Soil Fungal Pathogens in Pinus pinaster Mature Reforestation: Silvicultural Treatments Effects
Abstract
:1. Introduction
2. Methodology
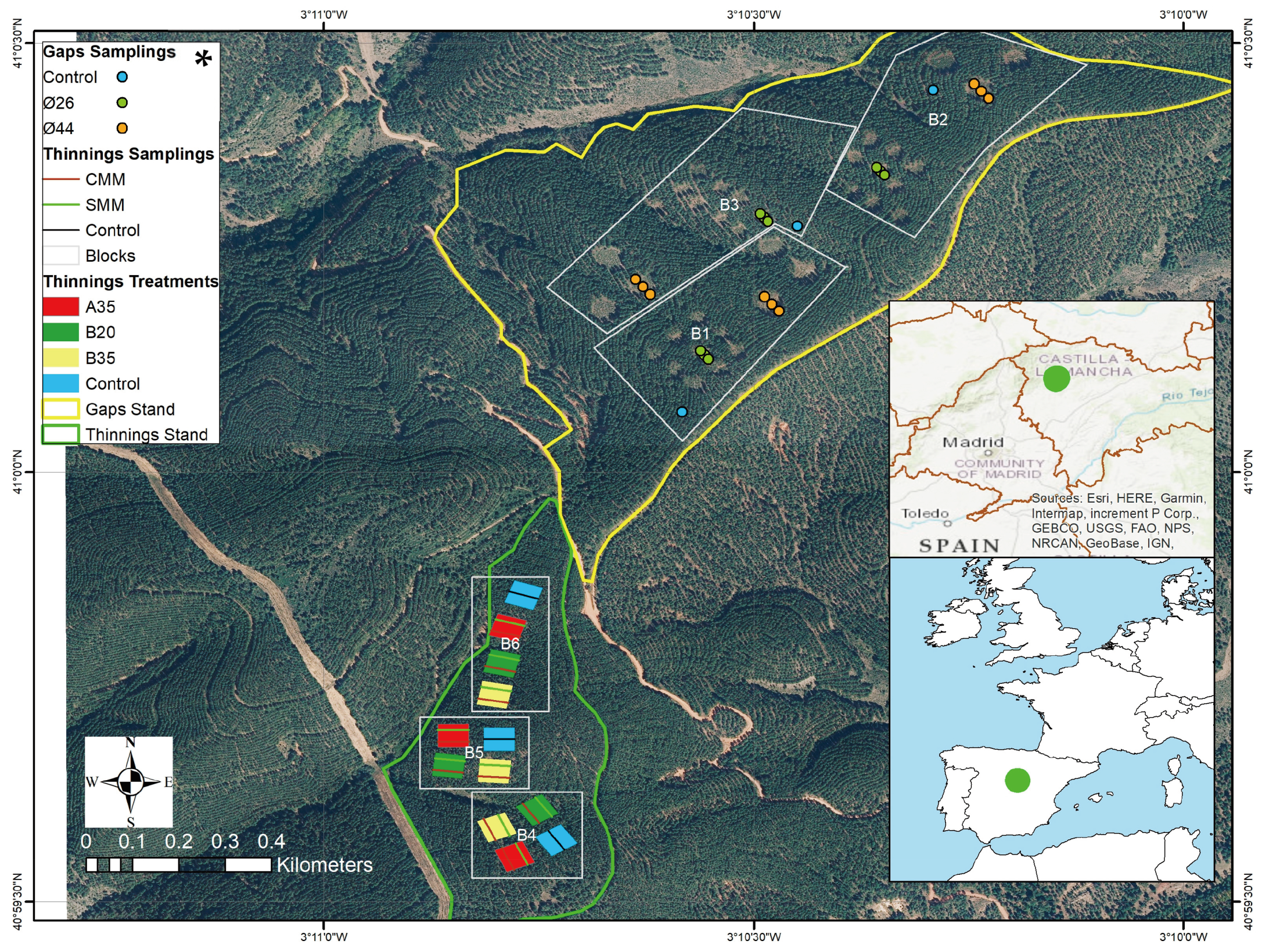
2.1. Area and Experimental Design
2.2. Soil Sampling and Processing
2.3. DNA Extraction, NGS Library Preparation, Sequencing and Analysis of ITS2 Sequencing Data
2.4. Environmental Data
2.5. Data Analysis
3. Results
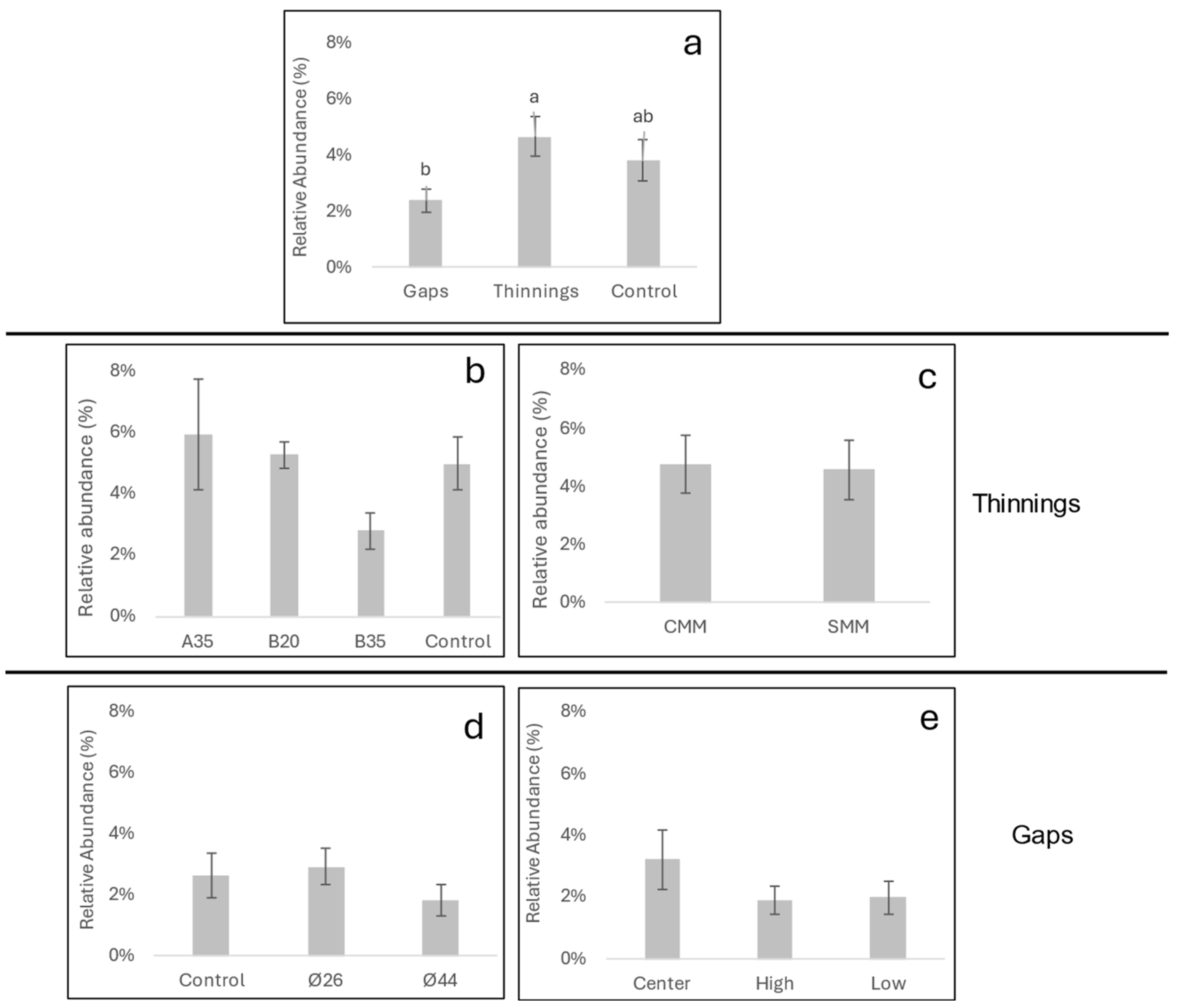
3.1. Abundance of Soil Pathogens
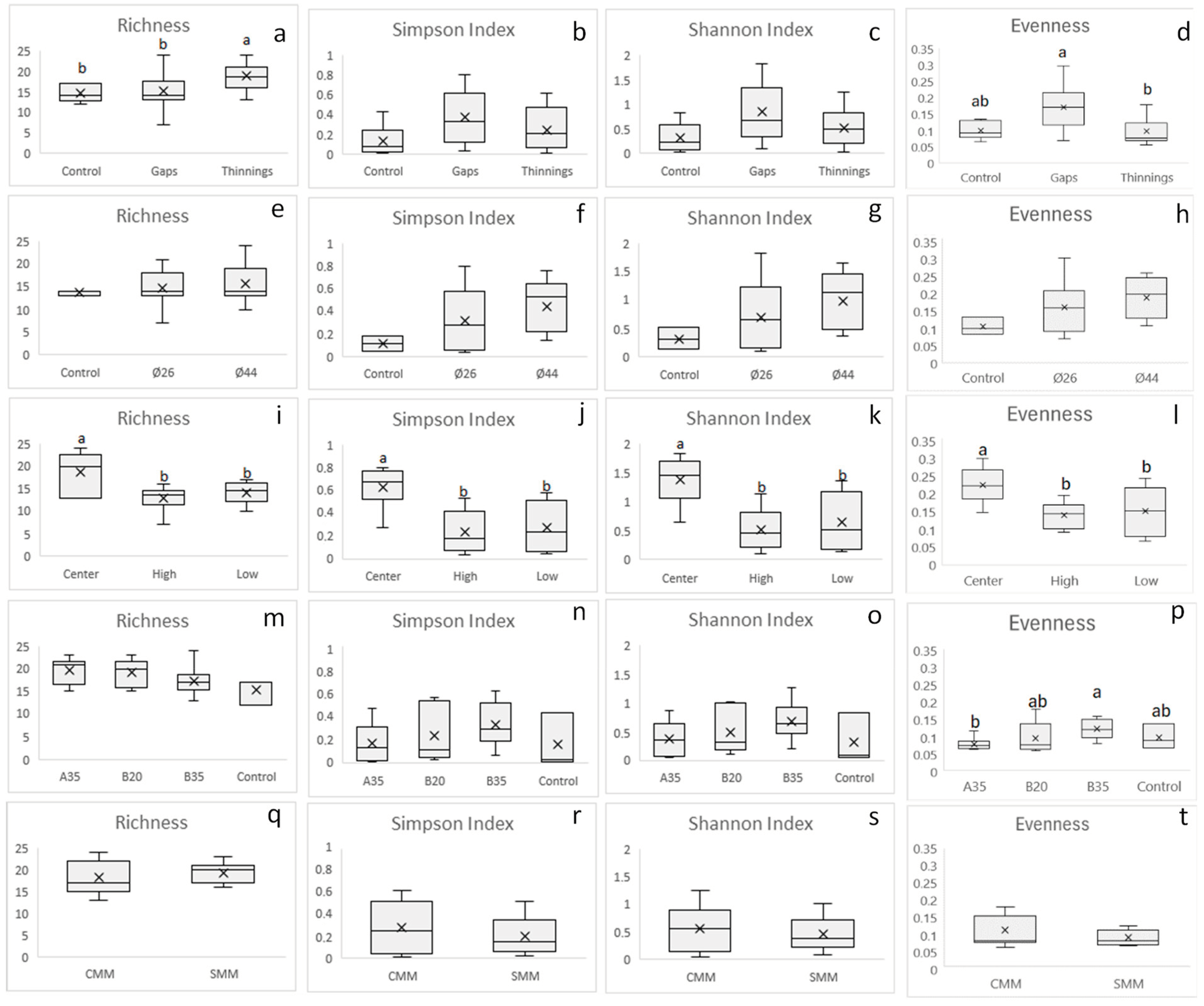
3.2. Diversity of Soil Plant Pathogens
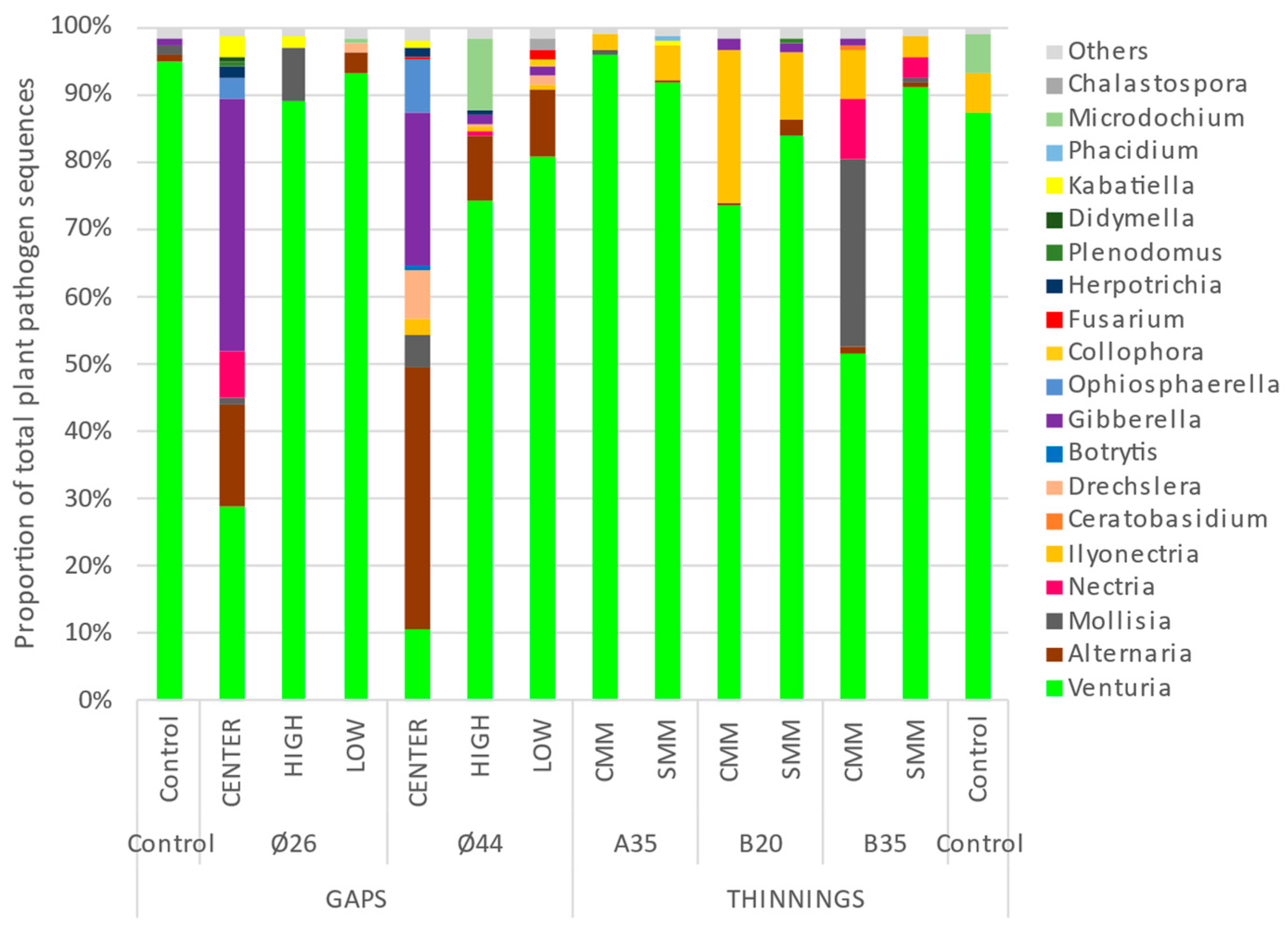
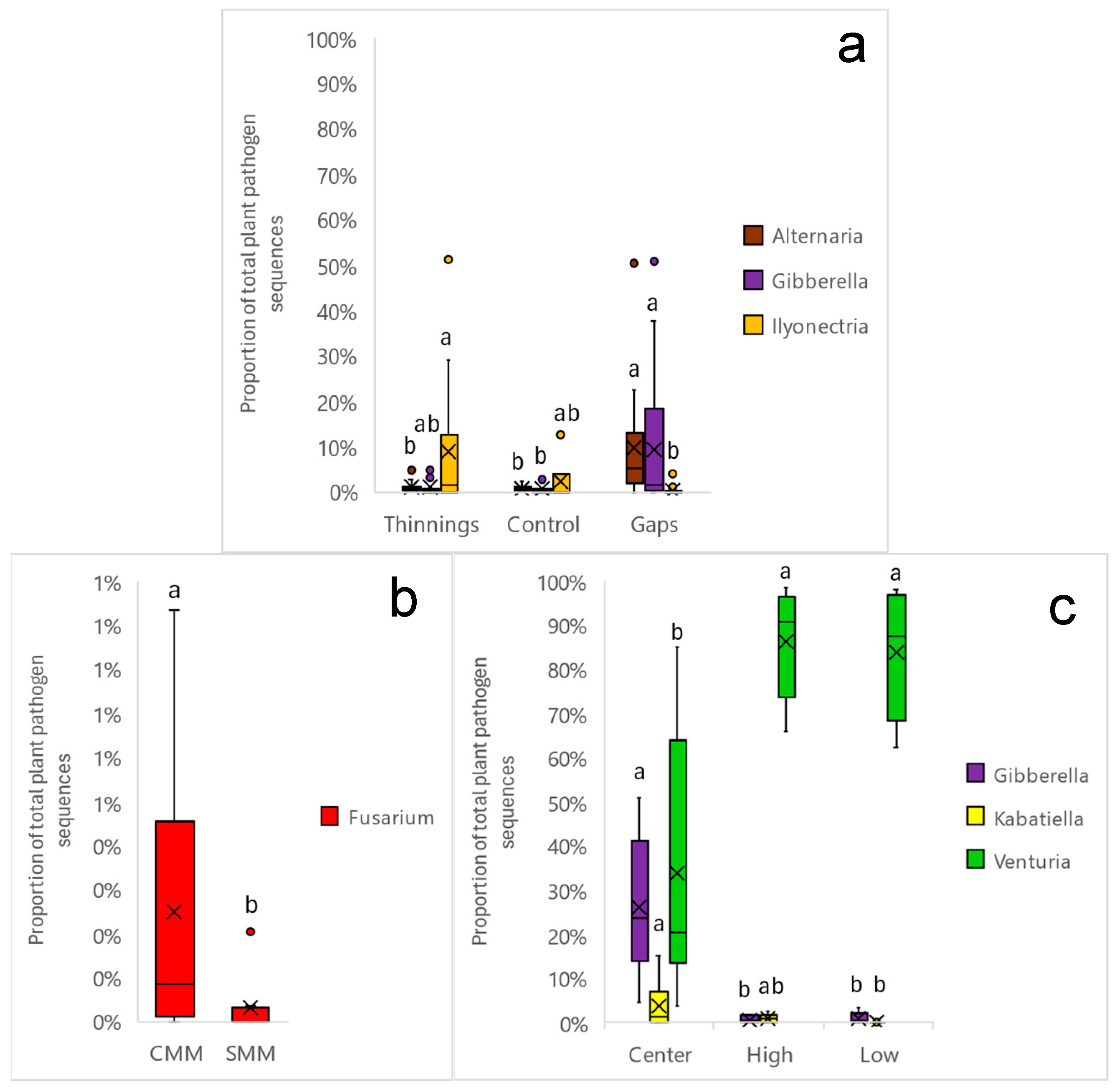
3.3. Dominance of Soil Plant Pathogen Taxa
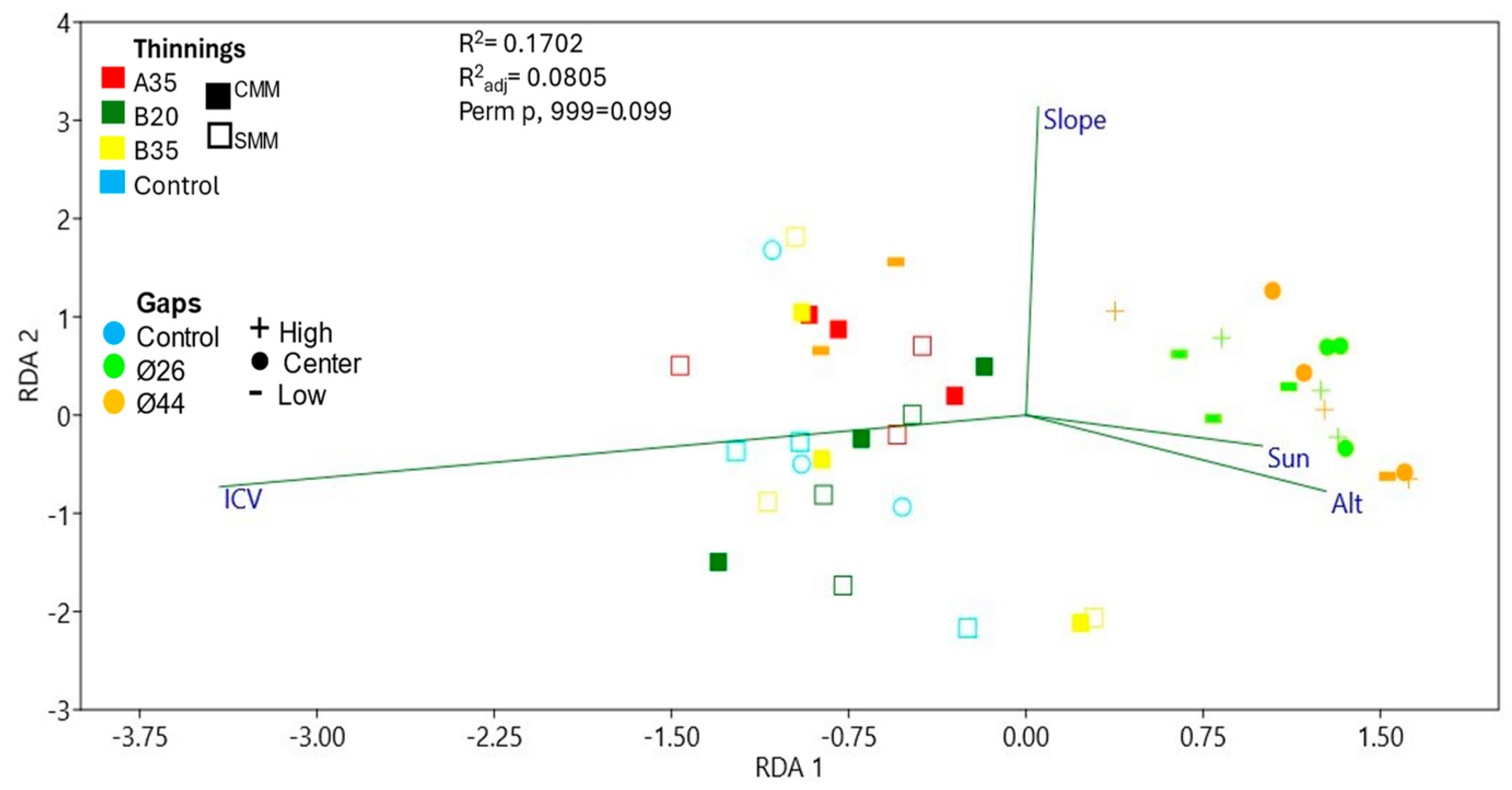
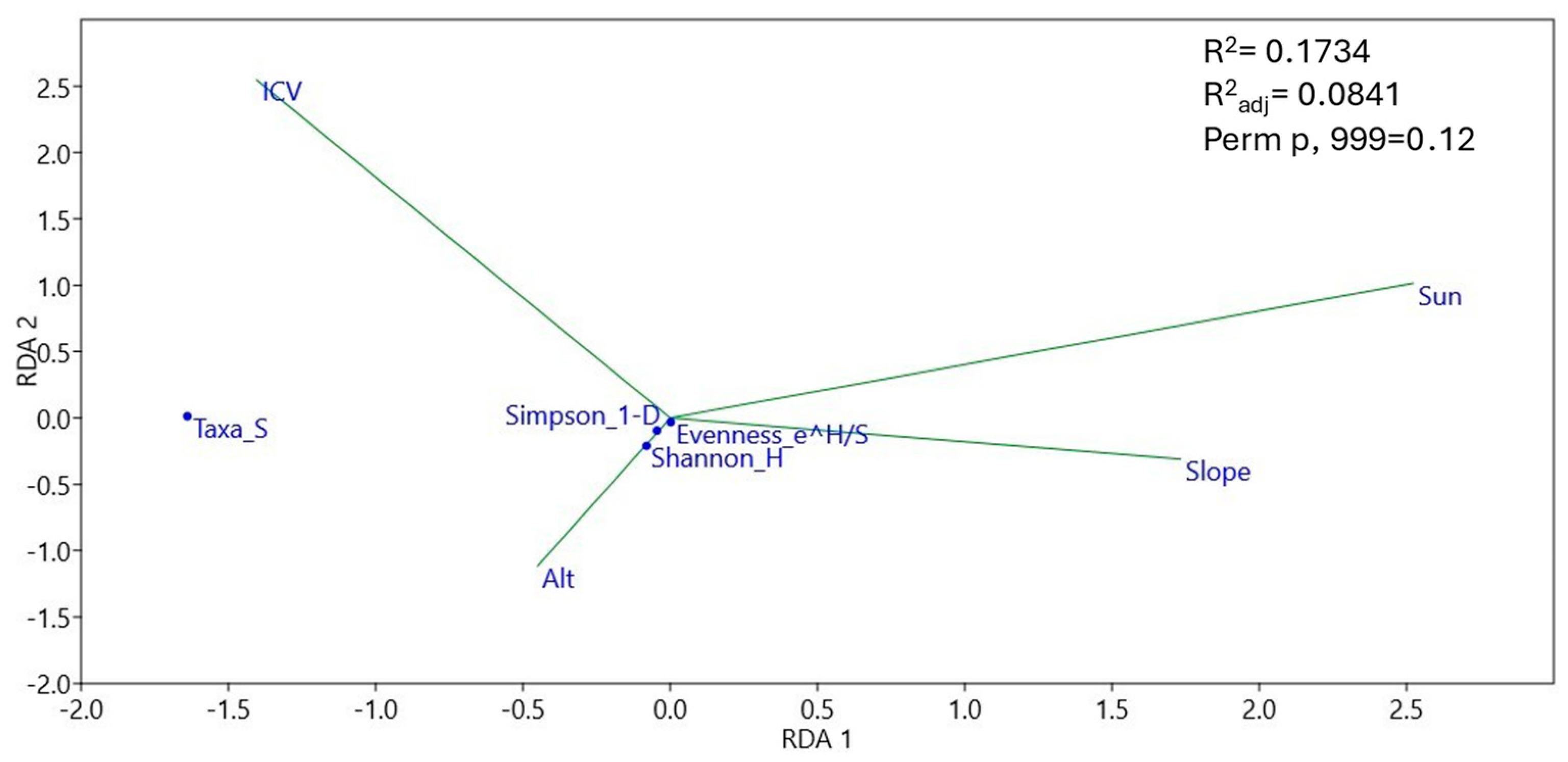
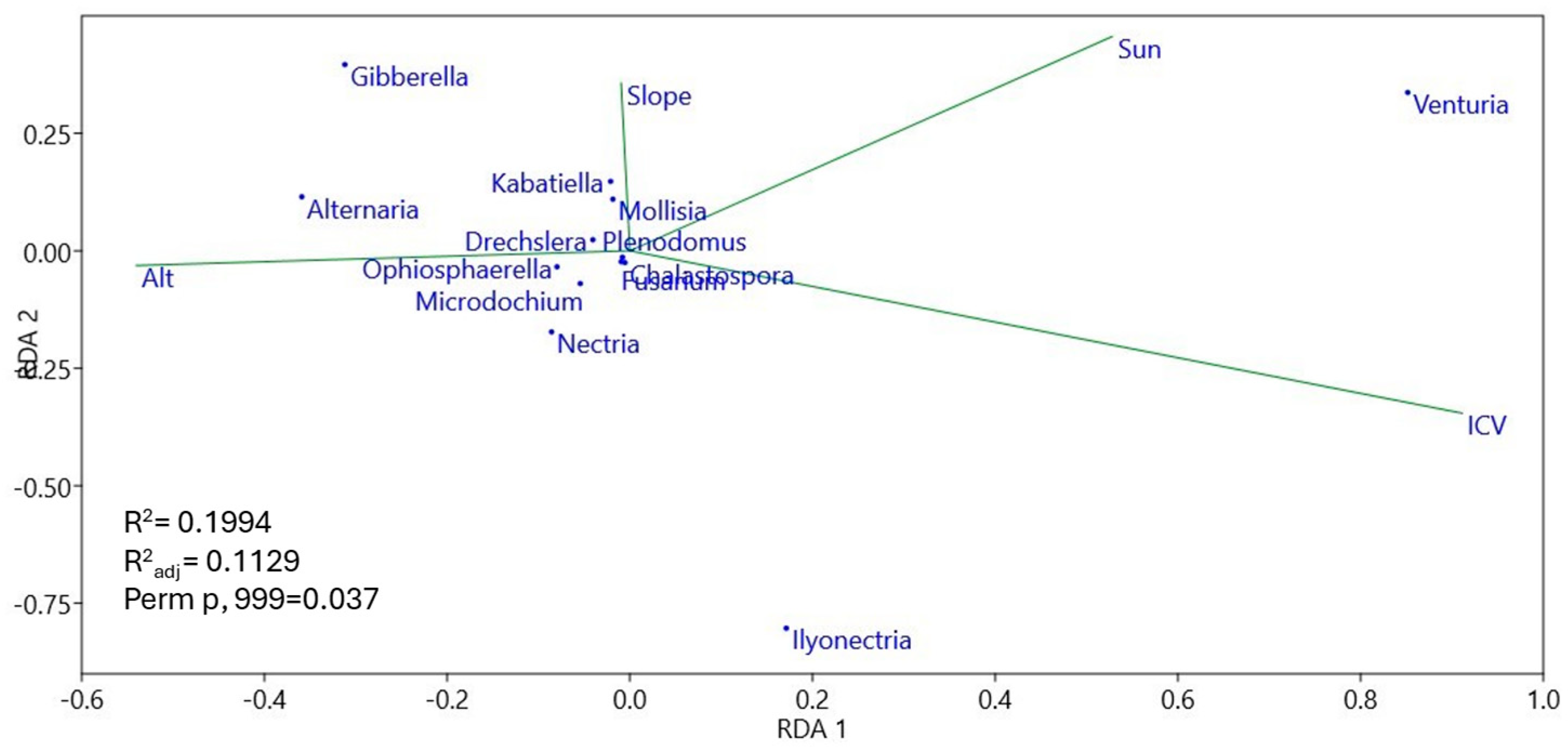
3.4. Community Structure and Environmental Effect on Soil Plant Pathogens
4. Discussion
4.1. Abundance of Soil Pathogens
