Rickettsia Species: Genetic Variability, Vectors, and Rickettsiosis—A Review
Abstract
:1. Introduction
2. Genetic Basis of Bacterial Classification
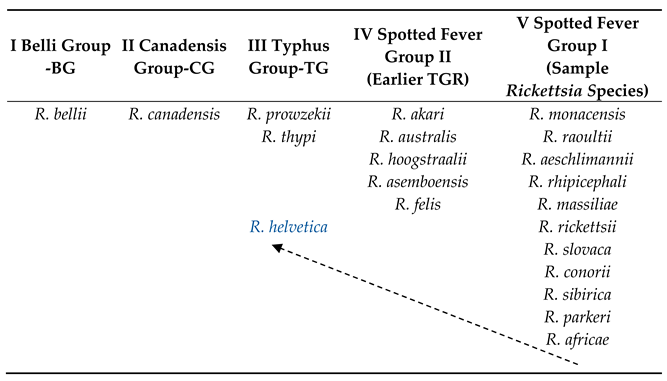
3. Rickettsia Genomes and Division into Bacterial Groups
4. Bacteria of the Genus Rickettsia in the Environment
4.1. Ticks as Vectors of Pathogens
4.2. Rickettsiae as Symbionts
4.3. The Lifecycle and Spread of Rickettsia
5. Rickettsiosis
6. Metagenomic Analyses and the Future in Research on Tick-Borne Pathogens
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Faccini-Martínez, Á.A.; García-Álvarez, L.; Hidalgo, M.; Oteo, J.A. Syndromic classification of rickettsioses: An approach for clinical practice. Int. J. Infect. Dis. 2014, 28, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Davison, H.R.; Pilgrim, J.; Wybouw, N.; Parker, J.; Pirro, S.; Hunter-Barnett, S.; Campbell, P.M.; Blow, F.; Darby, A.C.; Hurst, G.D.; et al. Genomic diversity across the Rickettsia and ‘Candidatus Megaira’ genera and proposal of genus status for the Torix group. Nat. Commun. 2022, 13, 2630. [Google Scholar] [CrossRef] [PubMed]
- Chaorattanakawee, S.; Korkusol, A.; Tippayachai, B.; Promsathaporn, S.; Poole-Smith, B.K.; Takhampunya, R. Amplicon-Based Next Generation Sequencing for rapid identification of Rickettsia and ectoparasite species from entomological surveillance in Thailand. Pathogens 2021, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.G.; Stewart, A.G.A. An update on the laboratory diagnosis of Rickettsia spp. infection. Pathogens 2021, 10, 1319. [Google Scholar] [CrossRef] [PubMed]
- Merhej, V.; Raoult, D. Rickettsial evolution in the light of comparative genomics. Biol. Rev. 2011, 86, 379–405. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, M.; Rymaszewska, A. Expansion of Tick-Borne Rickettsioses in the World. Microorganisms 2020, 8, 1906. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Frederiksen, W.; Garrity, G.M.; Grimont, P.A.D.; Kämpfer, P.; Maiden, M.C.J.; Nesme, X.; Rosselló-Mora, R.; Swings, J.; Trüper, H.G.; et al. Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 2002, 52, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.E.; Dumler, J.S.; Greub, G.; Zhang, J.; Wu, Y.; Raoult, D. Gene sequence-based criteria for identification of new rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J. Clin. Microbiol. 2003, 41, 5456–5465. [Google Scholar] [CrossRef]
- Diop, A.; El Karkouri, K.; Raoult, D.; Fournier, P.E. Genome sequence-based criteria for demarcation and definition of species in the genus Rickettsia. Int. J. Syst. Evol. Microbiol. 2020, 70, 1738–1750. [Google Scholar] [CrossRef]
- Gillespie, J.J.; Williams, K.; Shukla, M.; Snyder, E.E.; Nordberg, E.K.; Ceraul, S.M.; Dharmanolla, C.; Rainey, D.; Soneja, J.; Shallom, J.M.; et al. Rickettsia phylogenomics: Unwinding the intricacies of obligate intracellular life. PLoS ONE 2008, 3, e2018. [Google Scholar] [CrossRef]
- Gillespie, J.J.; Beier, M.S.; Rahman, M.S.; Ammerman, N.C.; Shallom, J.M.; Purkayastha, A.; Sobral, B.S.; Azad, A.F. Plasmids and rickettsial evolution: Insight from Rickettsia felis. PLoS ONE 2007, 2, e266. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702, Erratum in Clin. Microbiol. Rev. 2014, 27, 166. [Google Scholar] [CrossRef] [PubMed]
