Host-Pathogen Interaction and Resistance Mechanisms in Dermatophytes
Abstract
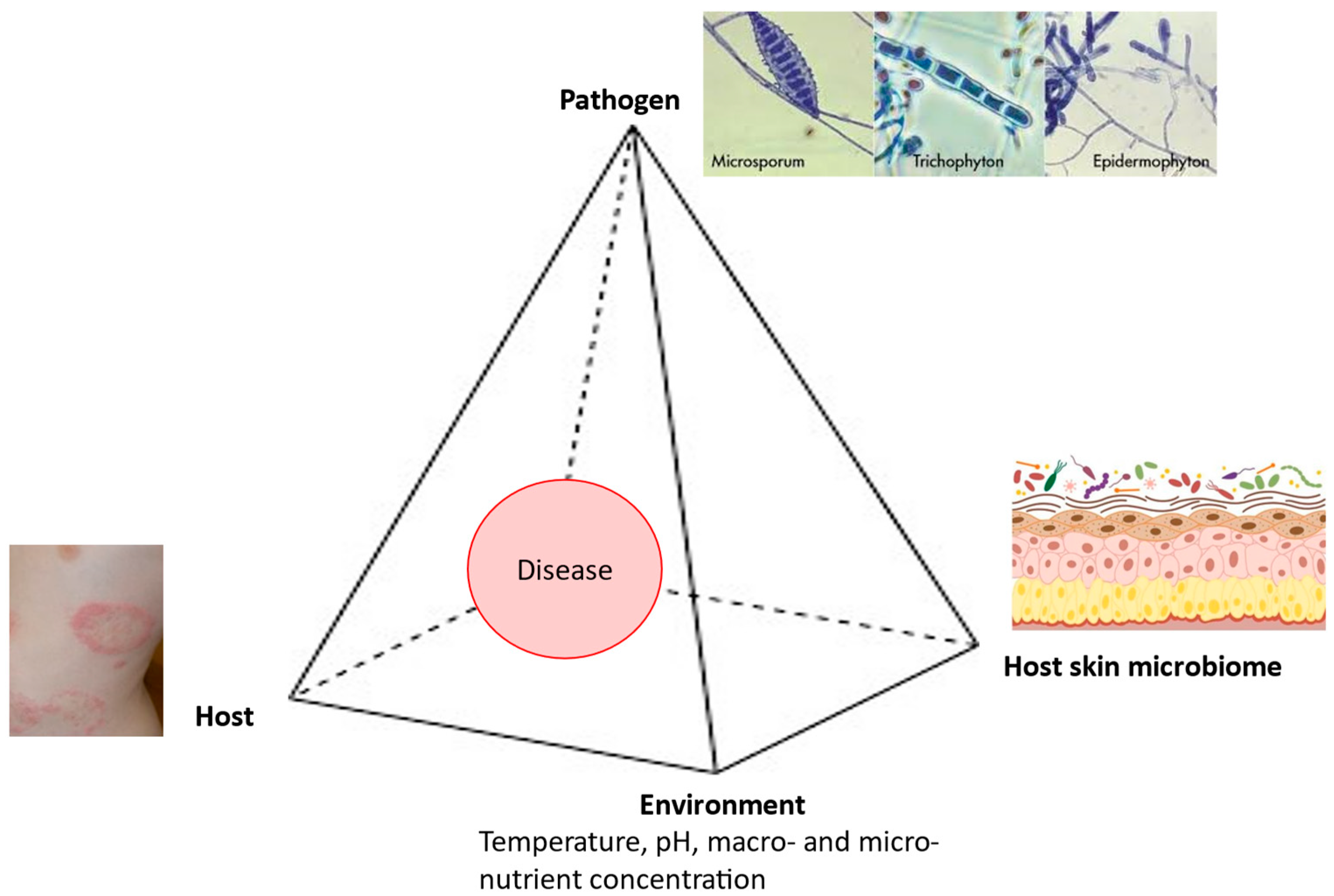
:1. Introduction
2. Pathogenesis
3. Pathogen
3.1. Adherence and Adhesins
3.2. Cell Wall Structure
3.3. Growth on Keratin Structures and Protein Degradation
3.4. Reduction in Cystine Disulfide Bridges
3.5. Secretory Proteases of Dermatophytes
3.6. Toxins
3.7. Biofilm Formation
3.8. Heat Shock Proteins
4. Host
5. Environment
5.1. Temperature
5.2. pH
5.3. Macro- and MicroNutrient Concentration
6. Host Microbiome
7. Dermatophytosis
Deep Dermatophytosis
8. Immune Response
8.1. Innate Immune Response
8.2. Acquired Immune Response
9. Antifungal Resistance
10. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- de Hoog, G.S.; Dukik, K.; Monod, M.; Packeu, A.; Stubbe, D.; Hendrickx, M.; Kupsch, C.; Stielow, J.B.; Freeke, J.; Göker, M.; et al. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 2017, 182, 5–31. [Google Scholar] [CrossRef]
- Ciesielska, A.; Kawa, A.; Kanarek, K.; Soboń, A.; Szewczyk, R. Metabolomic analysis of Trichophyton rubrum and Microsporum canis during keratin degradation. Sci. Rep. 2021, 11, 3959. [Google Scholar] [CrossRef]
- Gruby, M. Recherches sur la nature, le siége et le développment du Porrigo decalvans ou Phytoalopécie. CR Acad. Sci. Paris 1843, 17, 301–303. [Google Scholar]
- Graser, Y.; Kuijpers, A.F.; Presber, W.; de Hoog, G.S. Molecular taxonomy of the Trichophyton rubrum complex. J. Clin. Microbiol. 2000, 38, 3329–3336. [Google Scholar] [CrossRef] [PubMed]
- Sabouraud, R. Maladies du cuir chevelu. III. Les cryptogamiques. Les teignes. Masson Et Cie: Paris, France, 1910. [Google Scholar]
- Elewski, B.E.; Leyden, J.; Rinaldi, M.G.; Atillasoy, E. Office practice-based confirmation of onychomycosis: A US nationwide prospective survey. Arch. Intern. Med. 2002, 162, 2133–2138. [Google Scholar] [CrossRef]
- Graser, Y.; Scott, J.; Summerbell, R. The new species concept in dermatophytes—A polyphasic approach. Mycopathologia 2008, 166, 239–256. [Google Scholar] [CrossRef]
- Uhrlaß, S.; Verma, S.B.; Gräser, Y.; Rezaei-Matehkolaei, A.; Hatami, M.; Schaller, M.; Nenoff, P. Trichophyton indotineae—An Emerging Pathogen Causing Recalcitrant Dermatophytoses in India and Worldwide-A Multidimensional Perspective. J. Fungi 2022, 8, 757. [Google Scholar] [CrossRef] [PubMed]
- Graser, Y.; De Hoog, S.; Summerbell, R.C. Dermatophytes: Recognizing species of clonal fungi. Med. Mycol. 2006, 44, 199–209. [Google Scholar] [CrossRef]
- Taylor, J.W. One Fungus = One Name: DNA and fungal nomenclature twenty years after PCR. IMA Fungus 2011, 2, 113–120. [Google Scholar] [CrossRef]
- Gnat, S.; Lagowski, D.; Nowakiewicz, A. Major challenges and perspectives in the diagnostics and treatment of dermatophyte infections. J. Appl. Microbiol. 2020, 129, 212–232. [Google Scholar] [CrossRef]
