; StatPearls Publishing [Internet]: Treasure Island, FL, USA, 2023. [Google Scholar] [CrossRef]
- Coakley, G.; Harris, N.L. The Intestinal Epithelium at the Forefront of Host–Helminth Interactions. Trends Parasitol. 2020, 36, 761–772. [Google Scholar] [CrossRef] [PubMed]
- McClemens, J.; Kim, J.J.; Wang, H.; Mao, Y.-K.K.; Collins, M.; Kunze, W.; Bienenstock, J.; Forsythe, P.; Khan, W.I. Lactobacillus rhamnosus Ingestion Promotes Innate Host Defense in an Enteric Parasitic Infection. Clin. Vaccine Immunol. 2013, 20, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kim, J.J.; Denou, E.; Gallagher, A.; Thornton, D.J.; Sharif Shajib, M.; Xia, L.; Schertzer, J.D.; Grencis, R.K.; Philpott, D.J.; et al. New Role of Nod Proteins in Regulation of Intestinal Goblet Cell Response in the Context of Innate Host Defense in an Enteric Parasite Infection. Infect. Immun. 2016, 84, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Leon-Coria, A.; Kumar, M.; Chadee, K. The Delicate Balance between Entamoeba Histolytica, Mucus and Microbiota. Gut Microbes 2020, 11, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Leon-Coria, A.; Kumar, M.; Moreau, F.; Chadee, K. Defining Cooperative Roles for Colonic Microbiota and Muc2 Mucin in Mediating Innate Host Defense against Entamoeba histolytica. PLOS Pathog. 2018, 14, e1007466. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Hooper, L.V. Antimicrobial Defense of the Intestine. Immunity 2015, 42, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.L.; Hooper, L.V. Epithelial Antimicrobial Defence of the Skin and Intestine. Nat. Rev. Immunol. 2012, 12, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, C.; Sifuentes-Dominguez, L.; Zarek, C.M.; Propheter, D.C.; Kuang, Z.; Wang, Y.; Pendse, M.; Ruhn, K.A.; Hassell, B.; et al. Small Proline-Rich Protein 2A Is a Gut Bactericidal Protein Deployed during Helminth Infection. Science 2021, 374, eabe6723. [Google Scholar] [CrossRef]
- Ramanan, D.; Bowcutt, R.; Lee, S.C.; Tang, M.S.; Kurtz, Z.D.; Ding, Y.; Honda, K.; Gause, W.C.; Blaser, M.J.; Bonneau, R.A.; et al. Helminth Infection Promotes Colonization Resistance via Type 2 Immunity. Science 2016, 352, 608–612. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Jin, T.; Yi, C.; Ocansey, D.K.W.; Mao, F. The Role of NOD2 in Intestinal Immune Response and Microbiota Modulation: A Therapeutic Target in Inflammatory Bowel Disease. Int. Immunopharmacol. 2022, 113, 109466. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, D.; Tang, M.S.; Bowcutt, R.; Loke, P.; Cadwell, K. Bacterial Sensor Nod2 Prevents Inflammation of the Small Intestine by Restricting the Expansion of the Commensal Bacteroides vulgatus. Immunity 2014, 41, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Pullan, R.L.; Smith, J.L.; Jasrasaria, R.; Brooker, S.J. Global Numbers of Infection and Disease Burden of Soil Transmitted Helminth Infections in 2010. Parasites Vectors 2014, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Diemert, D. Update on Prevention and Treatment of Intestinal Helminth Infections. Curr. Infect. Dis. Rep. 2015, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, D.H.; Hotez, P.J.; Fenwick, A. “Rapid-Impact Interventions”: How a Policy of Integrated Control for Africa’s Neglected Tropical Diseases Could Benefit the Poor. PLoS Med. 2005, 2, e336. [Google Scholar] [CrossRef] [PubMed]