- El Karkouri, K.; Ghigo, E.; Raoult, D.; Fournier, P.E. Genomic evolution and adaptation of arthropod-associated Rickettsia. Sci. Rep. 2022, 12, 3807. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Qin, T.; Liu, Z.; Feng, H.; Sun, Y.; Zhang, M. Meta-transcriptional detection of Rickettsia canadensis from Ixodes persulcatus in China. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Philip, R.N.; Casper, E.A.; Anacker, R.L. Rickettsia bellii sp. nov.: A tick-borne Rickettsia, widely distributed in the United States, that is distinct from the spotted fever and typhus biogroups. Int. J. Syst. Bacteriol. 1983, 33, 94–106. [Google Scholar] [CrossRef]
- Stothard, D.R.; Clark, J.B.; Fuerst, P.A. Ancestral divergence of Rickettsia bellii from the spotted fever and typhus groups of Rickettsia and antiquity of the genus Rickettsia. Int. J. Syst. Bacteriol. 1994, 44, 798–804. [Google Scholar] [CrossRef]
- Krawczak, F.S.; Labruna, M.B.; Hecht, J.A.; Paddock, C.D.; Karpathy, S.E. Genotypic characterization of Rickettsia bellii reveals distinct lineages in the United States and South America. BioMed Res. Int. 2018, 2018, 8505483. [Google Scholar] [CrossRef] [PubMed]
- Hajduskova, E.; Literak, I.; Papousek, I.; Costa, F.B.; Novakova, M.; Labruna, M.B.; Zdrazilova-Dubska, L. ‘Candidatus Rickettsia mendelii’, a novel basal group rickettsia detected in Ixodes ricinus ticks in the Czech Republic. Ticks Tick-Borne Dis. 2016, 7, 482–486. [Google Scholar] [CrossRef]
- Igolkina, Y.; Nikitin, A.; Verzhutskaya, Y.; Gordeyko, N.; Tikunov, A.; Epikhina, T.; Tikunova, N.; Rar, V. Multilocus genetic analysis indicates taxonomic status of “Candidatus Rickettsia mendelii” as a separate basal group. Ticks Tick-Borne Dis. 2023, 14, 102104. [Google Scholar] [CrossRef]
- Horak, I.G.; Camicas, J.L.; Keirans, J.E. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida): A world list of valid tick names. Exp. Appl. Acarol. 2002, 28, 27–54. [Google Scholar] [CrossRef]
- Guglielmone, A.A.; Nava, S. Names for Ixodidae (Acari: Ixodoidea): Valid, synonyms, incertae sedis, nomina dubia, nomina nuda, lapsus, incorrect and suppressed names—With notes on confusions and misidentifications. Zootaxa 2014, 3767, 1–256. [Google Scholar] [CrossRef] [PubMed]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Pena, I.G.; Horak, I.G.; Shao, R.; Barker, S.C. The Agrasidae, Ixodidae and Nuttalliedae (Acari: Ixodida) of the world: A list of valid species names. Zootaxa 2010, 2528, 1–28. [Google Scholar] [CrossRef]
- Sharifah, N.; Heo, C.C.; Ehlers, J.; Houssaini, J.; Tappe, D. Ticks and tick-borne pathogens in animals and humans in the island nations of Southeast Asia: A review. Acta Trop. 2020, 209, 105527. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; de la Fuente, J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral. Res. 2014, 108, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Kahl, O.; Gern, L.; Eisen, L.; Lane, R.S. Ecological research on Borrelia burgdorferi sensu lato: Terminology and some methodological pitfalls. In Lyme Borreliosis Biology Epidemiology and Control (2002) CABI Books; Gray, J.S., Kahl, O., Lane, R.S., Stanek, G., Eds.; CABI International: Wallingford, UK, 2002; pp. 29–46. [Google Scholar] [CrossRef]
- Beati, L.; Meskini, M.; Their, B.; Raoult, D. Rickettsia aeschlimannii sp. nov., a new spotted fever group rickettsia associated with Hyalomma marginatum ticks. Int. J. Syst. Bacteriol. 1997, 47, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Eremeeva, J.A.; Bossermana, E.A.; Demma, L.J.; Zambrano, M.L.; Blau, D.M.; Dasch, G.A. Isolation and identification of Rickettsia massiliae from Rhipicephalus sanguineus ticks collected in Arizona. Appl. Environ. Microbiol. 2006, 72, 5569–5577. [Google Scholar] [CrossRef]