- Dubljanin, E.; Dzamic, A.; Vujcic, I.; Mijatovic, S.; Crvenkov, T.; Grujicic, S.S.; Calovski, I.C. Correlation of clinical characteristics, by calculation of SCIO index, with the laboratory diagnosis of onychomycosis. Braz. J. Microbiol. 2022, 53, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Arsić-Arsenijević, V.S.; Branković, M.; Čolović, I.; Džamić, A.M.; Mitrović, S.; Ratkov, E. Antimiycotics susceptibility testing of dermatophytes. Srp. Arh. Celok. Lek. 2010, 138, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Ratkov, E.; Vidović, A.; Minić, P.; Janić, D.; Šipetić-Grujičić, S.; Džamić, A.; Arsić-Arsenijević, V. Detection of laboratory biomarkes in haematological and pulmonology patients at high risk for aspergillosis. Srp. Arh. Celok. Lek. 2012, 140, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Dubljanin, E.; Čolović Čalovski, I.C.; Vujčić, I.; Džamić, A.; Arendrup, M.C.; Petersen, R.F.; Jensen, R.H. Clinical evaluation of a T. rubrum-specific polymerase chain reaction and pandermatophyte polymerase chain reaction in the diagnosis of suspected onychomycosis in 183 Serbian patients [letter]. Br. J. Dermatol. 2014, 171, 1593–1595. [Google Scholar] [CrossRef] [PubMed]
- Gnat, S.; Lagowski, D.; Nowakiewicz, A. Genetic Predisposition and its Heredity in the Context of Increased Prevalence of Dermatophytoses. Mycopathologia 2021, 186, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Celestrino, G.A.; Verrinder Veasey, J.; Benard, G.; Sousa, M.G.T. Host immune responses in dermatophytes infection. Mycoses 2021, 64, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Sardana, K.; Gupta, A.; Mathachan, S.R. Immunopathogenesis of Dermatophytoses and Factors Leading to Recalcitrant Infections. Indian Dermatol. Online J. 2021, 12, 389–399. [Google Scholar] [CrossRef]
- AL-Khikani, F.H. Dermatophytosis a worldwide contiguous fungal infection: Growing challenge and few solutions. Biomed. Biotechnol. Res. J. 2020, 4, 117–122. [Google Scholar] [CrossRef]
- Rokas, A. Evolution of the human pathogenic lifestyle in fungi. Nat. Microbiol. 2022, 7, 607–619. [Google Scholar] [CrossRef]
- Toussaint, F.; Sticherling, M. Multiple dermal abscesses by Trichophyton rubrum in an immunocompromised patient. Front. Med. 2019, 6, 97. [Google Scholar] [CrossRef]
- Long, S.; Carveth, H.; Chang, Y.M.; O’Neill, D.; Bond, R. Isolation of dermatophytes from dogs and cats in the South of England between 1991 and 2017. Veterinary Record 2020, 187, e87. [Google Scholar] [CrossRef] [PubMed]
- Effendy, I.; Lecha, M.; Feuilhade de Chauvin, M.; Di Chiacchio, N.; Baran, R. Epidemiology and clinical classification of onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Dubljanin, E.; Dzamic, A.; Vujcic, I.; Grujicic, S.S.; Arsenijević, V.A.; Mitrovic, S.; Calovski, I.C. Epidemiology of onychomycosis in Serbia: A laboratory-based survey and risk factor identification. Mycoses 2017, 60, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Dubljanin, E.; Crvenkov, T.; Vujcic, I.; Grujicic, S.S.; Dubljanin, J.; Dzamic, A. Fungal contamination of medical students’ mobile phones from the University of Belgrade, Serbia: A cross-sectional study. Sci. Rep. 2022, 12, 16852. [Google Scholar] [CrossRef]
