Multidrug-Resistant Escherichia coli Accumulated by Freshwater Bivalves: An Underestimated Risk for Public Health?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Freshwater Bivalve Collection
2.2. Sample Processing, Isolation, and Identification of Bacteria
2.3. Antimicrobial Susceptibility Test
2.4. Phylogenetic Determination of E. coli Isolates
2.5. Determination of E. coli Pathotypes
3. Results
3.1. Bivalve Characterization
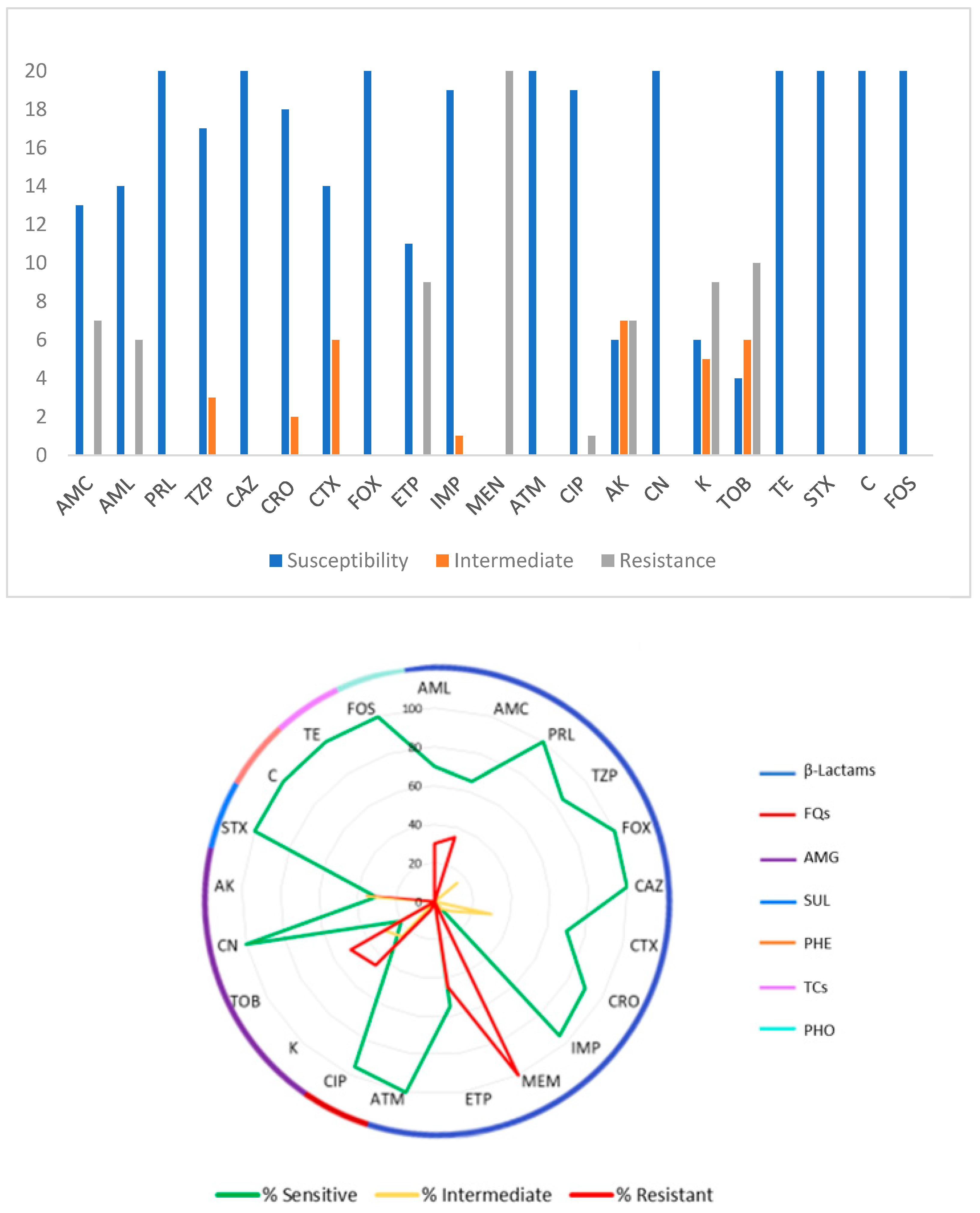
3.2. Antimicrobial Susceptibility Tests
3.3. Multiresistant Isolates
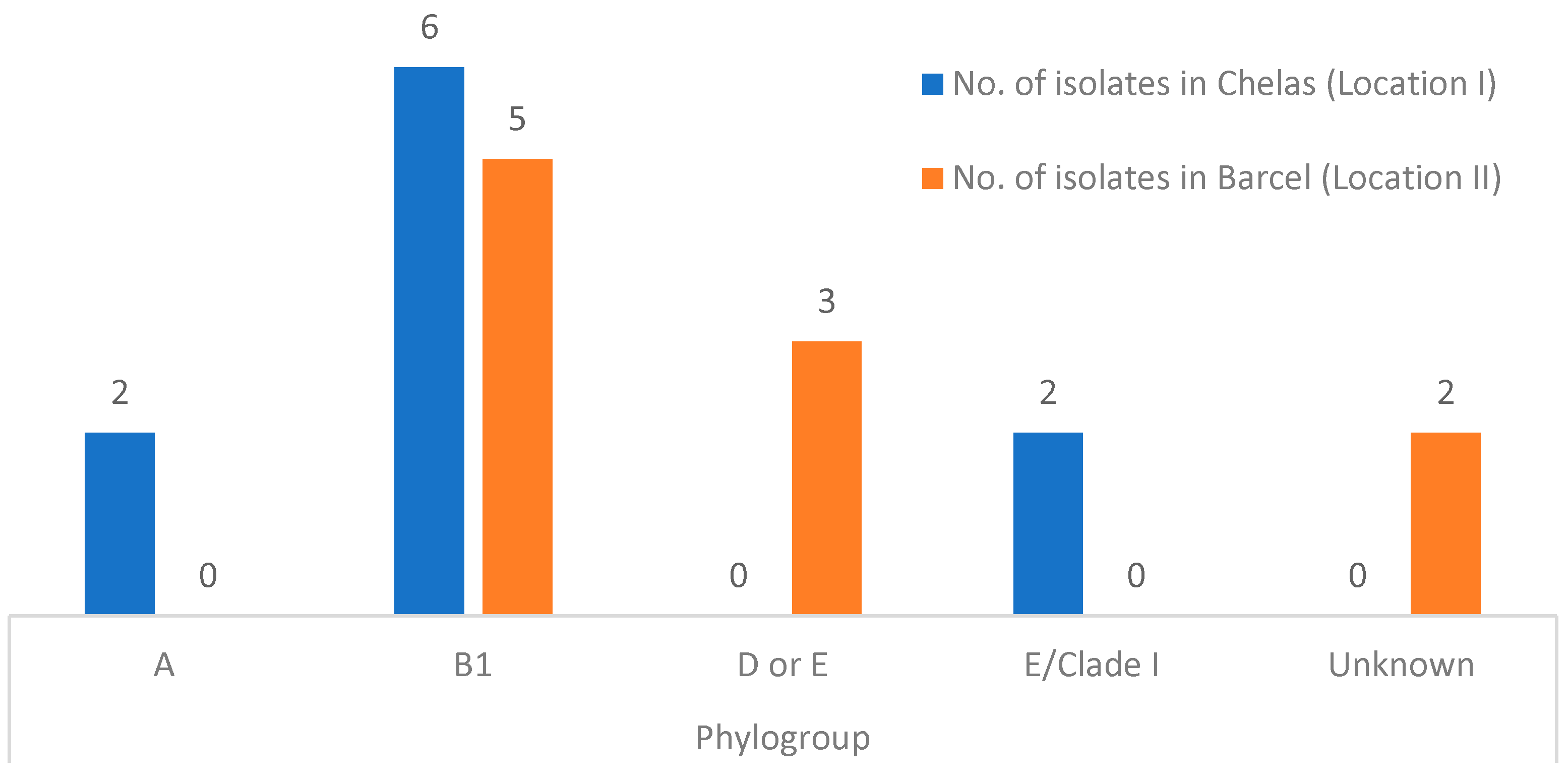
3.4. Phylogenetic Analysis and E. coli Pathotype Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araujo, R.; Reis, J.; Machordom, A.; Toledo, C.; Madeira, M.J.; Gómez, I.; Velasco Marcos, J.C.; Morales, J.; Barea, J.M.; Ondina, P.; et al. Las náyades de la península Ibérica As náiades da Península Ibérica the naiads of the Iberian Peninsula. Soc. Española Malacol. Iberus. 2009, 27, 7–72. [Google Scholar]
- Saavedra, M.J.; Fernandes, C.; Teixeira, A.; Álvarez, X.; Varandas, S. Multiresistant bacteria: Invisible enemies of freshwater mussels. Environ. Pollut. 2022, 295, 118671. [Google Scholar] [CrossRef]
- Dias, A.R.; Teixeira, A.; Lopes-Lima, M.; Varandas, S.; Sousa, R. From the lab to the river: Determination of ecological hosts of Anodonta anatina. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 988–999. [Google Scholar] [CrossRef]
- European Food Safety Authority. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2019–2020. EFSA J. 2022, 20, 7209. [Google Scholar]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- O’Neill, J. Tackling Drug-Resistance Infections Globally: Final Report and Recommendations. The Review on Antimicrobial Resistance Chaired by Jim O’Neill. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 13 May 2024).