- Merhej, V.; Angelakis, E.; Socolovschi, C.; Raoult, D. Genotyping, evolution and epidemiological findings of Rickettsia species. Infect. Genet. Evol. 2014, 25, 122–137. [Google Scholar] [CrossRef]
- Macaluso, K.R.; Sonenshine, D.E.; Shane, M.; Azad, C.A.F. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 2002, 39, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Steiner, F.E.; Pinger, R.R.; Vann, C.N.; Grindle, N.; Civitello, D.; Clay, K.; Fuqua, C. Infection and co-infection rates of Anaplasma phagocytophilum variants, Babesia spp., Borrelia burgdorferi, and the rickettsial endosymbiont in Ixodes scapularis (Acari: Ixodidae) from sites in Indiana, Maine, Pennsylvania, and Wisconsin. J. Med. Entomol. 2008, 45, 289–297. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Sameshima, S.; Kitade, O.; Kojima, J.; Fukatsu, T. Novel clade of Rickettsia spp. from leeches. Appl. Environ. Microbiol. 2002, 68, 999–1004. [Google Scholar] [CrossRef]
- Perlman, S.J.; Hunter, M.S.; Zchori-Fein, E. The emerging diversity of Rickettsia. Proc. Biol. Sci. 2006, 273, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Hurst, G.D.D.; Zhang, W.; Breeuwer, J.A.J.; Stouthamer, R.; Majerus, M.E.N. Rickettsial relative associated with male killing in the ladybird beetle (Adalia bipunctata). J. Bacteriol. 1994, 176, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Caspi-Fluger, A.; Inbar, M.; Mozes-Daube, N.; Katzir, N.; Portnoy, V.; Belausov, E.; Hunter, M.S.; Zchori-Fein, E. Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proc. Biol. Sci. 2012, 279, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Kagemann, J.; Clay, K. Effects of infection by Arsenophonus and Rickettsia bacteria on the locomotive ability of the ticks Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis. J. Med. Entomol. 2013, 50, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.F.; Burgdorfer, W.; Aeschlimann, A. Sexual transmission of spotted fever group rickettsiae by infected male ticks: Detection of rickettsiae in immature spermatozoa of Ixodes ricinus. Infect. Immun. 1980, 27, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Raoult, D. Ticks and Tickborne Bacterial Diseases in Humans: An Emerging Infectious Threat. Clin. Infect. Dis. 2001, 32, 897–928. [Google Scholar] [CrossRef] [PubMed]
- Hauck, D.; Jordan, D.; Springer, A.; Schunack, B.; Pachnicke, S.; Fingerle, V.; Strube, C. Transovarial transmission of Borrelia spp., Rickettsia spp. and Anaplasma phagocytophilum in Ixodes ricinus under field conditions extrapolated from DNA detection in questing larvae. Parasites Vectors 2020, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- Wechtaisong, W.; Bonnet, S.I.; Chomel, B.B.; Lien, Y.Y.; Chuang, S.T.; Tsai, Y.L. Investigation of transovarial transmission of Bartonella henselae in Rhipicephalus sanguineus sensu lato ticks using artificial feeding. Microorganisms 2021, 9, 2501. [Google Scholar] [CrossRef] [PubMed]
- Buczek, W.; Buczek, A.; Witecka, J.; Asman, M. Prevalence of pathogens in sympatric Ixodes ricinus and Dermacentor reticulatus ticks in Eastern Poland and their potential impact on oral-anal contacts between ticks. Ann. Agric. Environ. Med. 2023, 30, 259–265. [Google Scholar] [CrossRef]