- Dubljanin, E.; Dzamic, A.; Mitrovic, S.; Arsic Arsenijevic, V.; Colovic Calovski, I. Onychomycosis: Clinical findings, etiological agents and evaluation of laboratory methods. Arch. Biol. Sci. 2014, 66, 587–594. [Google Scholar] [CrossRef]
- Wang, L.; Ma, L.; Leng, W.; Liu, T.; Yu, L.; Yang, J.; Yang, L.; Zhang, W.; Zhang, Q.; Dong, J.; et al. Analysis of the dermatophyte Trichophyton rubrum expressed sequence tags. BMC Genomics. 2006, 7, 255. [Google Scholar] [CrossRef]
- Vermout, S.; Tabart, J.; Baldo, A.; Mathy, A.; Losson, B.; Mignon, B. Pathogenesis of dermatophytosis. Mycopathologia 2008, 166, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, D.; Alviano, C.S.; de Souza, W.; Rozental, S. The influence of surface carbohydrates during in vitro infection of mammalian cells by the dermatophyte Trichophyton rubrum. Res. Microbiol. 2004, 155, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Monod, M.; Lechenne, B.; Jousson, O.; Grand, D.; Zaugg, C.; Stocklin, R.; Grouzmann, E. Aminopeptidases and dipeptidyl-peptidases secreted by the dermatophyte Trichophyton rubrum. Microbiology 2005, 15, 145–155. [Google Scholar] [CrossRef]
- Kaufman, G.; Horwitz, B.A.; Duek, L.; Ullman, Y.; Berdicevsky, I. Infection stages of the dermatophyte pathogen Trichophyton: Microscopic characterization and proteolytic enzymes. Med. Mycol. 2007, 45, 149–155. [Google Scholar] [CrossRef]
- De Bernardis, F.; Liu, H.; O’Mahony, R.; La Valle, R.; Bartollino, S.; Sandini, S.; Grant, S.; Brewis, S.; Tonlinson, I.; Basset, R.C.; et al. Human domain antibodies against virulence traits of Candida albicans inhibit fungus adherence to vaginal epithelium and protect against experimental vaginal candidiasis. J. Infect. Dis. 2007, 195, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rossi, N.M.; Peres, N.T.A.; Bitencourt, T.A.; Martins, M.P.; Rossi, A. State-of-the-Art Dermatophyte Infections: Epidemiology Aspects, Pathophysiology, and Resistance Mechanisms. J. Fungi 2021, 7, 629. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, A.E.; Kajava, A.V.; Steinert, P.M. Epithelial barrier function: Assembly and structural features of the cornified cell envelope. BioEssays 2000, 24, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Monod, M. Secreted Proteases from Dermatophytes. Mycopathologia 2008, 166, 285–294. [Google Scholar] [CrossRef]
- Chua, W.; Poh, S.E.; Li, H. Secretory Proteases of the Human Skin Microbiome. Infect. Immun. 2022, 90, e0039721. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.; Gonca, S.; Kandemir, H.; Döğen, A.; Hilmioğlu-Polat, S.; Ilkit, M.; Tanaka, R.; Yaguchi, T.; Uhrlaβ, S.; Nenoff, P. Genes Encoding Proteolytic Enzymes Fungalysin and Subtilisin in Dermatophytes of Human and Animal Origin: A Comparative Study. Mycopathologia 2020, 185, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Khedmati, E.; Hazaveh, S.J.H.; Bayat, M.; Amini, K. Identification of subtilisin virulence genes (SUB1-7) in Epidermophyton floccosum isolated from patients with dermatophytosis in Iran. Gene Rep. 2020, 20, 100748. [Google Scholar] [CrossRef]