4.2. Diversity of Soil Plant Pathogens
4.3. Dominance of Soil Plant Pathogen Taxa
4.4. Community Structure and Environmental Effect on Soil Plant Pathogens
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Joergensen, R.G.; Emmerling, C. Methods for evaluating human impact on soil microorganisms based ontheir activity, biomass, and diversity in agricultural soils. J. Plant Nutrit. Soil Sci. 2006, 169, 295–309. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Kohout, P.; Charvátová, M.; Štursová, M.; Mašínová, T.; Tomšovský, M.; Baldrian, P. Clearcutting alters decomposition processes and initiates complex restructuring of fungal communities in soil and tree roots. ISME 2018, 12, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.H.; Hytteborn, H. Gap structure, disturbance and regeneration in a primeval Picea abies forest. J. Veg. Sci. 1991, 2, 391–402. [Google Scholar] [CrossRef]
- Chavarriaga, D.; Bodles, W.J.A.; Leifert, C.; Belbahri, L.; Woodward, S. Phytophthora cinnamomi and other fine root pathogens in north temperate pine forests. FEMS Microbiol. Lett. 2007, 276, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Bever, J.D.; Mangan, S.A.; Alexander, H.M. Maintenance of plant species diversity by pathogens. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 305–325. [Google Scholar] [CrossRef]
- Ciccarone, C.; Rambelli, A. A study on micro-fungi in arid areas: Notes on soil saprotrophs and animal opportunistic pathogens. Plant Biosyst. 2000, 134, 25–29. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Guerra, C.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.-T.; Eisenhauer, N.; Singh, B.K.; Maestre, F.T. The proportion of soil-borne pathogens increases with warming at the global scale. Nat. Clim. Chang. 2020, 10, 550–554. [Google Scholar] [CrossRef]