- Buczek, A.; Bartosik, K.; Buczek, W.; Buczek, A.M.; Kulina, D.; Kulisz, J.; Tomasiewicz, K. A unique phenomenon of oral-anal contact between ticks observed in two tick species Ixodes ricinus and Dermacentor reticulatus. Ann. Agric. Environ. Med. 2018, 25, 686–689. [Google Scholar] [CrossRef]
- Helminiak, L.; Mishra, S.; Kim, H.K. Pathogenicity and virulence of Rickettsia. Virulence 2022, 13, 1752–1771. [Google Scholar] [CrossRef] [PubMed]
- Talleklint, L.; Jaenson, T.G. Infestation of mammals by Ixodes ricinus ticks (Acari: Ixodidae) in south-central Sweden. Exp. Appl. Acarol. 1997, 21, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Guglielmone, A.A.; Nava, S. Worldwide host associations of the tick genus Ixodes suggest relationships based on environmental sharing rather than on co-phylogenetic events. Parasit. Vec. 2023, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.F.; Beard, C.B. Rickettsial Pathogens and Their Arthropod Vectors. Emerg. Infect. Dis. 1998, 4, 179–186. [Google Scholar] [CrossRef]
- Thepparit, C.; Hirunkanokpun, S.; Popov, V.L.; Foil, L.D.; Macaluso, K.R. Dissemination of bloodmeal acquired Rickettsia felis in cat fleas, Ctenocephalides felis. Parasites Vectors 2013, 6, 149. [Google Scholar] [CrossRef] [PubMed]
- Schorderet-Weber, S.; Noack, S.; Selzer, P.M.; Kaminsky, R. Blocking transmission of vector-borne diseases. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 90–109. [Google Scholar] [CrossRef] [PubMed]
- Halos, L.; Lebon, W.; Chalvet-Monfray, K.; Larsen, D.; Beugnet, F. Immediate efficacy and persistent speed of kill of a novel oral formulation of afoxolaner (NexGardTM) against induced infestations with Ixodes ricinus ticks. Parasites Vectors 2014, 7, 452. [Google Scholar] [CrossRef] [PubMed]
- Beugnet, F.; Halos, L.; Liebenberg, J.; Fourie, J. Assessment of the prophylactic speed of kill of Frontline Tri-Act(®) against ticks (Ixodes ricinus and Rhipicephalus sanguineus) on dogs. Parasite 2016, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Six, R.H.; Geurden, T.; Carter, L.; Everett, W.R.; McLoughlin, A.; Mahabir, S.P.; Myers, M.R.; Slootmans, N. Evaluation of the speed of kill of sarolaner (Simparica™) against induced infestations of three species of ticks (Amblyomma maculatum, Ixodes scapularis, Ixodes ricinus) on dogs. Vet. Parasitol. 2016, 30, 37–42. [Google Scholar] [CrossRef]
- Rochlin, I.; Toledo, A. Emerging tick-borne pathogens of public health importance: A mini-review. J. Med. Microbiol. 2020, 69, 781–791. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef] [PubMed]
- Volunteer for Clinical Studies. Available online: https://www.niaid.nih.gov/ (accessed on 22 April 2024).
- Buckingham, S.C.; Marshall, G.S.; Schutze, G.E.; Woods, C.R.; Jackson, M.A.; Patterson, L.E.; Jacobs, R.F. Clinical and laboratory features, hospital course, and outcome of Rocky Mountain spotted fever in children. J. Pediatr. 2007, 150, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Horta, M.C.; Labruna, M.B.; Pinter, A.; Linardi, P.M.; Schumaker, T.T. Rickettsia infection in five areas of the state of São Paulo, Brazil. Memórias Inst. Oswaldo Cruz 2007, 102, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Brazil Has 49 Cases of Spotted Fever in 2023, Six Deaths. Available online: https://agenciabrasil.ebc.com.br/en/saude/noticia/2023-06/brazil-has-49-cases-spotted-fever-2023-six-deaths (accessed on 9 February 2024).
