Investigating the Hepatitis E Virus (HEV) Diversity in Rat Reservoirs from Northern Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection and RNA Extractions
2.2. HEV RNA Detection by Real-Time and Conventional Reverse Transcription PCR
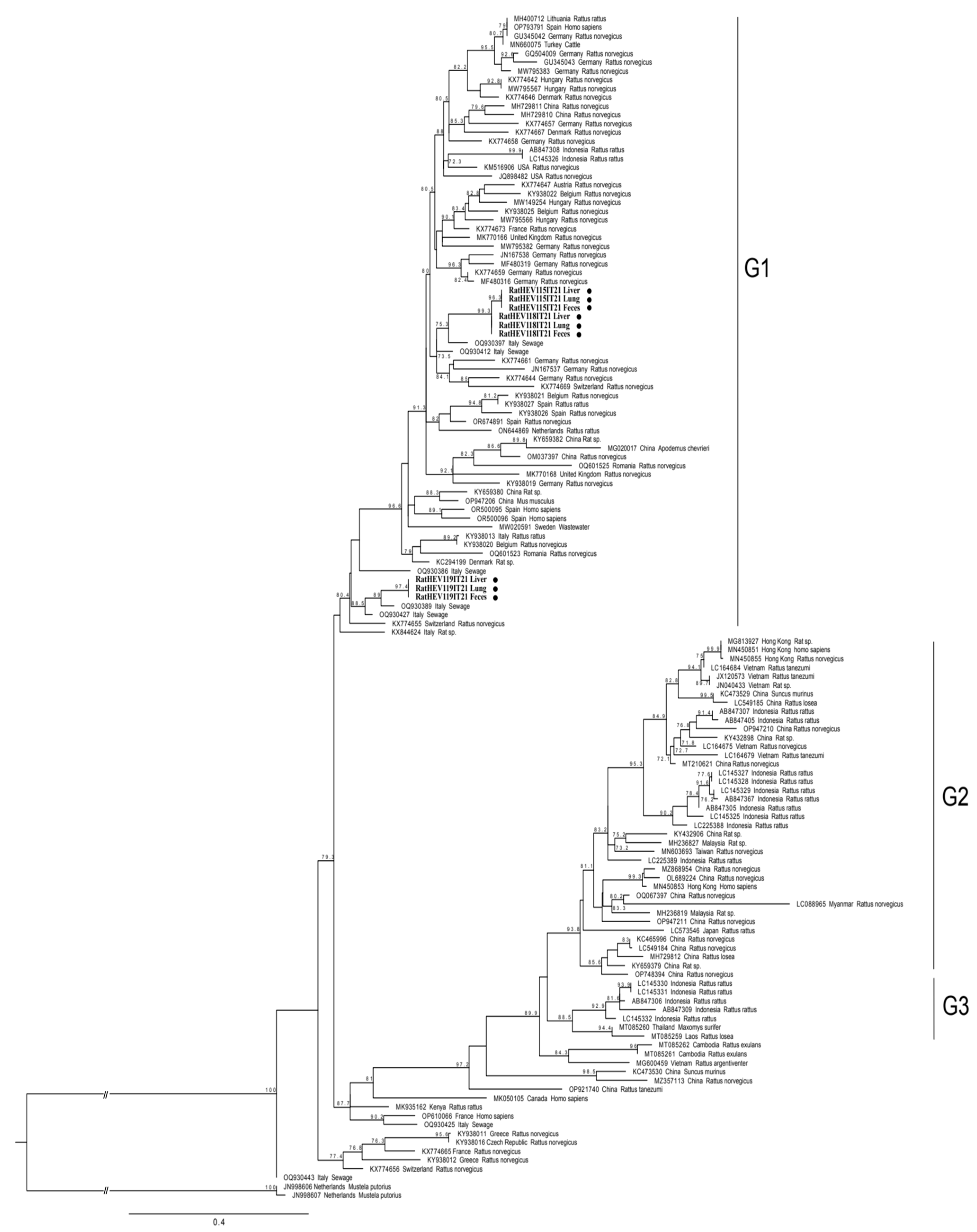
2.3. Sequencing and Phylogenetic Analyses
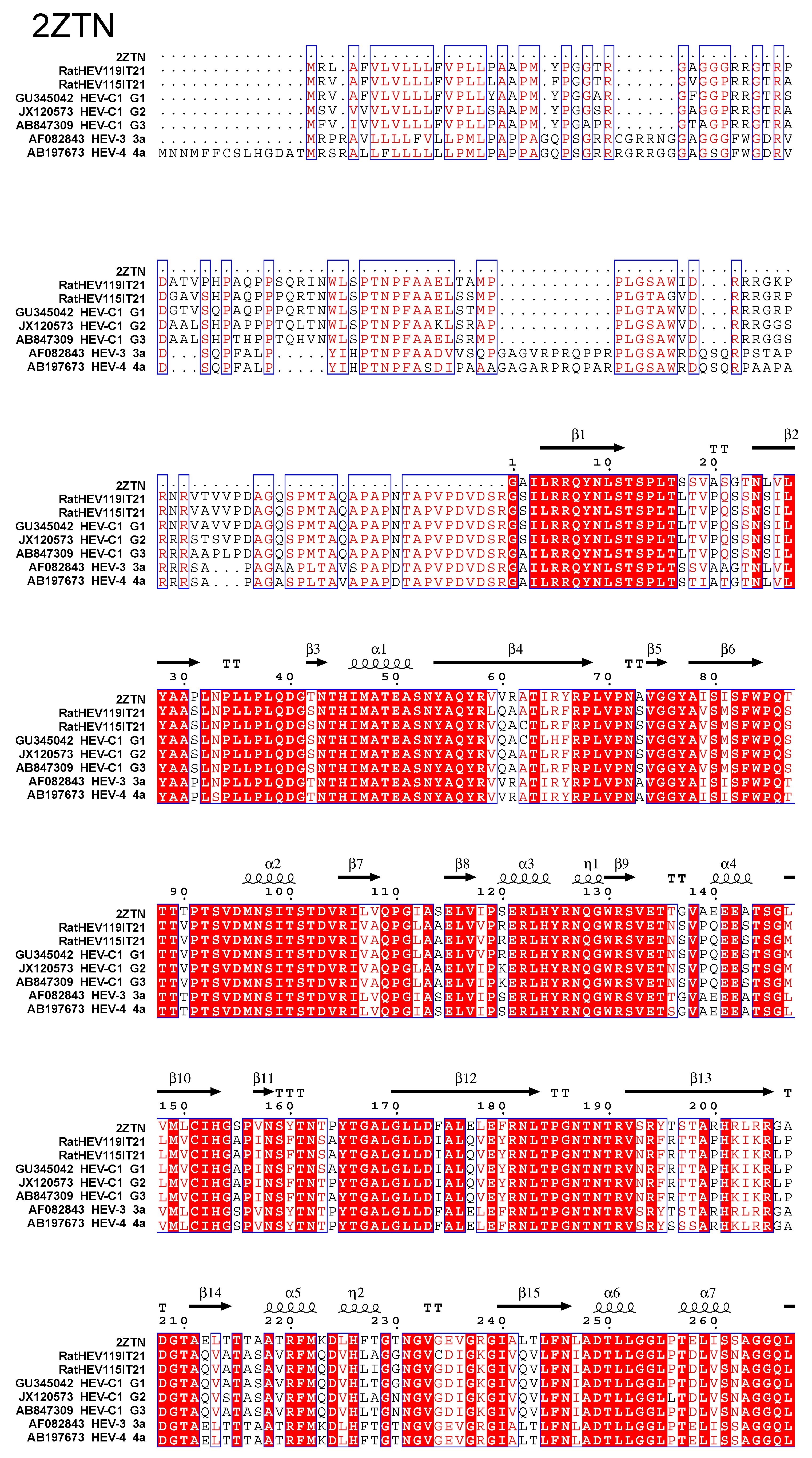
2.4. Capsid Proteins Alignment
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Harms, D.; Yang, X.L.; Bock, C.T. Orthohepevirus C: An Expanding Species of Emerging Hepatitis E Virus Variants. Pathogens 2020, 9, 154. [Google Scholar] [CrossRef]
- Johne, R.; Heckel, G.; Plenge-Bönig, A.; Kindler, E.; Maresch, C.; Reetz, J.; Schielke, A.; Ulrich, R.G. Novel hepatitis E virus genotype in Norway rats, Germany. Emerg. Infect. Dis. 2010, 16, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Benavent, S.; Carlos, S.; Reina, G. Rocahepevirus ratti as an Emerging Cause of Acute Hepatitis Worldwide. Microorganisms 2023, 11, 2996. [Google Scholar] [CrossRef]
- Kabrane-Lazizi, Y.; Fine, J.B.; Elm, J.; Glass, G.E.; Higa, H.; Diwan, A.; Gibbs, C.J., Jr.; Meng, X.J.; Emerson, S.U.; Purcell, R.H. Evidence for widespread infection of wild rats with hepatitis E virus in the United States. Am. J. Trop. Med. Hyg. 1999, 61, 331–335. [Google Scholar] [CrossRef]
- Johne, R.; Dremsek, P.; Kindler, E.; Schielke, A.; Plenge-Bönig, A.; Gregersen, H.; Wessels, U.; Schmidt, K.; Rietschel, W.; Groschup, M.H.; et al. Rat hepatitis E virus: Geographical clustering within Germany and serological detection in wild Norway rats (Rattus norvegicus). Infect. Genet. Evol. 2012, 12, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Ding, X.; Li, T.C.; Takeda, N.; Kawabata, H.; Koizumi, N.; Kadosaka, T.; Goto, I.; Masuzawa, T.; Nakamura, M.; et al. Evidence for widespread infection of hepatitis E virus among wild rats in Japan. Hepatol. Res. 2003, 27, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Widen, F.; Ayral, F.; Artois, M.; Olofson, A.S.; Lin, J. PCR detection and analysis of potentially zoonotic Hepatitis E virus in French rats. Virol. J. 2014, 11, 90. [Google Scholar] [CrossRef]
