A Retrospective Study of Genetic Characterization in Suspected Visceral Leishmaniasis Cases in Greece, 2005 to 2020
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Case Definition
2.3. L. infantum Cells Preparation for Immunofluorescence Assay (IFA)
2.4. Immunofluorescence Assay (IFA)
2.5. Molecular Assay
2.6. Cohen’s Kappa Coefficient
2.7. Genotyping and Phylogenetic Analysis
2.8. Statistical Analysis
2.9. Ethics Approval Statement
3. Results
3.1. VL Cases
3.2. Clinical Manifestations
3.3. Seasonality
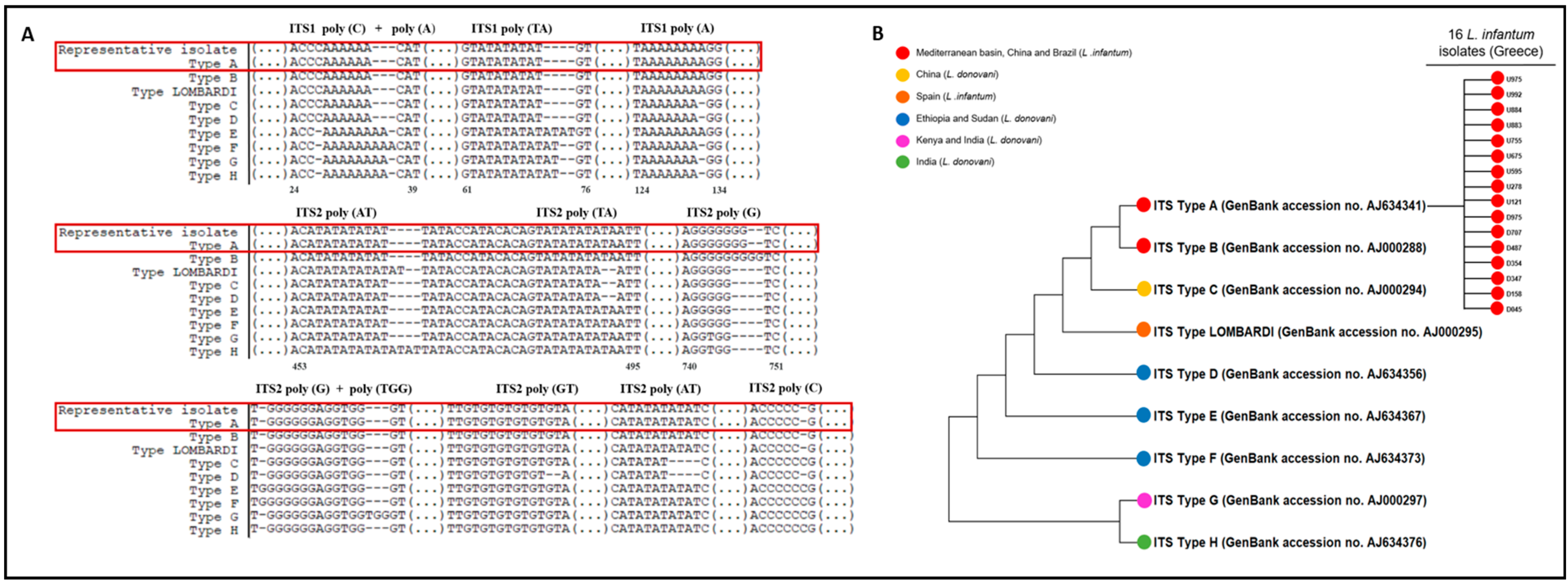
3.4. Phylogenetic Tree
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). Available online: http://www.cdc.gov/parasites/leishmaniasis/disease.html (accessed on 2 March 2024).
- Ntais, P.; Sifaki-Pistola, D.; Christodoulou, V.; Messaritakis, I.; Pratlong, F.; Poupalos, G.; Antoniou, M. Leishmaniases in Greece. Am. J. Trop. Med. Hyg. 2013, 89, 906–915. [Google Scholar] [CrossRef]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- Pan American Health Organization/World Health Organization Regional Office of the Americas (PAHO/WHO). Available online: https://www.paho.org/en/topics/leishmaniasis (accessed on 2 March 2024).