- Gasser, M.; Zingg, W.; Cassini, A.; Kronenberg, A. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in Switzerland. Lancet Infect. Dis. 2019, 19, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Reports of Selected E. coli Outbreak Investigations. 2018. Available online: https://www.cdc.gov/ecoli/outbreaks.html (accessed on 28 March 2024).
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A review of the mechanisms that confer antibiotic resistance in pathotypes of E. coli. Front. Cell Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef] [PubMed]
- Braz, V.S.; Melchior, K.; Moreira, C.G. Escherichia coli as a multifaceted pathogenic and versatile bacterium. Front. Cell. Infect. Microbiol. 2020, 10, 548492. [Google Scholar] [CrossRef]
- Sora, V.M.; Meroni, G.; Martino, P.A.; Soggiu, A.; Bonizzi, L.; Zecconi, A. Extraintestinal Pathogenic Escherichia coli: Virulence Factors and Antibiotic Resistance. Pathogens 2021, 10, 1355. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, R.J.; Roque, F.; Teixeira Rodrigues, A.; Herdeiro, M.T.; Ramalheira, E. Use of antibiotics and bacterial resistances: Brief notes on its evolution. Rev. Port. Saude Publica 2016, 34, 77–84. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Longcore, T.; Barbieri, M.; Dabritz, H.; Hill, D.; Klein, P.N.; Lepczyk, C.; Lilly, E.L.; McLeod, R.; Milcarsky, J.; et al. The One Health Approach to Toxoplasmosis: Epidemiology, Control, and Prevention Strategies. Ecohealth 2019, 16, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Grenni, P.; Ancona, V.; Barra Caracciolo, A. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Guo, F.; Wang, Y.; Peng, J.; Huang, H.; Tu, X.; Zhao, H.; Zhan, N.; Rao, Z.; Zhao, G.; Yang, H. Occurrence, Distribution, and Risk Assessment of Antibiotics in the Aquatic Environment of the Karst Plateau Wetland of Yangtze River Basin, Southwestern China. Int. J. Environ. Res. Public Health 2022, 19, 7211. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Manaia, C.M.; Macedo, G.; Fatta-Kassinos, D.; Nunes, O.C. Antibiotic resistance in urban aquatic environments: Can it be controlled? Appl. Microbiol. Biotechnol. 2016, 100, 1543–1557. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.; Nehra, K.; Rana, J.S.; Dahiya, T. Antibiotic resistance in agriculture: Perspectives on upcoming strategies to overcome upsurge in resistance. Curr. Res. Microb. Sci. 2021, 2, 100030. [Google Scholar] [CrossRef] [PubMed]
- Schar, D.; Zhao, C.; Wang, Y.; Larsson, D.G.J.; Gilbert, M.; Van Boeckel, T.P. Twenty-year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nat. Commun. 2021, 12, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, C.; Qu, Z.; Li, M. The combined toxicity of binary mixtures of antibiotics against the cyanobacterium Microcystis is dose-dependent: Insight from a theoretical nonlinear combined toxicity assessment method. Environ. Sci. Pollut. Res. 2022, 29, 11612–11624. [Google Scholar] [CrossRef]