- Takahashi, M.; Kunita, S.; Kawakami, M.; Kadosaka, T.; Fujita, H.; Takada, N.; Miyake, M.; Kobayashi, T.; Ohnishi, H.; Nagashima, S.; et al. First detection and characterization of rat hepatitis E Virus (HEV-C1) in Japan. Virus Res. 2022, 314, 198766. [Google Scholar] [CrossRef]
- Porea, D.; Raileanu, C.; Crivei, L.A.; Gotu, V.; Savuta, G.; Pavio, N. First Detection of Hepatitis E Virus (Rocahepevirus ratti Genotype C1) in Synanthropic Norway Rats (Rattus norvegicus) in Romania. Viruses 2023, 15, 1337. [Google Scholar] [CrossRef]
- De Sabato, L.; Ianiro, G.; Monini, M.; De Lucia, A.; Ostanello, F.; Di Bartolo, I. Detection of hepatitis E virus RNA in rats caught in pig farms from Northern Italy. Zoonoses Public Health 2020, 67, 62–69. [Google Scholar] [CrossRef]
- Kanai, Y.; Miyasaka, S.; Uyama, S.; Kawami, S.; Kato-Mori, Y.; Tsujikawa, M.; Yunoki, M.; Nishiyama, S.; Ikuta, K.; Hagiwara, K. Hepatitis E virus in Norway rats (Rattus norvegicus) captured around a pig farm. BMC Res. Notes. 2012, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Marion, O.; Lhomme, S.; Nayrac, M.; Dubois, M.; Pucelle, M.; Requena, M.; Migueres, M.; Abravanel, F.; Peron, J.M.; Carrere, N.; et al. Hepatitis E virus replication in human intestinal cells. Gut 2020, 69, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Yip, C.C.Y.; Wu, S.; Cai, J.; Zhang, A.-X.; Leung, K.-H.; Chung, T.W.H.; Chan, J.F.W.; Chan, W.-M.; Teng, J.L.L.; et al. Rat hepatitis E virus as cause. of persistent hepatitis after liver transplant. Emerg. Infect. Dis. 2018, 24, 2241–2250. [Google Scholar] [CrossRef]
- Sridhar, S.; Yip, C.C.; Wu, S.; Chew, N.F.; Leung, K.H.; Chan, J.F.; Zhao, P.S.; Chan, W.M.; Poon, R.W.; Tsoi, H.W.; et al. Transmission of Rat Hepatitis E Virus Infection to Humans in Hong Kong: A Clinical and Epidemiological Analysis. Hepatology 2021, 73, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Yip, C.C.Y.; Lo, K.H.Y.; Wu, S.; Situ, J.; Chew, N.F.S.; Leung, K.H.; Chan, H.S.Y.; Wong, S.C.Y.; Leung, A.W.S.; et al. Hepatitis E Virus Species C Infection in Humans, Hong Kong. Clin. Infect. Dis. 2022, 75, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Andonov, A.; Robbins, M.; Borlang, J.; Cao, J.; Hatchette, T.; Stueck, A.; Deschambault, Y.; Murnaghan, K.; Varga, J.; Johnston, L. Rat Hepatitis E Virus Linked to Severe Acute Hepatitis in an Immunocompetent Patient. J. Infect. Dis. 2019, 220, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Marchand, S.; Sessa, A.; Cappy, P.; Pawlotsky, J.M. Orthohepevirus C hepatitis, an underdiagnosed disease? J. Hepatol. 2023, 79, e39–e41. [Google Scholar] [CrossRef]