- Horton, J. Global Anthelmintic Chemotherapy Programs: Learning from History. Trends Parasitol. 2003, 19, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Farthing, M.J.G. Treatment Options for the Eradication of Intestinal Protozoa. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Sarjapuram, N.; Mekala, N.; Singh, M.; Tatu, U. The Potential of Lactobacillus casei and Entercoccus faecium Combination as a Preventive Probiotic Against Entamoeba. Probiotics Antimicrob. Proteins 2017, 9, 142–149. [Google Scholar] [CrossRef]
- Shukla, G.; Devi, P.; Sehgal, R. Effect of Lactobacillus casei as a Probiotic on Modulation of Giardiasis. Dig. Dis. Sci. 2008, 53, 2671–2679. [Google Scholar] [CrossRef]
- Shukla, G.; Goyal, N.; Tiwari, R.P. Lactobacillus rhamnosus GG as an Effective Probiotic for Murine Giardiasis. Interdiscip. Perspect. Infect. Dis. 2011, 2011, 795219. [Google Scholar] [CrossRef]
- Dinleyici, E.C.; Eren, M.; Dogan, N.; Reyhanioglu, S.; Yargic, Z.A.; Vandenplas, Y. Clinical Efficacy of Saccharomyces boulardii or Metronidazole in Symptomatic Children with Blastocystis hominis Infection. Parasitol. Res. 2011, 108, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Eren, M.; Dinleyici, E.C.; Vandenplas, Y. Clinical Efficacy Comparison of Saccharomyces boulardii and Yogurt Fluid in Acute Non-Bloody Diarrhea in Children: A Randomized, Controlled, Open Label Study. Am. J. Trop. Med. Hyg. 2010, 82, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Mansour-Ghanaei, F.; Dehbashi, N.; Yazdanparast, K.; Shafaghi, A. Efficacy of Saccharomyces boulardii with Antibiotics in Acute Amoebiasis. World J. Gastroenterol. 2003, 9, 1832. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Sonnenburg, J.L. The Ancestral and Industrialized Gut Microbiota and Implications for Human Health. Nat. Rev. Microbiol. 2019, 17, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Cortés, A.; Rooney, J.; Bartley, D.J.; Nisbet, A.J.; Cantacessi, C. Helminths, Hosts, and Their Microbiota: New Avenues for Managing Gastrointestinal Helminthiases in Ruminants. Expert Rev. Anti. Infect. Ther. 2020, 18, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Hodžić, A.; Dheilly, N.M.; Cabezas-Cruz, A.; Berry, D. The Helminth Holobiont: A Multidimensional Host–Parasite–Microbiota Interaction. Trends Parasitol. 2023, 39, 91–100. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Sonnenburg, E.D. Vulnerability of the Industrialized Microbiota. Science 2019, 366, eaaw9255. [Google Scholar] [CrossRef] [PubMed]
- Negrao-Correa, D.; Teixeira, M.M. The Mutual Influence of Nematode Infection and Allergy. Chem. Immunol. Allerg. 2006, 90, 14–28. [Google Scholar]
- Yazdanbakhsh, M.; Wahyuni, S. The Role of Helminth Infections in Protection from Atopic Disorders. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 386–391. [Google Scholar] [CrossRef]
- Weinstock, J.V.; Summers, R.W.; Elliott, D.E.; Qadir, K.; Urban, J.F.; Thompson, R. The Possible Link between De-Worming and the Emergence of Immunological Disease. J. Lab. Clin. Med. 2002, 139, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.A.; Raine, T.; Cooke, A.; Lawrence, C.E. Inhibition of Autoimmune Type 1 Diabetes by Gastrointestinal Helminth Infection. Infect. Immun. 2007, 75, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Panelli, S.; Epis, S.; Cococcioni, L.; Perini, M.; Paroni, M.; Bandi, C.; Drago, L.; Zuccotti, G.V. Inflammatory Bowel Diseases, the Hygiene Hypothesis and the Other Side of the Microbiota: Parasites and Fungi. Pharmacol. Res. 2020, 159, 104962. [Google Scholar] [CrossRef]