- Vite-Garín, T.; Estrada-Cruz, N.A.; Hernández-Castro, R.; Fuentes-Venado, C.E.; Zarate-Segura, P.B.; Frías-De-León, M.G.; Martínez-Castillo, M.; Martínez-Herrera, E.; Pinto-Almazán, R. Remarkable Phenotypic Virulence Factors of Microsporum canis and Their Associated Genes: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 2533. [Google Scholar] [CrossRef] [PubMed]
- Baumbach, C.M.; Michler, J.K.; Nenoff, P.; Uhrlaß, S.; Schrödl, W. Visualising virulence factors: Trichophyton benhamiaes subtilisins demonstrated in a guinea pig skin ex vivo model. Mycoses 2020, 63, 970–978. [Google Scholar] [CrossRef]
- Naeimipour, F.; Hashemi, S.J.; Rezaie, S.; Bayat, M. Subtilisin Gene Activity in Dermatophytes: A study on the Presence of the Subtilisin Gene in Trichophyton verrucosum and Microsporum gypseum in Clinical and Nonclinical Samples in Tehran, Iran. Arch. Razi Inst. 2021, 76, 253–259. [Google Scholar]
- Mercer, D.K.; Stewart, C.S. Keratin hydrolysis by dermatophytes. Med. Mycol. 2019, 57, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Martins-Santana, L.; Petrucelli, M.F.; Sanches, P.R.; Martinez-Rossi, N.M.; Rossi, A. Peptidase Regulation in Trichophyton rubrum Is Mediated by the Synergism Between Alternative Splicing and StuA-Dependent Transcriptional Mechanisms. Front. Microbiol. 2022, 13, 930398. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rossi, N.M.; Persinoti, G.F.; Peres, N.T.; Rossi, A. Role of pH in the pathogenesis of dermatophytoses. Mycoses 2012, 55, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Neves-da-Rocha, J.; Santos-Saboya, M.J.; Lopes, M.E.R.; Rossi, A.; Martinez-Rossi, N.M. Insights and Perspectives on the Role of Proteostasis and Heat Shock Proteins in Fungal Infections. Microorganisms 2023, 11, 1878. [Google Scholar] [CrossRef] [PubMed]
- Burstein, V.L.; Beccacece, I.; Guasconi, L.; Mena, C.J.; Cervi, L.; Chiapello, L.S. Skin Immunity to Dermatophytes: From Experimental Infection Models to Human Disease. Front. Immunol. 2020, 11, 605644. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, C.A. Candida biofilms. Curr. Opin. Microbiol. 2002, 5, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ding, H.; Ke, W.; Wang, L. Quorum Sensing in Fungal Species. Annu. Rev. Microbiol. 2021, 75, 449–469. [Google Scholar] [CrossRef]
- Kovács, R.; Majoros, L. Fungal Quorum-Sensing Molecules: A Review of Their Antifungal Effect against Candida Biofilms. J. Fungi 2020, 6, 99. [Google Scholar] [CrossRef]
- Costa-Orlandi, C.B.; Sardi, J.C.O.; Pitangui, N.S.; De Oliveira, H.C.; Scorzoni, L.; Galeane, M.C.; Medina-Alarcón, K.P.; Melo, W.C.M.A.; Marcelino, M.Y.; Braz, J.D.; et al. Fungal Biofilms and Polymicrobial Diseases. J. Fungi 2017, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Markantonatou, A.M.; Samaras, K.; Vyzantiadis, T.A. Dermatophytic Biofilms: Characteristics, Significance and Treatment Approaches. J. Fungi 2023, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Wang, T.; Cooper, E.A. Dermatophytomas: Clinical Overview and Treatment. J. Fungi 2022, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rossi, N.M.; Jacob, T.R.; Sanches, P.R.; Peres, N.T.; Lang, E.A.; Martins, M.P.; Rossi, A. Heat Shock Proteins in Dermatophytes: Current Advances and Perspectives. Curr. Genomics 2016, 17, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Horianopoulos, L.C.; Kronstad, J.W. Chaperone Networks in Fungal Pathogens of Humans. J. Fungi 2021, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- García-Romero, M.T.; Arenas, R. New insights into genes, immunity, and the occurrence of dermatophytosis. J. Investig. Dermatol. 2015, 135, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.A.; Franco, R.M.; Lionakis, M.S. Human CARD9: A critical molecule of fungal immune surveillance. Front. Immunol. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Gnat, S.; Nowakiewitcz, A.; Lagowski, D.; Zieba, P. Host- and pathogen- dependent susceptibility and predisposition to dermatophytosis. J. Med. Microbiol. 2019, 68, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Pathan, S.; Vincent, Q.B.; Liu, L.; Cypowyj, S.; Prando, C.; Migaud, M.; Taibi, L.; Ammar-Khodja, A.; Stambouli, O.B.; et al. Deep dermatophytosis and inherited CARD9 deficiency. N. Engl. J. Med. 2013, 369, 1704–1714. [Google Scholar] [CrossRef]
- Peixoto, R.R.G.B.; Meneses, O.M.S.; da Silva, F.O.; Donati, A.; Veasey, J.V. Tinea Capitis: Correlation of Clinical Aspects, Findings on Direct Mycological Examination, and Agents Isolated from Fungal Culture. Int. J. Trichol. 2019, 11, 232–235. [Google Scholar] [CrossRef]
- Moriello, K.A.; Verbrugge, M.J.; Kesting, R.A. Effects of temperature variations and light exposure on the time to growth of dermatophytes using six different fungal culture media inoculated with laboratory strains and samples obtained from infected cats. J. Feline Med. Surg. 2010, 12, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Keshwania, P.; Kaur, N.; Chauhan, J.; Sharma, G.; Afzal, O.; Alfawaz Altamimi, A.S.; Almalki, W.H. Superficial Dermatophytosis across the World’s Populations: Potential Benefits from Nanocarrier-Based Therapies and Rising Challenges. ACS Omega 2023, 8, 31575–31599. [Google Scholar] [CrossRef] [PubMed]
- Pontes, Z.B.; Oliveira, A.C.; Guerra, F.Q.; Pontes, L.R.; Santos, J.P. Distribution of dermatophytes from soils of urban and rural areas of cities of Paraiba State, Brazil. Rev. Inst. Med. Trop. São Paulo 2013, 55, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Lübeck, M.; Lübeck, P.S. Fungal Cell Factories for Efficient and Sustainable Production of Proteins and Peptides. Microorganisms 2022, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.K.; Stewart, C.S.; Miller, L.; Robertson, J.; Duncan, V.M.S.; O’Neil, D.A. Improved Methods for Assessing Therapeutic Potential of Antifungal Agents against Dermatophytes and Their Application in the Development of NP213, a Novel Onychomycosis Therapy Candidate. Antimicrob. Agents Chemother. 2019, 63, e02117–e02118. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Flowers, L.; Grice, E.A. The Skin Microbiota: Balancing Risk and Reward. Cell Host Microbe 2020, 28, 190–200. [Google Scholar] [CrossRef]