- Dinoor, A.; Eshed, N. The role and importance of pathogens in natural plant communities. Annu. Rev. Phytopathol. 1984, 22, 443–466. [Google Scholar] [CrossRef]
- Barrett, L.G.; Kniskern, J.M.; Bodenhausen, N.; Zhang, W.; Bergelson, J. Continua of specificity and virulence in plant host-pathogen interactions: Causes and consequences. New Phytol. 2009, 183, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Delavaux, C.S.; Schemanski, J.L.; House, G.L.; Tipton, A.G.; Sikes, B.; Bever, J.D. Root pathogen diversity and composition varies with climate in undisturbed grasslands, but less so in anthropogenically disturbed grasslands. ISME J. 2021, 15, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Baral, S.; Thapa-Magar, K.B.; Karki, G.; Devkota, S.; Shrestha, B.B. Macrofungal diversity in community-managed sal (Shorea robusta) forests in central Nepal. Mycology 2015, 6, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.R.; Wang, P.H.; Chen, M.C.; Kuo, Y.L.; Chiang, P.N.; Wang, M.K. The impacts of thinning on the fruiting of saprophytic fungi in Cryptomeria japonica plantations in central Taiwan. For. Ecol. Manag. 2015, 336, 183–193. [Google Scholar] [CrossRef]
- Maghnia, F.Z.; Sanguin, H.; Abbas, Y.; Verdinelli, M.; Kerdouh, B.; El Ghachtouli, N.; Lancellotti, E.; Bakkali Yakhlef, S.E.; Duponnois, R. Impact du mode de gestión de la subéraie de la Maâmora (Maroc) sur la diversité des champignons ectomycorhiziens associés à Quercus suber. C. R. Biol. 2017, 340, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Engel, H.; Blaschke, M. Assemblages of wood-inhabiting fungi related to silvicultural management intensity in beech forests in southern Germany. Eur. J. For. Res. 2007, 126, 513–527. [Google Scholar] [CrossRef]
