Geospatial Analysis of Lumpy Skin Disease Outbreaks among Cattle in Uttar Pradesh, India, 2021–2022
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Data Collection
LSD Case and Population Data
2.3. Data Analysis
2.3.1. Spatial Analysis
Disease Mapping
Spatial Statistics
3. Results
3.1. Descriptive Analysis
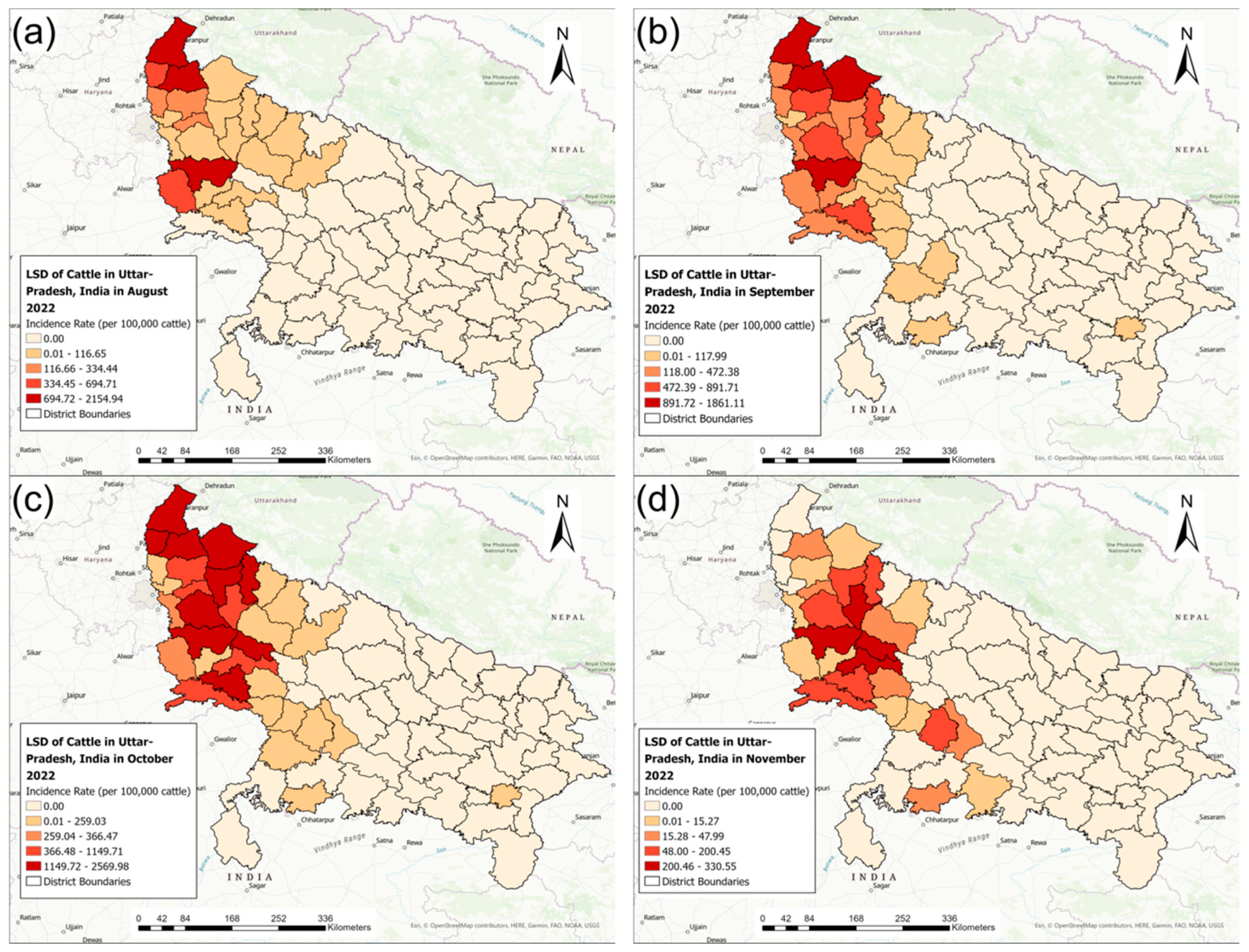
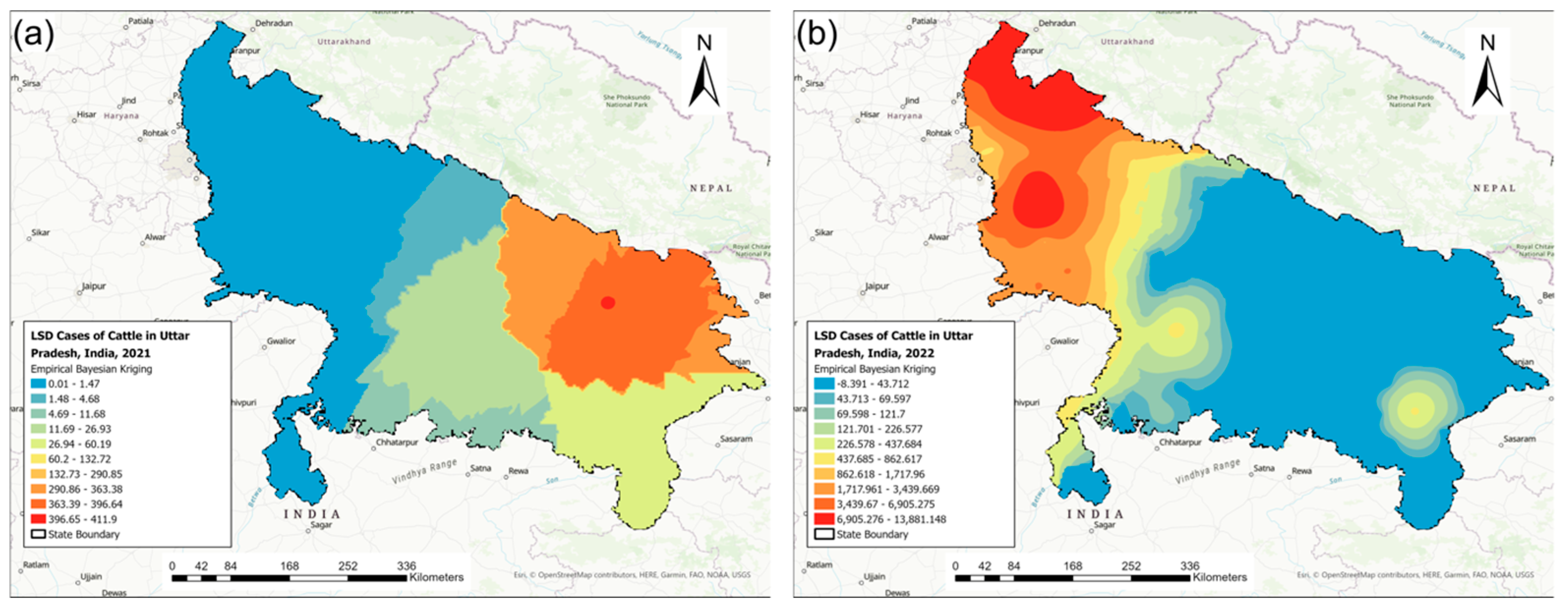
3.2. Disease Mapping
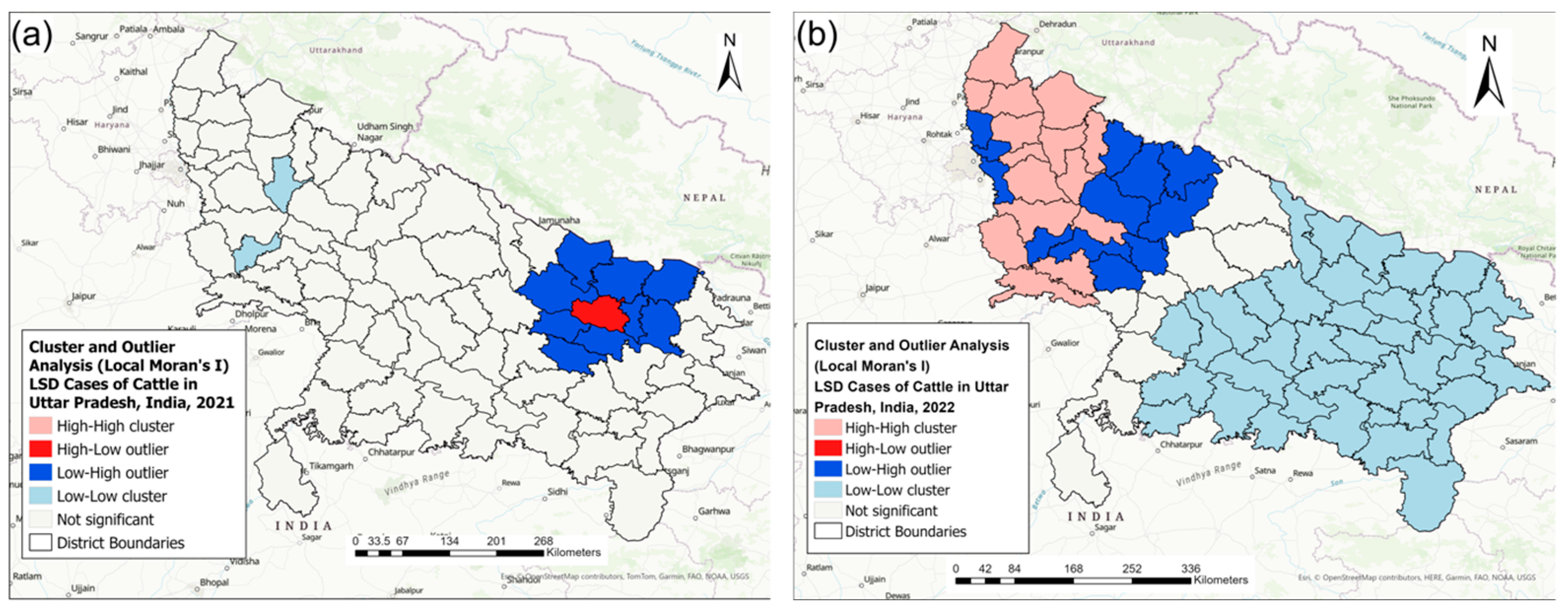
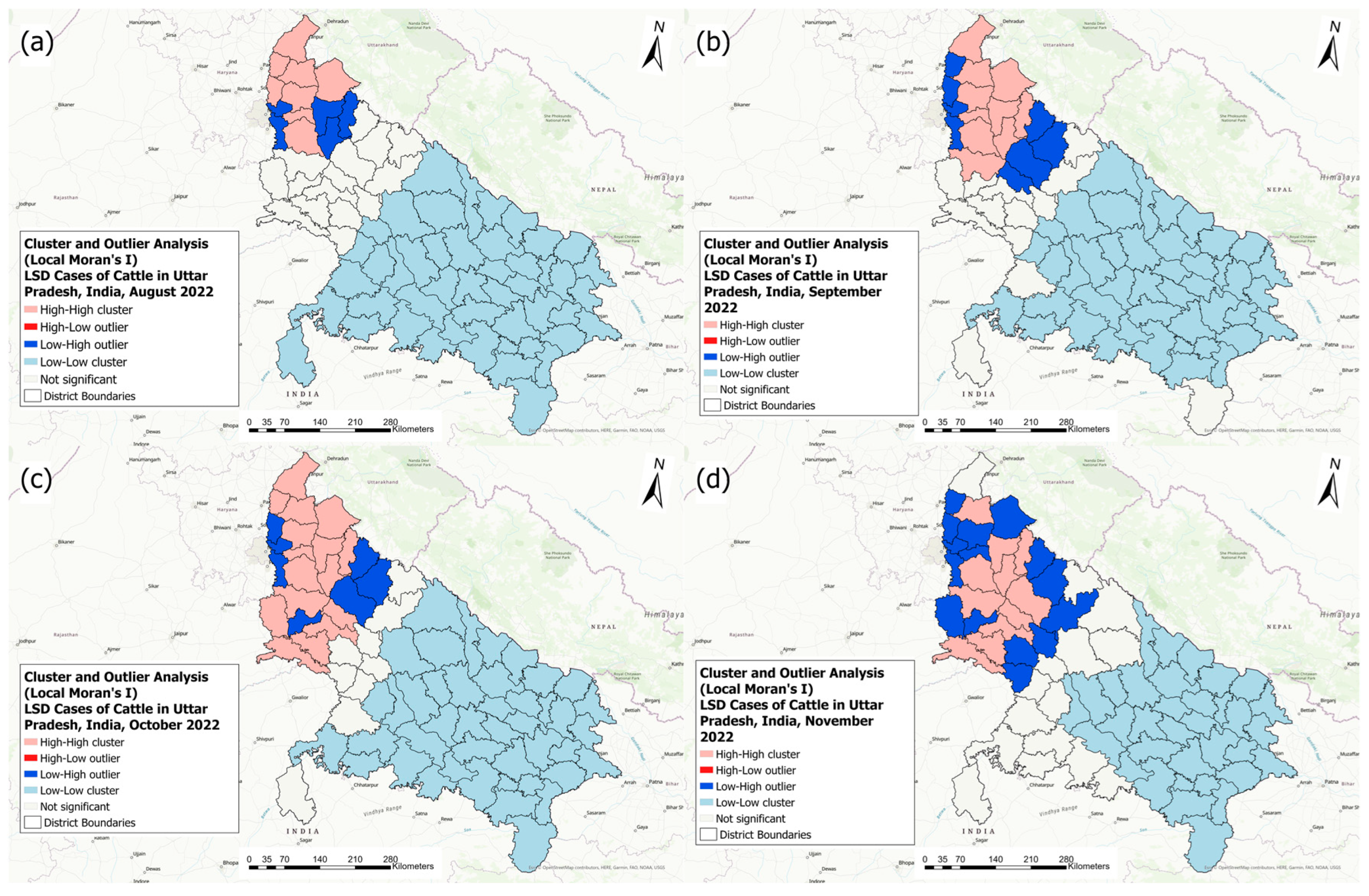
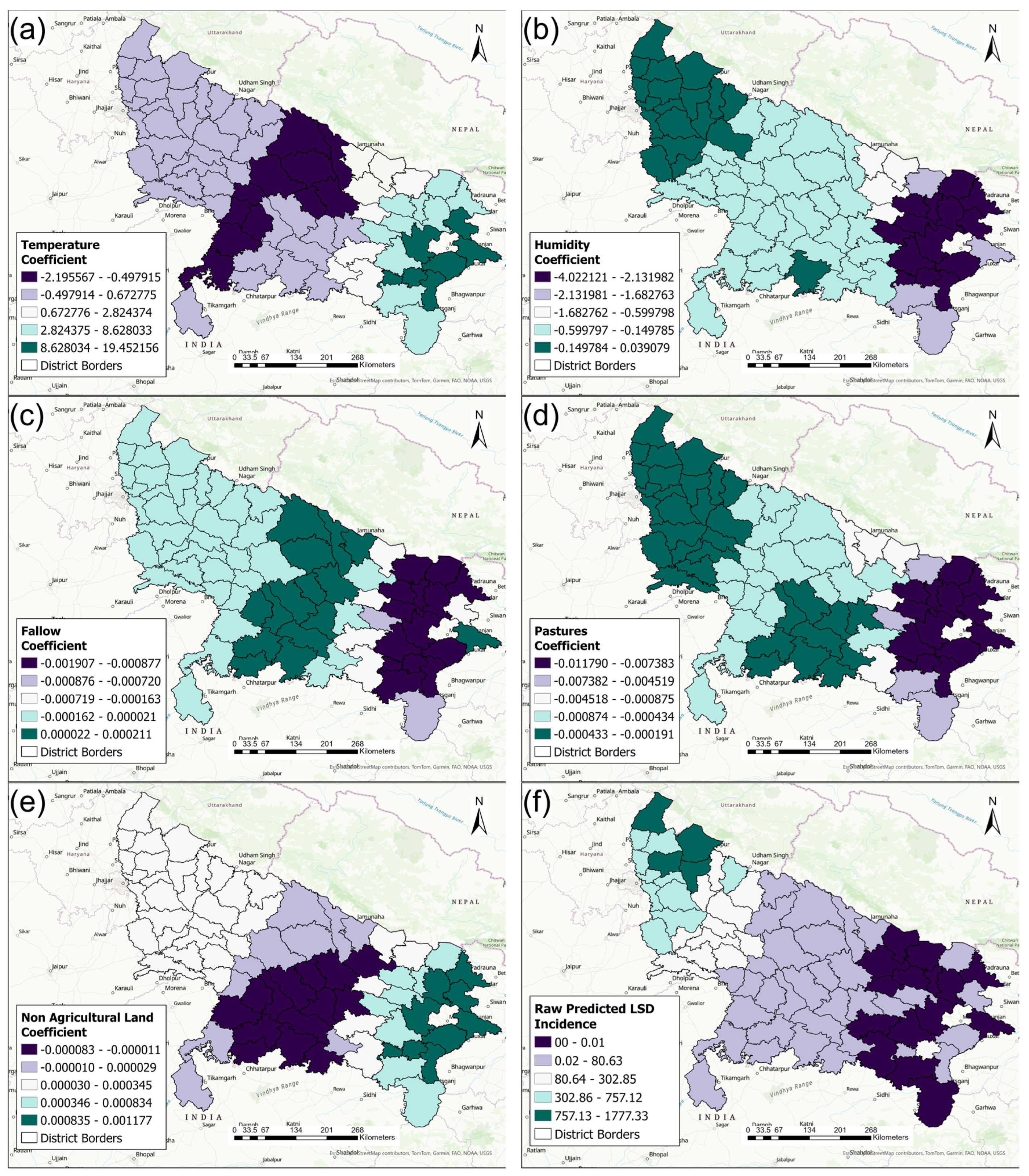
3.3. Spatial Statistics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eom, H.J.; Lee, E.-S.; Yoo, H.S. Lumpy Skin Disease as an Emerging Infectious Disease. J. Vet. Sci. 2023, 24, e42. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.A. Scientific Opinion on Lumpy Skin Disease. EFSA J. 2015, 13, 3986. [Google Scholar]