- Pinto, I.; Simões, M.; Gomes, I.B. An Overview of the Impact of Pharmaceuticals on Aquatic Microbial Communities. Antibiotics 2022, 11, 1700. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Ying, G.-G.; Liu, Y.S.; Chen, F.; Yang, J.F.; Wang, L.; Yang, X.B.; Stauber, J.L.; Warne, M.S.J. Occurrence and a screening-level risk assessment of human pharmaceuticals in the pearl river system, South China. Environ. Toxicol. Chem. 2010, 29, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Nappier, S.P.; Liguori, K.; Ichida, A.M.; Stewart, J.R.; Jones, K.R. Antibiotic Resistance in Recreational Waters: State of the Science. Int. J. Environ. Res. Public Health 2020, 17, 8034. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Fiayyaz, F.; Khurshid, M.; Sabir, S.; Akash, M.S.H. Chapter 2—Antibiotics and antimicrobial resistance: Temporal and global trends in the environment. In Antibiotics and Antimicrobial Resistance Genes in the Environment; Hashmi, M.Z., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 7–27. [Google Scholar] [CrossRef]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The population genetics of pathogenic Escherichia coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Lasa, A.; Romalde, J.L. Coevolution of Molluscs and Their Microbes. In Microbes: The Foundation Stone of the Biosphere; Hurst, C.J., Ed.; Advances in Environmental Microbiology; Springer: Cham, Switzerland, 2021; Volume 8. [Google Scholar] [CrossRef]
- Masanja, F.; Yang, K.; Xu, Y.; He, G.; Liu, X.; Xu, X.; Jiang, X.; Luo, X.; Mkuye, R.; Deng, Y.; et al. Bivalves and microbes: A mini-review of their relationship and potential implications for human health in a rapidly warming ocean. Front. Mar. Sci. 2023, 10, 1182438. [Google Scholar] [CrossRef]
- Ferri, G.; Olivieri, V.; Olivastri, A.; Pennisi, L.; Vergara, A. Multidrug resistant Vibrio spp. identified from mussels farmed for human consumption in Central Italy. J. Appl. Microbiol. 2024, 135, lxae098. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Fernandes, C.; Monteiro, S.; Cabecinha, E.; Teixeira, A.; Varandas, S.; Saavedra, M.J. The role of aquatic ecosystems (River Tua, Portugal) as reservoirs of multidrug-resistant Aeromonas spp. Water 2021, 13, 698. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 13.0. 2023. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 13 May 2024).
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2022; Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 13 May 2024).
- Rafailidis, P.; Panagopoulos, P.; Koutserimpas, C.; Samonis, G. Current Therapeutic Approaches for Multidrug-Resistant and Extensively Drug-Resistant Acinetobacter baumannii Infections. Antibiotics 2024, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Instituto Superiore di Sanità. ACTIVITIES: European Union Reference Laboratory for Escherichia coli. Instituto Superiore di Sanità. 2021. Available online: https://www.iss.it/en/vtec-laboratory-methods (accessed on 13 May 2024).
- ISO/TS 13136:2012; Microbiology of Food and Animal Feed—Real-Time Polymerase Chain Reaction (PCR)-Based Method for the Detection of Food-Borne Pathogens—Horizontal Method for the Detection of Shiga Toxin-Producing Escherichia coli (STEC) and the Determ. International Organization for Standardization: Geneva, Switzerland, 2012; p. 22. Available online: https://www.iso.org/standard/53328.html (accessed on 15 February 2024).
- Cuttelod, A.; Seddon, M.; Neubert, E. European Red List of Non-Marine Molluscs; Publications Office of the European Union: Luxembourg, 2011; pp. 1–93. [Google Scholar]
- Lopes-lima, M. Anodonta anatina. IUCN Red List. Threat. Species 2014, 8235. Available online: http://www.iucnredlist.org/details/155667/0 (accessed on 15 February 2024).