- Luoma, D.L.; Eberhart, J.L.; Molina, R.; Amaranthus, M.P. Response of ectomycorrhizal fungus sporocarp production to varying levels and patterns of green-tree retention. For. Ecol. Manag. 2004, 202, 337–354. [Google Scholar] [CrossRef]
- Rosenvald, R.; Lohmus, A. For what, when, and where is green-tree retention better than clear-cutting? A review of the biodiversity aspects. For. Ecol. Manag. 2008, 255, 1–15. [Google Scholar] [CrossRef]
- Varenius, K.; Lindahl, B.D.; Dahlberg, A. Retention of seed trees fails to lifeboat ectomycorrhizal fungal diversity in harvested Scots pine forests. FEMS Microbiol. Ecol. 2017, 93, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dove, N.C.; Keeton, W.S. Structural complexity enhancement increases fungal species richness in northern hardwood forests. Fungal Ecol. 2015, 13, 181–192. [Google Scholar] [CrossRef]
- Salerni, E.; Perini, C. Experimental study for increasing productivity of Boletus edulis s.l. in Italy. For. Ecol. Manag. 2004, 201, 161–170. [Google Scholar] [CrossRef]
- Baar, J.; Ter Braak, C.J.F. Ectomycorrhizal sporocarp occurrence as affected by manipulation of litter and humus layers in Scots pine stands of different age. Appl. Soil Ecol. 1996, 4, 61–73. [Google Scholar] [CrossRef]
- Treseder, K.K.; Lennon, J.T. Fungal Traits That Drive Ecosystem Dynamics on Land. Microbiol. Mol. Biol. Rev. 2015, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- FAO. Soil Map of the World; FAO-Unesco: Rome, Italy, 1988. [Google Scholar]
- Oliet, J.; Bravo, A.; Rubio, A.; de Frutos, S.; Roig, S. Descripción del Dispositivo Experimental del Proyecto FORADMIT: Acciones Selvícolas Para la Diversificación de Pinares Artificiales. Proyecto FORTRESS. 2022. PID2021-127241OB-I00. Available online: https://oa.upm.es/80752/3/80752.pdf (accessed on 20 April 2024).