- Rivero-Juarez, A.; Frias, M.; Perez, A.B.; Pineda, J.A.; Reina, G.; Fuentes-Lopez, A.; Freyre-Carrillo, C.; Ramirez-Arellano, E.; Alados, J.C.; Rivero, A. Orthohepevirus C infection as an emerging cause of acute hepatitis in Spain: First report in Europe. J. Hepatol. 2022, 77, 326–331. [Google Scholar] [CrossRef]
- Faber, M.; Wenzel, J.J.; Erl, M.; Stark, K.; Schemmerer, M. No Evidence for Orthohepevirus C in Archived Human Samples in Germany, 2000-2020. Viruses 2022, 14, 742. [Google Scholar] [CrossRef]
- Dremsek, P.; Wenzel, J.J.; Johne, R.; Ziller, M.; Hofmann, J.; Groschup, M.H.; Werdermann, S.; Mohn, U.; Dorn, S.; Motz, M.; et al. Seroprevalence study in forestry workers from eastern Germany using novel genotype 3- and rat hepatitis E virus-specific immunoglobulin G ELISAs. Med. Microbiol. Immunol. 2012, 201, 189–200. [Google Scholar] [CrossRef]
- Parraud, D.; Lhomme, S.; Péron, J.M.; Da Silva, I.; Tavitian, S.; Kamar, N.; Izopet, J.; Abravanel, F. Rat Hepatitis E Virus: Presence in Humans in South-Western France? Front. Med. 2021, 8, 726363. [Google Scholar] [CrossRef] [PubMed]
- Ryll, R.; Bernstein, S.; Heuser, E.; Schlegel, M.; Dremsek, P.; Zumpe, M.; Wolf, S.; Pépin, M.; Bajomi, D.; Müller, G.; et al. Detection of rat hepatitis E virus in wild Norway rats (Rattus norvegicus) and Black rats (Rattus rattus) from 11 European countries. Vet. Microbiol. 2017, 208, 58–68. [Google Scholar] [CrossRef]
- Palombieri, A.; Di Profio, F.; Sarchese, V.; Fruci, P.; Suffredini, E.; Martella, V.; Veneri, C.; Bonanno Ferraro, G.; Mancini, P.; La Rosa, G.; et al. Surveillance for rat hepatitis E in wastewater networks, Italy. Microbiol. Spectr. 2023, 11, e0267523. [Google Scholar] [CrossRef] [PubMed]