- Arjkumpa, O.; Suwannaboon, M.; Boonrod, M.; Punyawan, I.; Liangchaisiri, S.; Laobannue, P.; Lapchareonwong, C.; Sansri, C.; Kuatako, N.; Panyasomboonying, P.; et al. The First Lumpy Skin Disease Outbreak in Thailand (2021): Epidemiological Features and Spatio-Temporal Analysis. Front. Vet. Sci. 2022, 8, 799065. [Google Scholar] [CrossRef]
- Kumar, N.; Tripathi, B.N. A Serious Skin Virus Epidemic Sweeping through the Indian Subcontinent Is a Threat to the Livelihood of Farmers. Virulence 2022, 13, 1943–1944. [Google Scholar] [CrossRef] [PubMed]
- Babiuk, S.; Bowden, T.R.; Boyle, D.B.; Wallace, D.B.; Kitching, R.P. Capripoxviruses: An Emerging Worldwide Threat to Sheep, Goats and Cattle. Transbound. Emerg. Dis. 2008, 55, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Datten, B.; Chaudhary, A.A.; Sharma, S.; Singh, L.; Rawat, K.D.; Ashraf, M.S.; Alneghery, L.M.; Aladwani, M.O.; Rudayni, H.A.; Dayal, D.; et al. An Extensive Examination of the Warning Signs, Symptoms, Diagnosis, Available Therapies, and Prognosis for Lumpy Skin Disease. Viruses 2023, 15, 604. [Google Scholar] [CrossRef] [PubMed]
- Paslaru, A.I.; Maurer, L.M.; Vögtlin, A.; Hoffmann, B.; Torgerson, P.R.; Mathis, A.; Veronesi, E. Putative Roles of Mosquitoes (Culicidae) and Biting Midges (Culicoides spp.) as Mechanical or Biological Vectors of Lumpy Skin Disease Virus. Med. Vet. Entomol. 2022, 36, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Sprygin, A.; Pestova, Y.; Wallace, D.B.; Tuppurainen, E.; Kononov, A.V. Transmission of Lumpy Skin Disease Virus: A Short Review. Virus Res. 2019, 269, 197637. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, J.; Simons, X.; Humblet, M.-F.; Saegerman, C. Lumpy Skin Disease: A Systematic Review of Mode of Transmission, Risk of Emergence and Risk Entry Pathway. Viruses 2023, 15, 1622. [Google Scholar] [CrossRef]