- Ihrmark, K.; Bödeker, I.T.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region—Evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Põlme, S.; Abarenkov, K.; Henrik Nilsson, R.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. FungalTraits: A user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers. 2020, 105, 1–16. [Google Scholar] [CrossRef]
- Pemán García, J.; Iriarte Goñi, I.; Lario Leza, F.J. (Eds.) La Restauración Forestal de España. 75 Años de una Ilusión; Ministerio de Agricultura, Alimentación y Medio Ambiente, Sociedad Española de Ciencias Forestales: Madrid, Spain, 2017; pp. 176–226. [Google Scholar]
- Strauss, S.Y. Benefits and risks of biotic exchange between Eucalyptus plantations and native Australian forests. Austral. Ecol. 2001, 26, 447–457. [Google Scholar] [CrossRef]
- Liu, H.; Li, J. The study of the ecological problems of Eucalyptus plantation and sustainable development in maoming xiaoliang. J. Sustain. Dev. 2010, 3, 197–201. [Google Scholar] [CrossRef]
- Yu, L.; Zi, H.; Zhu, H.; Liao, Y.; Xu, X.; Li, X. Rhizosphere microbiome of forest trees is connected to their resistance to soil-borne pathogens. Plant Soil 2022, 479, 143–158. [Google Scholar] [CrossRef]
- Liu, Y.; He, F. Incorporating the diseasetriangle framework for testing the effect of soil-bornepathogens on tree species diversity. Funct. Ecol. 2019, 33, 1211–1222. [Google Scholar] [CrossRef]
- Hannula, S.E.; Morriën, E.; de Hollander, M.; van der Putten, W.H.; van Veen, J.A.; de Boer, W. Shifts in rhizosphere fungal community during secondary succession following abandonment from agriculture. ISME J 2017, 11, 2294–2304. [Google Scholar] [CrossRef] [PubMed]
- DeBellis, T.; Kernaghan, G.; Widden, P. Plant community influences on soil microfungal assemblages in boreal mixed-wood forests. Mycologia 2007, 99, 356–367. [Google Scholar] [CrossRef]
- Mäkipää, R.; Rajala, T.; Schigel, D.; Rinne, K.T.; Pennanen, T.; Abrego, N.; Ovaskainen, O. Interactions between soil- and dead wood-inhabiting fungal communities during the decay of Norway spruce logs. ISME J. 2017, 11, 1964–1974. [Google Scholar] [CrossRef]
- Castaño, C.; Alday, J.G.; Lindahl, B.D.; de Aragón, J.M.; de-Miguel, S.; Colinas, C.; Parladé, J.; Pera, J.; Bonet, J.A. Lack of thinning effects over inter-annual changes in soil fungal community and diversity in a Mediterranean pine forest. For. Ecol. Manag. 2018, 424, 420–427. [Google Scholar] [CrossRef]
- Rodriguez-Ramos, J.C.; Cale, J.A.; Cahill, J.F.; Simard, S.W.; Karst, J.; Erbilgin, N. Changes in soil fungal community composition depend on functional group and forest disturbance type. New Phytol. 2021, 229, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pinto, P.; Sanz-Benito, I.; Santos, M.; Oria-de-Rueda, J.A.; Geml, J. Anthropological impacts determine the soil fungal distribution of Mediterranean oak stands. Ecol. Indic. 2021, 132, 108343. [Google Scholar] [CrossRef]
- Costa, D.; Tavares, R.M.; Baptista, P.; Lino-Neto, T. Cork oak endophytic fungi as potential biocontrol agents against Biscogniauxia mediterranea and Diplodia corticola. J. Fungi 2020, 6, 287. [Google Scholar] [CrossRef] [PubMed]
- Mora-Sala, B.; Cabral, A.; León, M.; Agustí-Brisach, C.; Armengol, J.; Abad-Campos, P. Survey, identification, and characterization of Cylindrocarpon-like asexual morphs in Spanish forest nurseries. Plant Dis. 2018, 102, 2083–2100. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, M.; Etienne, R.S.; Wang, Y.; Staehelin, C.; Yu, S. Experimental evidence for a phylogenetic Janzen-Connell effect in a subtropical forest. Ecol. Lett. 2012, 15, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Winder, R.S.; Shamoun, S.F. Forest pathogens: Friend or foe to biodiversity? Can. J. Plant Pathol. 2006, 28, S221–S227. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martos, I.; Domínguez-Núñez, J.A. Soil Fungal Pathogens in Pinus pinaster Mature Reforestation: Silvicultural Treatments Effects. Pathogens 2024, 13, 637. https://doi.org/10.3390/pathogens13080637
Martos I, Domínguez-Núñez JA. Soil Fungal Pathogens in Pinus pinaster Mature Reforestation: Silvicultural Treatments Effects. Pathogens. 2024; 13(8):637. https://doi.org/10.3390/pathogens13080637
Chicago/Turabian StyleMartos, Iciar, and Jose Alfonso Domínguez-Núñez. 2024. "Soil Fungal Pathogens in Pinus pinaster Mature Reforestation: Silvicultural Treatments Effects" Pathogens 13, no. 8: 637. https://doi.org/10.3390/pathogens13080637