- Vilges de Oliveira, S.; Faccini-Martínez, Á.A.; Adelino, T.E.R.; de Lima Duré, A.Í.; Barbieri, A.R.M.; Labruna, M.B. Needlestick-Associated Rocky Mountain Spotted Fever, Brazil. Emerg. Infect. Dis. 2020, 26, 815–816. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Berbis, P.; Roux, V.; Xu, W.; Maurin, M. A new tick-transmitted disease due to Rickettsia slovaca. Lancet 1997, 12, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Oteo, J.A.; Ibarra, V.; Blanco, J.R.; Martínez de Artola, V.; Márquez, F.J.; Portillo, A.; Raoult, D.; Anda, P. Dermacentor-borne necrosis erythema and lymphadenopathy: Clinical and epidemiological features of a new tick-borne disease. Clin. Microbiol. Infect. 2004, 10, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Gouriet, F.; Rolain, J.M.; Raoult, D. Rickettsia slovaca infection, France. Emerg. Infect. Dis. 2006, 12, 521–523. [Google Scholar] [CrossRef]
- Angelakis, E.; Pulcini, C.; Waton, J.; Imbert, P.; Socolovschi, C.; Edouard, S.; Dellamonica, P.; Raoult, D. Scalp eschar and neck lymphadenopathy caused by Bartonella henselae after tick bite. Clin. Infect. Dis. 2010, 15, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Rickettsial Diseases. CDC Yellow Book 2024. Travel-Associated Infections & Diseases. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/rickettsial-diseases (accessed on 9 February 2024).
- Talhelm, C.F.; Helms, J.L.; Tran, L.T.; Contreras, B.X.; Stevens, M.L.; Sierra-Hoffman, M.; Castro-Lainez, M.T.; Deliz, R.J. Rickettsia typhi central nervous system infection. IDCases 2020, 29, e00852. [Google Scholar] [CrossRef]
- Rickettsiosis Subcommittee Report to the Tick-Borne Disease Working Group. Available online: https://www.hhs.gov/ash/advisory-committees/tickbornedisease/reports/rickettsiosis-subcomm-2020/index.html (accessed on 9 February 2024).
- Walker, D.H.; Myers, C.T.E.; Blanton, L.S.; Bloch, K.C.; Fowler, V.G., Jr.; Gaines, D.N.; Paddock, C.D.; Yaglom, H.D. Rickettsiosis subcommittee report to the tick-borne disease working group. Ticks Tick-Borne Dis. 2022, 13, 101855. [Google Scholar] [CrossRef]
- Knapp, K.L.; Rice, N.A. Human Coinfection with Borrelia burgdorferi and Babesia microti in the United States. J. Parasitol. Res. 2015, 2015, 587131. [Google Scholar] [CrossRef]
- Lou, Y.; Liu, L.; Gao, D. Modeling co-infection of Ixodes tick-borne pathogens. Math. Biosci. Eng. 2017, 14, 1301–1316. [Google Scholar] [CrossRef]
- Belongia, E.A. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector Borne Zoonotic Dis. 2002, 2, 265–273. [Google Scholar] [CrossRef]
- Nyarko, E.; Grab, D.J.; Dumler, J.S. Anaplasma phagocytophilum-infected neutrophils enhance transmigration of Borrelia burgdorferi across the human blood brain barrier in vitro. Int. J. Parasitol. 2006, 36, 601–605. [Google Scholar] [CrossRef]
- Boyer, P.H.; Lenormand, C.; Jaulhac, B.; Talagrand-Reboul, E. Human co-infections between Borrelia burgdorferi s.l. and other Ixodes-borne microorganisms: A systematic review. Pathogens 2022, 11, 282. [Google Scholar] [CrossRef]
- Tijsse-Klasen, E.; Sprong, H.; Pandak, N. Co-infection of Borrelia burgdorferi sensu lato and Rickettsia species in ticks and in an erythema migrans patient. Parasites Vectors 2013, 6, 347. [Google Scholar] [CrossRef] [PubMed]