- Martinez-Rossi, N.M.; Bitencourt, T.A.; Peres, N.T.A.; Lang, E.A.S.; Gomes, E.V.; Quaresemin, N.R.; Martins, M.P.; Lopes, L.; Rossi, A. Dermatophyte Resistance to Antifungal Drugs: Mechanisms and Prospectus. Front. Microbiol. 2018, 9, 1108. [Google Scholar] [CrossRef]
- Wang, R.; Huang, C.; Zhang, Y.; Li, R. Invasive dermatophyte infection: A systematic review. Mycoses 2021, 64, 340–348. [Google Scholar] [CrossRef]
- Rouzaud, C.; Hay, R.; Chosidow, O.; Dupin, N.; Puel, A.; Lortholary, O.; Lanternier, F. Severe Dermatophytosis and Acquired or Innate Immunodeficiency: A Review. J. Fungi 2015, 2, 4. [Google Scholar] [CrossRef]
- Blechert, O.; Xiong, S.; Chen, J.; Brand, A.C.; Zhan, P. Nutritional requirements of the human pathogenic fungus, Trichophyton rubrum, and nutritional immunity of the human skin as barrier against colonization. Fungal Biol. Rev. 2023, 45, 100330. [Google Scholar] [CrossRef]
- Sugita, K.; Kabashima, K.; Atarashi, K.; Shimauchi, T.; Kobayashi, M.; Tokura, Y. Innate immunity mediated by epidermal keratinocytes promotes acquired immunity involving Langerhans cells and T cells in the skin. Clin. Exp. Immunol. 2007, 147, 176–183. [Google Scholar] [CrossRef]
- Almeida, S.R. Immunology of dermatophytosis. Micopathologia 2008, 166, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Hau, C.S.; Tada, Y.; Kanda, N.; Watanabe, S. Immunoresponses in dermatomycoses. J. Dermatol. 2015, 42, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Das, S.; Gaurav, V.; Singh, P.K.; Rai, G.; Datt, S.; Tigga, R.A.; Pandhi, D.; Bhattacharya, S.N.; Ansari, M.A.; et al. Review on host-pathogen interaction in dermatophyte infections. J. Mycol. Med. 2023, 33, 101331. [Google Scholar] [CrossRef]
- Campos, M.R.M.; Russo, M.; Gomes, E.; Almeida, S.R. Stimulation, inhibition and death of macrophages infected with Trichophyton rubrum. Microbes Infect. 2006, 8, 372–379. [Google Scholar] [CrossRef]
- Kopp, E.; Medzhitov, R. Recognition of microbial infection by Toll-like receptors. Curr. Opin. Immunol. 2003, 15, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.J.; Gordon, S. Dectin-1 mediates the biological effects of b-glucans. J. Exp. Med. 2003, 197, 1119–1124. [Google Scholar] [CrossRef]
- Sato, K.; Yang, X.L.; Yudate, T.; Chung, J.S.; Wu, J.; Luby-Phelps, K.; Kimberly, R.P.; Underhill, D.; Cruz, P.D., Jr.; Ariizumi, K. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor c chain to induce innate immune responses. J. Biol. Chem. 2006, 28, 38854–38866. [Google Scholar] [CrossRef]
- Woodfolk, J.A.; Platts-Mills, T.A. Diversity of the human allergen-specific T cell repertoire associated with distinct skin test reactions: Delayed-type hypersensitivity-associated major epitopes induce Th1- and Th2-dominated responses. J. Immunol. 2001, 167, 5412–5419. [Google Scholar] [CrossRef]