- Lopes-Lima, M.; Reis, J.; Alvarez, M.G.; Anastácio, P.M.; Banha, F.; Beja, P.; Castro, P.; Gama, M.; Gil, M.G.; Gomes-Dos-Santos, A.; et al. The silent extinction of freshwater mussels in Portugal. Biol. Conserv. 2023, 285, 110244. [Google Scholar] [CrossRef]
- Varandas, S.; Fernandes, C.; Cabecinha, E.; Gomes, S.; da Silva, G.J.; Saavedra, M.J. Escherichia coli Phylogenetic and Antimicrobial Pattern as an Indicator of Anthropogenic Impact on Threatened Freshwater Mussels. Antibiotics 2023, 12, 1401. [Google Scholar] [CrossRef]
- Bighiu, M.A.; Norman Haldén, A.; Goedkoop, W.; Ottoson, J. Assessing microbial contamination and antibiotic resistant bacteria using zebra mussels (Dreissena polymorpha). Sci. Total Environ. 2019, 650, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Ekakoro, J.E.; Guptill, L.F.; Hendrix, G.K.; Dorsey, L.; Ruple, A. Antimicrobial Susceptibility of Bacteria Isolated from Freshwater Mussels in the Wildcat Creek Watershed, Indiana, United States. Antibiotics 2023, 12, 728. [Google Scholar] [CrossRef] [PubMed]
- Meletis, G. Carbapenem resistance: Overview of the problem and future perspectives. Ther. Adv. Infect. Dis. 2016, 3, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, N.; Poirel, L.; Bessa, L.J.; Barbosa-vasconcelos, A. VIM-1, VIM-34, and IMP-8 Carbapenemase-Producing Escherichia coli Strains Recovered from a Portuguese River. Antimicrob. Agents Chemother. 2016, 60, 2585–2586. [Google Scholar] [CrossRef] [PubMed]
- Tacão, M.; Laço, J.; Teixeira, P.; Henriques, I. CTX-M-Producing Bacteria Isolated from a Highly Polluted River System in Portugal. Int. J. Environ. Res. Public Health 2022, 19, 11858. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance Global Report on Surveillance; Microbiology Australia: Clayton, VIC, Australia, 2014; Volume 40, pp. 55–56. [Google Scholar]
- Balière, C.; Rincé, A.; Thevenot, D.; Gourmelon, M. Successful detection of pathogenic Shiga-toxin-producing Escherichia coli in shellfish, environmental waters and sediment using the ISO/TS-13136 method. Lett. Appl. Microbiol. 2015, 60, 315–320. [Google Scholar] [CrossRef]
- Sawa, T.; Kooguchi, K.; Moriyama, K. Molecular diversity of extended-spectrum β-lactamases and carbapenemases, and antimicrobial resistance. J. Intensive Care 2020, 8, 13. [Google Scholar] [CrossRef]
- Bush, K. Past and present perspectives on β-lactamases. Antimicrob. Agents Chemother. 2018, 62, e01076-18. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, F.; Pezzi, A.; Galletti, G.; Tamba, M.; Merialdi, G.; Piva, S.; Serraino, A.; Rubini, S. Antimicrobial resistance patterns in Salmonella enterica subsp. enterica and Escherichia coli isolated from bivalve molluscs and marine environment. Food Control. 2021, 121, 107590. [Google Scholar] [CrossRef]
- Bong, C.W.; Low, K.Y.; Chai, L.C.; Lee, C.W. Prevalence and Diversity of Antibiotic Resistant Escherichia coli from Anthropogenic-Impacted Larut River. Front. Public Health 2022, 10, 794513. [Google Scholar] [CrossRef] [PubMed]
- Stoppe, N.d.C.; Silva, J.S.; Carlos, C.; Sato MI, Z.; Saraiva, A.M.; Ottoboni LM, M.; Torres, T.T. Worldwide Phylogenetic Group Patterns of Escherichia coli from Commensal Human and Wastewater Treatment Plant Isolates. Front. Microbiol. 2017, 8, 2512. [Google Scholar] [CrossRef] [PubMed]