- Rios-Muñoz, L.; Gonzálvez, M.; Caballero-Gomez, J.; Castro-Scholten, S.; Casares-Jimenez, M.; Agulló-Ros, I.; Corona-Mata, D.; García-Bocanegra, I.; Lopez-Lopez, P.; Fajardo, T.; et al. Detection of Rat Hepatitis E Virus in Pigs, Spain, 2023. Emerg. Infect. Dis. 2024, 30, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Jothikumar, N.; Cromeans, T.L.; Robertson, B.H.; Meng, X.J.; Hill, V.R. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J. Virol. Methods 2006, 131, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.F.; Seelen, A.; Corman, V.M.; Fumie Tateno, A.; Cottontail, V.; Melim Zerbinati, R.; Gloza-Rausch, F.; Klose, S.M.; Adu-Sarkodie, Y.; Oppong, S.K.; et al. Bats worldwide carry hepatitis E virus-related viruses that form a putative novel genus within the family Hepeviridae. J. Virol. 2012, 86, 9134–9147. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wen, Y.; Xiong, Y.; Zhang, M.; Cheng, M.; Chen, Q. The prevalence and genomic characteristics of hepatitis E virus in murine rodents and house shrews from several regions in China. BMC Vet. Res. 2018, 14, 414. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
- Depamede, S.N.M.; Sriasih, M.; Takahashi, M.; Nagashima, S.; Jirintai, S.; Nishizawa, T.; Okamoto, H. Frequent detection and characterization of hepatitis E virus variants in wild rats (Rattus rattus) in Indonesia. Arch. Virol. 2013, 158, 87–96. [Google Scholar] [CrossRef]
- Wang, B.; Cai, C.L.; Li, B.; Zhang, W.; Zhu, Y.; Chen, W.H.; Zhuo, F.; Shi, Z.L.; Yang, X.L. Detection and characterization of three zoonotic viruses in wild rodents and shrews from Shenzhen city, China. Virol. Sin. 2017, 32, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, J.; Noh, J.; Kim, K.; Yang, E.; Kim, S.G.; Cho, H.K.; Byun, K.S.; Kim, J.H.; Lee, Y.S.; et al. First detection and characterization of hepatitis E virus (Rocahepevirus ratti) from urban Norway rats (Rattus norvegicus) in the Republic of Korea. J. Med. Virol. 2024, 96, e29401. [Google Scholar] [CrossRef]
- Osanyinlusi, S.A.; Salu, O.B.; James, A.B.; Orenolu, R.M.; Omilabu, S.A. Molecular Detection of Hepatitis E Virus in Rattus norvegicus in Lagos, Nigeria. Nig. J. Microbiol. 2020, 34, 5197–5203. [Google Scholar]
- Ding, Q.; Hu, B.; Yao, X.; Gan, M.; Chen, D.; Zhang, N.; Wei, J.; Cai, K.; Zheng, Z. Prevalence and molecular characterization of hepatitis E virus (HEV) from wild rodents in Hubei Province, China. Infect. Genet. Evol. 2024, 121, 105602. [Google Scholar] [CrossRef] [PubMed]