- Danese, S.; Fiocchi, C. Etiopathogenesis of Inflammatory Bowel Diseases. World J. Gastroenterol. 2006, 12, 4807–4812. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The Gut Microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The Epidemiology of Inflammatory Bowel Disease: East Meets West. J. Gastroenterol. Hepatol. 2019, 35, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The Global Burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Arai, T.; Lopes, F. Potential of Human Helminth Therapy for Resolution of Inflammatory Bowel Disease: The Future Ahead. Exp. Parasitol. 2022, 232, 108189. [Google Scholar] [CrossRef]
- Khan, W.I.; Blennerhasset, P.A.; Varghese, A.K.; Chowdhury, S.K.; Omsted, P.; Deng, Y.; Collins, S.M. Intestinal Nematode Infection Ameliorates Experimental Colitis in Mice. Infect. Immun. 2002, 70, 5931–5937. [Google Scholar] [CrossRef]
- Motomura, Y.; Wang, H.; Deng, Y.; El-Sharkawy, R.T.; Verdu, E.F.; Khan, W.I. Helminth Antigen-Based Strategy to Ameliorate Inflammation in an Experimental Model of Colitis. Clin. Exp. Immunol. 2009, 155, 88–95. [Google Scholar] [CrossRef]
- Summers, R.W.; Elliot, D.E.; Urban, J.F.; Thompson, R.; Weinstock, J.V. Trichuris Suis Therapy in Crohn’s Disease. Gut 2005, 54, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.W.; Elliott, D.E.; Urban, J.F.; Thompson, R.A.; Weinstock, J.V. Trichuris Suis Therapy for Active Ulcerative Colitis: A Randomized Controlled Trial. Gastroenterology 2005, 128, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Shields, V.E.; Cooper, J. Use of Helminth Therapy for Management of Ulcerative Colitis and Crohn’s Disease: A Systematic Review. Parasitology 2022, 149, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Shute, A.; Callejas, B.E.; Li, S.H.; Wang, A.; Jayme, T.S.; Ohland, C.; Lewis, I.A.; Layden, B.T.; Buret, A.G.; McKay, D.M. Cooperation between Host Immunity and the Gut Bacteria Is Essential for Helminth-Evoked Suppression of Colitis. Microbiome 2021, 9, 186. [Google Scholar] [CrossRef]
- Grainger, J.R.; Smith, K.A.; Hewitson, J.P.; McSorley, H.J.; Harcus, Y.; Filbey, K.J.; Finney, C.A.M.; Greenwood, E.J.D.; Knox, D.P.; Wilson, M.S.; et al. Helminth Secretions Induce de Novo T Cell Foxp3 Expression and Regulatory Function through the TGF-β Pathway. J. Exp. Med. 2010, 207, 2331–2341. [Google Scholar] [CrossRef]
- Pace, F.; Carvalho, B.M.; Zanotto, T.M.; Santos, A.; Guadagnini, D.; Silva, K.L.C.; Mendes, M.C.S.; Rocha, G.Z.; Alegretti, S.M.; Santos, G.A.; et al. Helminth Infection in Mice Improves Insulin Sensitivity via Modulation of Gut Microbiota and Fatty Acid Metabolism. Pharmacol. Res. 2018, 132, 33–46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grondin, J.A.; Jamal, A.; Mowna, S.; Seto, T.; Khan, W.I. Interaction between Intestinal Parasites and the Gut Microbiota: Implications for the Intestinal Immune Response and Host Defence. Pathogens 2024, 13, 608. https://doi.org/10.3390/pathogens13080608
Grondin JA, Jamal A, Mowna S, Seto T, Khan WI. Interaction between Intestinal Parasites and the Gut Microbiota: Implications for the Intestinal Immune Response and Host Defence. Pathogens. 2024; 13(8):608. https://doi.org/10.3390/pathogens13080608
Chicago/Turabian StyleGrondin, Jensine A., Asif Jamal, Sadrina Mowna, Tyler Seto, and Waliul I. Khan. 2024. "Interaction between Intestinal Parasites and the Gut Microbiota: Implications for the Intestinal Immune Response and Host Defence" Pathogens 13, no. 8: 608. https://doi.org/10.3390/pathogens13080608