- Zając, V.; Wójcik-Fatla, A.; Sawczyn, A.; Cisak, E.; Sroka, J.; Kloc, A.; Zając, Z.; Buczek, A.; Dutkiewicz, J.; Bartosik, K. Prevalence of infections and co-infections with 6 pathogens in Dermacentor reticulatus ticks collected in eastern Poland. Ann. Agric. Environ. Med. 2017, 24, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Raulf, M.K.; Jordan, D.; Fingerle, V.; Strube, C. Association of Borrelia and Rickettsia spp. and bacterial loads in Ixodes ricinus ticks. Ticks Tick-Borne Dis. 2018, 9, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Moutailler, S.; Valiente Moro, C.; Vaumourin, E.; Michelet, L.; Tran, F.H.; Devillers, E.; Cosson, J.F.; Gasqui, P.; Van, V.T.; Mavingui, P.; et al. Co-infection of ticks: The rule rather than the exception. PLoS Negl. Trop. Dis. 2016, 10, e0004539. [Google Scholar] [CrossRef]
- Rocha, S.C.; Velásquez, C.V.; Aquib, A.; Al-Nazal, A.; Parveen, N. Transmission cycle of tick-borne infections and co-infections, animal models and diseases. Pathogens 2022, 11, 1309. [Google Scholar] [CrossRef]
- Špitalská, E.; Boldiš, V.; Derdáková, M.; Selyemová, D.; Rusňáková Tarageľová, V. Rickettsial infection in Ixodes ricinus ticks in urban and natural habitats of Slovakia. Ticks Tick-Borne Dis. 2014, 5, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Kowalec, M.; Szewczyk, T.; Welc-Falęciak, R.; Siński, E.; Karbowiak, G.; Bajer, A. Ticks and the city—Are there any differences between city parks and natural forests in terms of tick abundance and prevalence of spirochaetes? Parasite Vectors 2017, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Heylen, D.; Lasters, R.; Adriaensen, F.; Fonville, M.; Sprong, H.; Matthysen, E. Ticks and tick-borne diseases in the city: Role of landscape connectivity and green space characteristics in a metropolitan area. Sci. Total Environ. 2019, 670, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Kolomiiets, V.; Rakowska, P.; Rymaszewska, A. New problems of environmental ecology: Ticks and tick-borne pathogens in city parks of Ukraine. Environ. Microbiol. Rep. 2022, 14, 591–594. [Google Scholar] [CrossRef]
- Polsomboon Nelson, S.; Ergunay, K.; Bourke, B.P.; Reinbold-Wasson, D.D.; Caicedo-Quiroga, L.; Kirkitadze, G.; Chunashvili, T.; Tucker, C.L.; Linton, Y.-M. Nanopore-based metagenomics reveal a new Rickettsia in Europe. Ticks Tick-Borne Dis. 2024, 15, 102305. [Google Scholar] [CrossRef]
- Kipp, E.J.; Lindsey, L.L.; Khoo, B.; Faulk, C.; Oliver, J.D.; Larsen, P.A. Metagenomic surveillance for bacterial tick-borne pathogens using nanopore adaptive sampling. Sci. Rep. 2023, 13, 10991. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rymaszewska, A.; Piotrowski, M. Rickettsia Species: Genetic Variability, Vectors, and Rickettsiosis—A Review. Pathogens 2024, 13, 661. https://doi.org/10.3390/pathogens13080661
Rymaszewska A, Piotrowski M. Rickettsia Species: Genetic Variability, Vectors, and Rickettsiosis—A Review. Pathogens. 2024; 13(8):661. https://doi.org/10.3390/pathogens13080661
Chicago/Turabian StyleRymaszewska, Anna, and Mariusz Piotrowski. 2024. "Rickettsia Species: Genetic Variability, Vectors, and Rickettsiosis—A Review" Pathogens 13, no. 8: 661. https://doi.org/10.3390/pathogens13080661
APA StyleRymaszewska, A., & Piotrowski, M. (2024). Rickettsia Species: Genetic Variability, Vectors, and Rickettsiosis—A Review. Pathogens, 13(8), 661. https://doi.org/10.3390/pathogens13080661