- Gupta, C.; Das, S.; Ramachandran, V.G.; Saha, R.; Bhattacharya, S.N.; Dar, S.A.; Atri, D. Possible role of trichophytin antigen in inducing impaired immunological clearance of fungus in onychomycosis. Mycopathologia 2016, 181, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Heinen, M.P.; Cambier, L.; Fievez, L.; Mignon, B. Are Th17 cells playing a role in immunity to dermatophytosis? Mycopathologia 2016, 182, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Heinen, M.P.; Cambier, L.; Antoine, N.; Gabriel, A.; Gillet, L.; Bureau, F.; Mignon, B. Th1 and Th17 Immune Responses Act Complementarily to Optimally Control Superficial Dermatophytosis. J. Investment Dermatol. 2019, 139, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Wang, X.; Li, R. Dermatophyte infection: From fungal pathogenicity to host immune responses. Front. Immunol. 2023, 14, 1285887. [Google Scholar] [CrossRef] [PubMed]
- Monod, M. Antifungal resistance in dermatophytes: Emerging problem and challenge for the medical community. J. Mycol. Med. 2019, 29, 283–284. [Google Scholar] [CrossRef]
- Khurana, A.; Sardana, K.; Chowdhary, A. Antifungal resistance in dermatophytes: Recent trends and therapeutic implications. Fungal Genet. Biol. 2019, 132, 103255. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Leidich, S.D.; Isham, N.; Leitner, I.; Ryder, N.S.; Ghannoum, M.A. Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob. Agents Chemother. 2003, 47, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.S.; Leitner, I.; Favre, B.; Ryder, N.S. Amino acid substitution in Trichophyton rubrum squalene epoxidase associated with resistance to terbinafine. Antimicrob. Agents Chemother. 2005, 49, 2840–2844. [Google Scholar] [CrossRef]
- Yamada, T.; Maeda, M.; Alshahni, M.M.; Tanaka, R.; Yaguchi, T.; Bontems, O.; Salamin, K.; Fratti, M.; Monod, M. Terbinafine resistance of Trichophyton clinical isolates caused by specific point mutations in the squalene epoxidase gene. Antimicrob. Agents Chemother. 2017, 61, e00115–e00117. [Google Scholar] [CrossRef]
- Hiruma, J.; Kitagawa, H.; Noguchi, H.; Kano, R.; Hiruma, M.; Kamata, H.; Harada, K. Terbinafine-resistant strain of Trichophyton interdigitale strain isolated from a tinea pedis patient. J. Dermatol. 2019, 46, 351–353. [Google Scholar] [CrossRef]
- Saunte, D.M.L.; Hare, R.K.; Jørgensen, K.M.; Jørgensen, R.; Deleuran, M.; Zachariae, C.O.; Thomsen, S.F.; Bjørnskov-Halkier, L.; Kofoed, K.; Arendrup, M.C. Emerging terbinafine resistance in Trichophyton: Clinical characteristics, squalene epoxidase gene mutations and a reliable EUCAST method for detection. Antimicrob. Agents Chemother. 2019, 63, e01126-19. [Google Scholar] [CrossRef] [PubMed]
- Nenoff, P.; Verma, S.B.; Ebert, A.; Süß, A.; Fischer, E.; Auerswald, E.; Dessoi, S.; Hofmann, W.; Schmidt, S.; Neubert, K.; et al. Spread of terbinafine-resistant trichophyton mentagrophytes type VIII (India) in Germany—“ the tip of the iceberg?”. J. Fungi 2020, 6, 207. [Google Scholar] [CrossRef] [PubMed]