- Reuter, G.; Boros, Á.; Pankovics, P. Review of Hepatitis E Virus in Rats: Evident Risk of Species Orthohepevirus C to Human Zoonotic Infection and Disease. Viruses 2020, 12, 1148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cremers, N.; Hendrickx, S.; Debing, Y.; Roskams, T.; Coelmont, L.; Neyts, J.; Kaptein, S.J.F. Establishment of a robust rat hepatitis E virus fecal-oral infection model and validation for antiviral studies. Antiviral. Res. 2023, 216, 105670. [Google Scholar] [CrossRef]
- Boswell, C.A.; Mundo, E.E.; Ulufatu, S.; Bumbaca, D.; Cahaya, H.S.; Majidy, N.; Van Hoy, M.; Schweiger, M.G.; Fielder, P.J.; Prabhu, S.; et al. Comparative physiology of mice and rats: Radiometric measurement of vascular parameters in rodent tissues. Mol. Pharm. 2014, 11, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Fousekis, F.S.; Mitselos, I.V.; Christodoulou, D.K. Extrahepatic manifestations of hepatitis E virus: An overview. Clin. Mol. Hepatol. 2020, 26, 16–23. [Google Scholar] [CrossRef]
- Guu, T.S.; Liu, Z.; Ye, Q.; Mata, D.A.; Li, K.; Yin, C.; Zhang, J.; Tao, Y.J. Structure of the hepatitis E virus-like particle suggests mechanisms for virus assembly and receptor binding. Proc. Natl. Acad. Sci. USA 2009, 106, 12992–12997. [Google Scholar] [CrossRef]
- Yamashita, T.; Mori, Y.; Miyazaki, N.; Cheng, R.H.; Yoshimura, M.; Unno, H.; Shima, R.; Moriishi, K.; Tsukihara, T.; Li, T.C.; et al. Biological and immunological characteristics of hepatitis E virus-like particles based on the crystal structure. Proc. Natl. Acad. Sci. USA 2009, 106, 12986–12991. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Yamada, K.; Hoshino, Y.; Takahashi, H.; Ichiyama, K.; Tanaka, T.; Okamoto, H. Monoclonal antibodies raised against the ORF3 protein of hepatitis E virus (HEV) can capture HEV particles in culture supernatant and serum but not those in feces. Arch. Virol. 2008, 153, 1703–1713. [Google Scholar] [CrossRef]
- Debing, Y.; Emerson, S.U.; Purcell, R.H.; Neyts, J.; Dallmeier, K. Complete genome sequence of a rat hepatitis e virus strain isolated in the United States. Genome Announc. 2014, 2, e01096-14. [Google Scholar] [CrossRef] [PubMed]