- Roche, X. Introduction and Spread of Lumpy Skin Disease in South, East and Southeast Asia: Qualitative Risk Assessment and Management; Étude FAO: Production et Santé Animales; FAO: Rome, Italy, 2020; ISBN 978-92-5-133563-5. [Google Scholar]
- Ratyotha, K.; Prakobwong, S.; Piratae, S. Lumpy Skin Disease: A Newly Emerging Disease in Southeast Asia. Vet. World 2022, 15, 2764–2771. [Google Scholar] [CrossRef]
- Mathivanan, E.; Raju, K.; Murugan, R. Outbreak of Lumpy Skin Disease in India 2022—An Emerging Threat to Livestock & Livelihoods. Glob. Biosecur. 2023, 5. [Google Scholar] [CrossRef]
- 20th Livestock Census. Available online: https://dahd.nic.in (accessed on 28 April 2024).
- Azeem, S.; Sharma, B.; Shabir, S.; Akbar, H.; Venter, E. Lumpy Skin Disease Is Expanding Its Geographic Range: A Challenge for Asian Livestock Management and Food Security. Vet. J. 2022, 279, 105785. [Google Scholar] [CrossRef] [PubMed]
- Limon, G.; Gamawa, A.A.; Ahmed, A.I.; Lyons, N.A.; Beard, P.M. Epidemiological Characteristics and Economic Impact of Lumpy Skin Disease, Sheeppox and Goatpox Among Subsistence Farmers in Northeast Nigeria. Front. Vet. Sci. 2020, 7, 492658. [Google Scholar] [CrossRef] [PubMed]
- Mahla, V.; Choudhary, V.K.; Saharan, J.S.; Yadav, M.L.; Kumar, S.; Choudhary, S. Study about Socio-Economic Status and Calf Rearing Management Practices Adopted by Cattle Keepers of Western Rajasthan, India. Indian J. Agric. Res. 2015, 49, 189–192. [Google Scholar] [CrossRef]
- Chandran, P.; Dey, A.; Barari, S.; Kamal, R.; Dayal, S.; Chakrabarti, A. Socio-Economic Status of Farmers Rearing Bachaur Cattle in Its Habitat under Middle Gangetic Plains. Indian J. Anim. Sci. 2014, 84, 1300–1303. [Google Scholar] [CrossRef]
- Agrawal, I.; Sharma, B.; Varga, C. Space-Time Clustering and Climatic Risk Factors for Lumpy Skin Disease of Cattle in Uttar Pradesh, India, 2022. Transbound Emerg Dis. 2024, 2024, 1343156. [Google Scholar] [CrossRef]
- Stackhouse, P.W.; Macpherson, B.; Broddle, M.; McNeil, C.; Barnett, A.J.; Mikovitz, C.; Zhang, T. Introduction to the Prediction of Worldwide Energy Resources (POWER) Project: NASA Applied Sciences Week 2021; NASA: Washington, DC, USA, 2021. [Google Scholar]
- University of Texas Libraries. Available online: https://geodata.lib.utexas.edu/catalog/stanford-sh819zz8121 (accessed on 20 November 2023).
- Jenks, G.F. The Data Model Concept in Statistical Mapping. Available online: https://api.semanticscholar.org/ (accessed on 15 February 2024).