- Łagowski, D.; Gnat, S.; Nowakiewicz, A.; Osińska, M.; Dyląg, M. Intrinsic resistance to terbinafine among human and animal isolates of Trichophyton mentagrophytes related to amino acid substitution in the squalene epoxidase. Infection 2020, 48, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Monod, M.; Feuermann, M.; Salamin, K.; Fratti, M.; Makino, M.; Alshahni, M.M.; Makimura, K.; Yamada, T. Trichophyton rubrum azole resistance mediated by a new ABC transporter, TruMDR3. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Sacheli, R.; Hayette, M.P. Antifungal Resistance in Dermatophytes: Genetic Considerations, Clinical Presentations and Alternative Therapies. J. Fungi 2021, 7, 983. [Google Scholar] [CrossRef] [PubMed]
- Kano, R.; Kimura, U.; Kakurai, M.; Hiruma, J.; Kamata, H.; Suga, Y.; Harada, K. Trichophyton indotineae sp. nov.: A New Highly Terbinafine-Resistant Anthropophilic Dermatophyte Species. Mycopathologia 2020, 185, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Kolarczyková, D.; Lysková, P.; Švarcová, M.; Kuklová, I.; Dobiáš, R.; Mallátová, N.; Kolařík, M.; Hubka, V. Terbinafine resistance in Trichophyton mentagrophytes and Trichophyton rubrum in the Czech Republic: A prospective multicentric study. Mycoses 2024, 67, e13708. [Google Scholar] [CrossRef]
- Kong, X.; Tang, C.; Singh, A.; Ahmed, S.A.; Al-Hatmi, A.M.; Chowdhary, A.; Nenoff, P.; Gräser, Y.; Hainsworth, S.; Zhan, P.; et al. Antifungal susceptibility and mutations in the squalene epoxidase gene in dermatophytes of the Trichophyton mentagrophytes species complex. Antimicrob. Agents Chemother. 2021, 65, 10–128. [Google Scholar] [CrossRef]
- Yamada, T.; Yaguchi, T.; Salamin, K.; Guenova, E.; Feuermann, M.; Monod, M. Mfs1, a pleiotropic transporter in dermatophytes that plays a key role in their intrinsic resistance to chloramphenicol and fluconazole. J. Fungi 2021, 7, 542. [Google Scholar] [CrossRef]
- Siopi, M.; Efstathiou, I.; Theodoropoulos, K.; Pournaras, S.; Meletiadis, J. Molecular Epidemiology and Antifungal Susceptibility of Trichophyton Isolates in Greece: Emergence of Terbinafine-Resistant Trichophyton mentagrophytes Type VIII Locally and Globally. J. Fungi 2021, 7, 419. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubljanin, E.; Zunic, J.; Vujcic, I.; Colovic Calovski, I.; Sipetic Grujicic, S.; Mijatovic, S.; Dzamic, A. Host-Pathogen Interaction and Resistance Mechanisms in Dermatophytes. Pathogens 2024, 13, 657. https://doi.org/10.3390/pathogens13080657
Dubljanin E, Zunic J, Vujcic I, Colovic Calovski I, Sipetic Grujicic S, Mijatovic S, Dzamic A. Host-Pathogen Interaction and Resistance Mechanisms in Dermatophytes. Pathogens. 2024; 13(8):657. https://doi.org/10.3390/pathogens13080657
Chicago/Turabian StyleDubljanin, Eleonora, Jelena Zunic, Isidora Vujcic, Ivana Colovic Calovski, Sandra Sipetic Grujicic, Stefan Mijatovic, and Aleksandar Dzamic. 2024. "Host-Pathogen Interaction and Resistance Mechanisms in Dermatophytes" Pathogens 13, no. 8: 657. https://doi.org/10.3390/pathogens13080657