- Xedzro, C.; Kimura, T.; Shimamoto, T.; Ahmed, A.M.; Shimamoto, T. Comparative molecular profiling of antimicrobial resistance and phylogenetic characterization of multidrug-resistant Escherichia coli isolated from meat sources in 2009 and 2021 in Japan. Int. J. Food Microbiol. 2023, 110146, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Osińska, A.; Korzeniewska, E.; Harnisz, M.; Niestępski, S. The prevalence and characterization of antibiotic-resistant and virulent Escherichia coli strains in the municipal wastewater system and their environmental fate. Sci. Total Environ. 2017, 577, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Mataseje, L.F.; Neumann, N.; Crago, B.; Baudry, P.; Zhanel, G.G.; Louie, M.; Mulvey, M.R. Characterization of cefoxitin-resistant Escherichia coli isolates from recreational beaches and private drinking water in Canada between 2004 and 2006. Antimicrob. Agents Chemother. 2009, 53, 3126–3130. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, Z.; Malekzadegan, Y.; Bahador, A.; Azimzadeh, M.; Haghighi, M.A. Phylogenetic study, distribution of virulence genes and antibiotic resistance profiles of Escherichia coli isolated from Bushehr coastal water. Gene Rep. 2022, 26, 101473. [Google Scholar] [CrossRef]
- Dioli, C.; Pappa, O.; Siatravani, E.; Bratakou, S.; Tatsiopoulos, A.; Giakkoupi, P.; Miriagou, V.; Beloukas, A. Molecular Characterization and Prevalence of Antimicrobial-Resistant Escherichia coli Isolates Derived from Clinical Specimens and Environmental Habitats. Microorganisms 2023, 11, 1399. [Google Scholar] [CrossRef] [PubMed]
- Berthe, T.; Ratajczak, M.; Clermont, O.; Denamur, E.; Petit, F. Evidence for coexistence of distinct Escherichia coli populations in various aquatic environments and their survival in estuary water. Appl. Environ. Microbiol. 2013, 79, 4684–4693. [Google Scholar] [CrossRef]
- Saraceno, M.; Gómez Lugo, S.; Ortiz, N.; Gómez, B.M.; Sabio y García, C.A.; Frankel, N.; Graziano, M. Unraveling the ecological processes modulating the population structure of Escherichia coli in a highly polluted urban stream network. Sci. Rep. 2021, 11, 14679. [Google Scholar] [CrossRef]
| Location | Isolates’ Identification |
|---|---|
| Chelas | LIFB1Ec1 |
| LIFB1Ec2 | |
| LIFB2Ec1 | |
| LIFB2Ec2 | |
| LIFB4Ec1 | |
| LIFB4Ec2 | |
| LIFB5Ec1 | |
| LIFB5Ec2 | |
| LIFB6Ec1 | |
| LIFB6Ec2 | |
| Barcel | LIIFB4Ec1 |
| LIIFB4Ec2 | |
| LIIFB5Ec1 | |
| LIIFB6Ec1 | |
| LIIFB7Ec1 | |
| LIIFB7Ec2 | |
| LIIFB7Ec3 | |
| LIIFB7Ec4 | |
| LIIFB8Ec1 | |
| LIIFB8Ec2 |
| Primer | Primer Sequences (5′-3′) | PCR Product (bp) |
|---|---|---|
| chuA | F: 5′-ATGGTACCGGACGAACCAAC-3′ R: 5′-TGCCGCCAGTACCAAAGACA-3′ | 288 |
| yjaA | F: 5′-CAAACGTGAAGTGTCAGGAG-3′ R: 5′-AATGCGTTCCTCAACCTGTG-3′ | 211 |
| Tsp.E4.C2 | F: 5′-CACTATTCGTAAGGTCATCC-3′ R: 5′-AGTTTATCGCTGCGGGTCGC-3′ | 152 |
| arpA | F: 5′-AACGCTATTCGCCAGCTTGC-3′ R: 5′-TCTCCCCATACCGTACGCTA-3′ | 400 |
| arpA (group E) | F: 5′-GATTCCATCTTGTCAAAATATGCC-3′ R: 5′-GAAAAGAAAAAGAATTCCCAAGAG-3′ | 301 |