- Pilz, J.; Spöck, G. Why Do We Need and How Should We Implement Bayesian Kriging Methods. Stoch. Environ. Res. Risk Assess. 2008, 22, 621–632. [Google Scholar] [CrossRef]
- Krivoruchko, K. Empirical Bayesian Kriging. ArcUser Fall 2012, 6, 1145. [Google Scholar]
- Anselin, L. Local Indicators of Spatial Association—LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- Moran, P.A.P. Notes on Continuous Stochastic Phenomena. Biometrika 1950, 37, 17–23. [Google Scholar] [CrossRef]
- Mitchel, A. The ESRI Guide to GIS Analysis, Volume 2: Spatial Measurements and Statistics; Esri Press: Redlands, CA, USA, 2005; Volume 2. [Google Scholar]
- Brunsdon, C.; Fotheringham, S.; Charlton, M. Geographically Weighted Regression. J. R. Stat. Soc. Ser. Stat. 1998, 47, 431–443. [Google Scholar] [CrossRef]
- Brunsdon, C.; Fotheringham, A.S.; Charlton, M.E. Geographically Weighted Regression: A Method for Exploring Spatial Nonstationarity. Geogr. Anal. 1996, 28, 281–298. [Google Scholar] [CrossRef]
- Getis, A.; Ord, J.K. The Analysis of Spatial Association by Use of Distance Statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Ord, J.K.; Getis, A. Local Spatial Autocorrelation Statistics: Distributional Issues and an Application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- Mitchell, A.; Scott Griffin, L.; Mitchell, A. The Esri Guide to GIS Analysis, 2nd ed; Esri Press: Redlands, CA, USA, 2020; ISBN 1-58948-579-3. [Google Scholar]
- Gautam, M.; Kattel, P.; Kaphle, K. Review on Lumpy Skin Disease and Its Emerging Threat to Livestock in Nepal. Vet. Sci. Res. Rev. 2022, 8, 43–51. [Google Scholar] [CrossRef]
- Manjunatha Reddy, G.B.; Pabbineedi, S.M.; Nagaraj, S.; Bijalwan, S.; Tadakod, S.; Bhutia, Z.; Palmu, D.; Rai, S.; Bhutia, P.D.; Bhutia, P.T.; et al. Lumpy Skin Disease (LSD) in Yak (Bos Grunniens): An Evidence of Species Spillover from Cattle in India. Microorganisms 2023, 11, 2823. [Google Scholar] [CrossRef] [PubMed]
- Akther, M.; Akter, S.H.; Sarker, S.; Aleri, J.W.; Annandale, H.; Abraham, S.; Uddin, J.M. Global Burden of Lumpy Skin Disease, Outbreaks, and Future Challenges. Viruses 2023, 15, 1861. [Google Scholar] [CrossRef]
- Mani, R.; Beillard, M.J. Lumpy Skin Disease Spreads to Northern States of India; GAIN: New Delhi, India, 2022; p. 3. [Google Scholar]
- Press Trust of India, PTI. Lumpy Disease: Uttar Pradesh Govt Bans Cattle Trade with 4 States; Restricts Movement of Animals in Some Districts. Deccan Herald, 23 September 2022. [Google Scholar]
- The National Network. Lumpy Skin Disease: UP First in Country to Give 1 Crore Doses of Goat Pox Vaccine. Times India, 11 October 2022. [Google Scholar]
- Zhugunissov, K.; Bulatov, Y.; Orynbayev, M.; Kutumbetov, L.; Abduraimov, Y.; Shayakhmetov, Y.; Taranov, D.; Amanova, Z.; Mambetaliyev, M.; Absatova, Z.; et al. Goatpox Virus (G20-LKV) Vaccine Strain Elicits a Protective Response in Cattle against Lumpy Skin Disease at Challenge with Lumpy Skin Disease Virulent Field Strain in a Comparative Study. Vet. Microbiol. 2020, 245, 108695. [Google Scholar] [CrossRef] [PubMed]
- Berke, O. Exploratory Disease Mapping: Kriging the Spatial Risk Function from Regional Count Data. Int. J. Health Geogr. 2004, 3, 18. [Google Scholar] [CrossRef]