- Niendorf, S.; Harms, D.; Hellendahl, K.F.; Heuser, E.; Böttcher, S.; Bock, C.T.; Ulrich, R.G. Presence and Diversity of Different Enteric Viruses in Wild Norway Rats (Rattus norvegicus). Viruses 2021, 13, 992. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Smits, S.L.; Pas, S.D.; Provacia, L.B.; Moorman-Roest, H.; Osterhaus, A.D.; Haagmans, B.L. Novel hepatitis E virus in ferrets, the Netherlands. Emerg. Infect. Dis. 2012, 18, 1369–1370. [Google Scholar] [CrossRef]
- Kobayashi, T.T.; Takahashi, M.; Jirintai, S.; Nishizawa, T.; Nagashima, S.; Nishiyama, T.; Kunita, S.; Hayama, E.; Tanaka, T.; Okamoto, H.M. An analysis of two open reading frames (ORF3 and ORF4) of rat hepatitis E virus genome using its infectious cDNA clones with mutations in ORF3 or ORF4. Virus Res. 2018, 249, 16–30. [Google Scholar] [CrossRef]
- Purcell, R.H.; Engle, R.E.; Rood, M.P.; Kabrane-Lazizi, Y.; Nguyen, H.T.; Govindarajan, S.; St Claire, M.; Emerson, S.U. Hepatitis E virus in rats, Los Angeles, California, USA. Emerg. Infect. Dis. 2011, 17, 2216–2222. [Google Scholar] [CrossRef]
- Suparyatmo, J.B.M.; Andayani, I.G.; Takahashi, M.K.; Ohnishi, H.; Jirintai, S.; Nagashima, S.; Nishizawa, T.; Okamoto, H. Marked genomic heterogeneity of rat hepatitis E virus strains in Indonesia demonstrated on a full-length genome analysis. Virus Res. 2014, 179, 102–112. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Sabato, L.; Monini, M.; Galuppi, R.; Dini, F.M.; Ianiro, G.; Vaccari, G.; Ostanello, F.; Di Bartolo, I. Investigating the Hepatitis E Virus (HEV) Diversity in Rat Reservoirs from Northern Italy. Pathogens 2024, 13, 633. https://doi.org/10.3390/pathogens13080633
De Sabato L, Monini M, Galuppi R, Dini FM, Ianiro G, Vaccari G, Ostanello F, Di Bartolo I. Investigating the Hepatitis E Virus (HEV) Diversity in Rat Reservoirs from Northern Italy. Pathogens. 2024; 13(8):633. https://doi.org/10.3390/pathogens13080633
Chicago/Turabian StyleDe Sabato, Luca, Marina Monini, Roberta Galuppi, Filippo Maria Dini, Giovanni Ianiro, Gabriele Vaccari, Fabio Ostanello, and Ilaria Di Bartolo. 2024. "Investigating the Hepatitis E Virus (HEV) Diversity in Rat Reservoirs from Northern Italy" Pathogens 13, no. 8: 633. https://doi.org/10.3390/pathogens13080633