| trpA (group C) | F: 5′-AGTTTTATGCCCAGTGCGAG-3′ R: 5′-TCTGCGCCGGTCACGCCC-3′ | 219 |
| E. coli Pathotype | Gene | Primer Sequences (5′-3′) |
|---|---|---|
| EIEC | ipah | F: CCT TTT CCG CGT TCC TTG A R: CGG AAT CCG GAG GTA TTG C′ P: Cy5-CGC CTT TCC GAT ACC GTC TCT GCA BHQ2 |
| ETEC | lt | F: TTCCCACCGGATCACCAA R: CAACCTTGTGGTGCATGATGA P: FAM-CTTGGAGAGAAGAACCCT BHQ1 |
| ETEC | sth | F: GCTAAACCAGYAGRGTCTTCAAAA R: CCCGGTACARGCAGGATTACAACA P: HEX-TGGTCCTGAAAGCATGAA-BHQ1 |
| ETEC | stp | F: TGAATCACTTGACTCTTCAAAA R: CCCCAGTTCARWGTRAGRTCMACRTC P: Cy5-TGAACAACACATTTTACTGCT BHQ2 |
| STEC | stx1 | F: TTTGTYACTGTSACAGCWGAAGCYTTACG R: CCCCAGTTCARWGTRAGRTCMACRTC P: FAM-CTGGATGATCTCAGTGGGCGTTCTTATGTAA-BHQ1 |
| STEC | stx2 | R: TTTGTYACTGTSACAGCWGAAGCYTTACG F: CCCCAGTTCARWGTRAGRTCMACRTC P:HEX-TCGTCAGGCACTGTCTGAAACTGCTCC-BHQ1 |
| EAEC | aggR | R: CCTAAAGGATGCCCTGATGA′ F: GAATCGTCAGCATCAGCTACA P: FAM-CGGACAACTGCAAGCATCTA-BHQ1 |
| EAEC | aaiC | R: CCTGATTTAGTTGATTCCCTACG F: CATTTCACGCTTTTTCAGGAAT P: HEX-CACATACAAGACCTTCTGGAGAA-BHQ1 |
| A. anatina | Length (mm) | Weight with Shell (g) | Weight of Soft Tissues (g) | |
|---|---|---|---|---|
| Location I | FB1 | 65 | 19.869 | 12.148 |
| FB2 | 72 | 27.694 | 16.159 | |
| FB3 | 83 | 30.522 | 17.736 | |
| FB4 | 74 | 29.745 | 15.847 | |
| FB5 | 81 | 33.917 | 19.064 | |
| FB6 | 81 | 39.682 | 20.144 | |
| FB7 | 73 | 37.190 | 20.120 | |
| Mean ± SD deviation | 75.6 ± 6.40 | 31.23 ± 6.563 | 17.63 ± 2.866 | |
| Location II | FB1 | 69 | 23.502 | 13.369 |
| FB2 | 71 | 23.755 | 12.749 | |
| FB3 | 75 | 34.533 | 18.081 | |
| FB4 | 69 | 22.942 | 13.002 | |
| FB5 | 72 | 24.785 | 11.492 | |
| FB6 | 99 | 61.260 | 29.847 | |
| FB7 | 98 | 56.498 | 29.250 | |
| FB8 | 121 | 118.169 | 39.133 | |
| Mean ± SD deviation | 86.4 ± 19.9 | 48.684 ± 34.406 | 21.936 ± 10.801 | |
| MDR Pattern | No. of Isolates in Chelas (Location I) | No. of Isolates in Barcel (Location II) | Total. No. of Isolates (%) |
|---|---|---|---|
| PEN-CEP-CARB-FQs-AMG | 1 | 0 | 5 |
| PEN-CEP-CARB-AMG | 1 | 0 | 5 |
| PEN-CARB-AMG | 7 | 0 | 35 |
| CEP-CARB-AMG | 0 | 3 | 15 |
| CARB-AMG | 1 | 7 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, J.C.L.; Gonçalves, A.; Fernandes, C.; Cabecinha, E.; Monteiro, S.; Guedes, H.; Almeida, G.; Garcia, J.; da Silva, G.J.; Varandas, S.; et al. Multidrug-Resistant Escherichia coli Accumulated by Freshwater Bivalves: An Underestimated Risk for Public Health? Pathogens 2024, 13, 617. https://doi.org/10.3390/pathogens13080617
Martins JCL, Gonçalves A, Fernandes C, Cabecinha E, Monteiro S, Guedes H, Almeida G, Garcia J, da Silva GJ, Varandas S, et al. Multidrug-Resistant Escherichia coli Accumulated by Freshwater Bivalves: An Underestimated Risk for Public Health? Pathogens. 2024; 13(8):617. https://doi.org/10.3390/pathogens13080617
Chicago/Turabian StyleMartins, Joana C. L., Ana Gonçalves, Conceição Fernandes, Edna Cabecinha, Sandra Monteiro, Hugo Guedes, Gonçalo Almeida, Juliana Garcia, Gabriela J. da Silva, Simone Varandas, and et al. 2024. "Multidrug-Resistant Escherichia coli Accumulated by Freshwater Bivalves: An Underestimated Risk for Public Health?" Pathogens 13, no. 8: 617. https://doi.org/10.3390/pathogens13080617