- Onovo, A.A.; Atobatele, A.; Kalaiwo, A.; Obanubi, C.; James, E.; Gado, P.; Odezugo, G.; Ogundehin, D.; Magaji, D.; Russell, M. Using Supervised Machine Learning and Empirical Bayesian Kriging to Reveal Correlates and Patterns of COVID-19 Disease Outbreak in Sub-Saharan Africa: Exploratory Data Analysis. MedRxiv 2020. [Google Scholar] [CrossRef]
- Ferraguti, M.; Magallanes, S.; Suarez-Rubio, M.; Bates, P.J.J.; Marzal, A.; Renner, S.C. Does Land-Use and Land Cover Affect Vector-Borne Diseases? A Systematic Review and Meta-Analysis. Landsc. Ecol. 2023, 38, 2433–2451. [Google Scholar] [CrossRef]
- Chala, B.; Hamde, F. Emerging and Re-Emerging Vector-Borne Infectious Diseases and the Challenges for Control: A Review. Front. Public Health 2021, 9, 715759. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.; Singh, M.; Roy, A.; Reddy, B.; Singh, N. Trends and Contribution of Grazing Resources to Livestock in Different States of India. Range Manag. Agrofor. 2015, 36, 204–210. [Google Scholar]
- Alkhamis, M.A.; VanderWaal, K. Spatial and Temporal Epidemiology of Lumpy Skin Disease in the Middle East, 2012–2015. Front. Vet. Sci. 2016, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Mertz, D.; Loeb, M. Assessing the Reporting Quality of Influenza Outbreaks in the Community. Influenza Other Respir. Viruses 2017, 11, 556–563. [Google Scholar] [CrossRef]
- Allam, A.M.; Elbayoumy, M.K.; Abdel-Rahman, E.H.; Hegazi, A.G.; Farag, T.K. Molecular Characterization of the 2018 Outbreak of Lumpy Skin Disease in Cattle in Upper Egypt. Vet. World 2020, 13, 1262–1268. [Google Scholar] [CrossRef]
- Orynbayev, M.B.; Nissanova, R.K.; Khairullin, B.M.; Issimov, A.; Zakarya, K.D.; Sultankulova, K.T.; Kutumbetov, L.B.; Tulendibayev, A.B.; Myrzakhmetova, B.S.; Burashev, E.D. Lumpy Skin Disease in Kazakhstan. Trop. Anim. Health Prod. 2021, 53, 166. [Google Scholar] [CrossRef]
- Punyapornwithaya, V.; Seesupa, S.; Phuykhamsingha, S.; Arjkumpa, O.; Sansamur, C.; Jarassaeng, C. Spatio-Temporal Patterns of Lumpy Skin Disease Outbreaks in Dairy Farms in Northeastern Thailand. Front. Vet. Sci. 2022, 9, 957306. [Google Scholar] [CrossRef]




| Year | Number of Districts Affected | LSD Cases | LSD Deaths |
|---|---|---|---|
| 2021 | |||
| Total | 6 | 5648 | Not available |
| 2022 | |||
| August 2022 | 22 | 18,569 | 150 |
| September 2022 | 29 | 32,871 | 252 |
| October 2022 | 32 | 56,300 | 226 |
| November 2022 | 25 | 4486 | 47 |
| Total | 33 | 112,226 | 675 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, I.; Sharma, B.; Singh, A.P.; Varga, C. Geospatial Analysis of Lumpy Skin Disease Outbreaks among Cattle in Uttar Pradesh, India, 2021–2022. Pathogens 2024, 13, 611. https://doi.org/10.3390/pathogens13080611
Agrawal I, Sharma B, Singh AP, Varga C. Geospatial Analysis of Lumpy Skin Disease Outbreaks among Cattle in Uttar Pradesh, India, 2021–2022. Pathogens. 2024; 13(8):611. https://doi.org/10.3390/pathogens13080611
Chicago/Turabian StyleAgrawal, Isha, Barkha Sharma, Ajay Pratap Singh, and Csaba Varga. 2024. "Geospatial Analysis of Lumpy Skin Disease Outbreaks among Cattle in Uttar Pradesh, India, 2021–2022" Pathogens 13, no. 8: 611. https://doi.org/10.3390/pathogens13080611