- Theocharidou, D.; Maltezos, E.; Constantinidis, T.C.; Papa, A. Human visceral leishmaniasis in northern Greece: Seroepidemiology and risk factors in endemic region. J. Vector Borne Dis. 2019, 56, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Gkolfinopoulou, K.; Bitsolas, N.; Patrinos, S.; Veneti, L.; Marka, A.; Dougas, G.; Pervanidou, D.; Detsis, M.; Triantafillou, E.; Georgakopoulou, T.; et al. Epidemiology of human leishmaniasis in Greece, 1981–2011. Euro Surveill. 2013, 18, 20532. [Google Scholar] [CrossRef]
- Tzani, M.; Barrasa, A.; Vakali, A.; Georgakopoulou, T.; Mellou, K.; Pervanidou, D. Surveillance data for human leishmaniasis indicate the need for a sustainable action plan for its management and control, Greece, 2004 to 2018. Euro Surveill. 2021, 26, 2000159. [Google Scholar] [CrossRef]
- Pedras, M.J.; de Gouvea Viana, L.; de Oliveira, E.J.; Rabello, A. Comparative evaluation of direct agglutination test, rK39 and soluble antigen ELISA and IFAT for the diagnosis of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 172–178. [Google Scholar] [CrossRef]
- de Assis, T.S.; Caligiorne, R.B.; Romero, G.A.; Rabello, A. Detection of Leishmania kDNA in human serum samples for the diagnosis of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 1269–1272. [Google Scholar] [CrossRef]
- Fissore, C.; Delaunay, P.; Ferrua, B.; Rosenthal, E.; Del Giudice, P.; Aufeuvre, J.P.; Le Fichoux, Y.; Marty, P. Convenience of serum for visceral leishmaniasis diagnosis by PCR. J. Clin. Microbiol. 2004, 42, 5332–5333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mary, C.; Faraut, F.; Lascombe, L.; Dumon, H. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J. Clin. Microbiol. 2004, 42, 5249–5255. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Richet, H.; Rolain, J.M.; La Scola, B.; Raoult, D. Comparison of real-time quantitative PCR and culture for the diagnosis of emerging Rickettsioses. PLoS Negl. Trop. Dis. 2012, 6, e1540. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. In Educational & Psychological Measurement, 1st ed.; Sage Publications: New York, NY, USA, 1960; pp. 37–46. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Kuhls, K.; Mauricio, I.L.; Pratlong, F.; Presber, W.; Schönian, G. Analysis of ribosomal DNA internal transcribed spacer sequences of the Leishmania donovani complex. Microbes Infect. 2005, 7, 1224–1234. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Kostoula, A.; Dimitriou, D.; Panagiou, A.; Bobojianni, C.; Antoniades, G. Human and canine leishmaniasis in asymptomatic and symptomatic population in Northwestern Greece. J. Infect. 2005, 50, 53–60. [Google Scholar] [CrossRef]
- Kyriakou, D.S.; Alexandrakis, M.G.; Passam, F.H.; Kourelis, T.V.; Foundouli, P.; Matalliotakis, E.; Maniatis, A.N. Quick detection of Leishmania in peripheral blood by flow cytometry. Is prestorage leucodepletion necessary for leishmaniasis prevention in endemic areas? Transfus. Med. 2003, 13, 59–62. [Google Scholar] [CrossRef]
- Georgiadou, S.P.; Stefos, A.; Spanakos, G.; Skrimpas, S.; Makaritsis, K.; Sipsas, N.V.; Dalekos, G.N. Current clinical, laboratory, and treatment outcome characteristics of visceral leishmaniasis: Results from a seven-year retrospective study in Greece. Int. J. Infect. Dis. 2015, 34, 46–50. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Stenzinger, A.; Nemeth, J.; Klauschen, F.; Schewe, C.; Ladhoff, A.M.; Muckenhuber, A.; Schurmann, M.; Schurmann, D.; Weichert, W. Visceral leishmaniasis in a patient with AIDS: Early pathological diagnosis using conventional histology, PCR and electron microscopy is the key for adequate treatment. Virchows Arch. 2012, 460, 357–360. [Google Scholar] [CrossRef]
- Pace, D.; Williams, T.N.; Grochowska, A.; Betts, A.; Attard-Montalto, S.; Boffa, M.J.; Vella, C. Manifestations of paediatric Leishmania infantum infections in Malta. Travel. Med. Infect. Dis. 2011, 9, 37–46. [Google Scholar] [CrossRef][Green Version]
- Medenica, S.; Jovanovic, S.; Dozic, I.; Milicic, B.; Lakicevic, N.; Rakocevic, B. Epidemiological Surveillance of Leishmaniasis in Montenegro, 1992–2013. Srp. Arh. Celok. Lek. 2015, 143, 707–711. [Google Scholar] [CrossRef]
- Petrela, R.; Kuneshka, L.; Foto, E.; Zavalani, F.; Gradoni, L. Pediatric visceral leishmaniasis in Albania: A retrospective analysis of 1210 consecutive hospitalized patients (1995–2009). PLoS Negl. Trop. Dis. 2010, 4, e814. [Google Scholar] [CrossRef] [PubMed]
- Harizanov, R.; Rainova, I.; Tzvetkova, N.; Kaftandjiev, I.; Bikov, I.; Mikov, O. Geographical distribution and epidemiological characteristics of visceral leishmaniasis in Bulgaria, 1988 to 2012. Euro Surveill. 2013, 18, 20531. [Google Scholar] [CrossRef] [PubMed]
- Arce, A.; Estirado, A.; Ordobas, M.; Sevilla, S.; Garcia, N.; Moratilla, L.; de la Fuente, S.; Martinez, A.M.; Perez, A.M.; Aranguez, E.; et al. Re-emergence of leishmaniasis in Spain: Community outbreak in Madrid, Spain, 2009 to 2012. Euro Surveill. 2013, 18, 20546. [Google Scholar] [CrossRef] [PubMed]
- Haralambous, C.; Antoniou, M.; Pratlong, F.; Dedet, J.P.; Soteriadou, K. Development of a molecular assay specific for the Leishmania donovani complex that discriminates L. donovani/Leishmania infantum zymodemes: A useful tool for typing MON-1. Diagn. Microbiol. Infect. Dis. 2008, 60, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Reimao, J.Q.; Coser, E.M.; Lee, M.R.; Coelho, A.C. Laboratory Diagnosis of Cutaneous and Visceral Leishmaniasis: Current and Future Methods. Microorganisms 2020, 8, 1632. [Google Scholar] [CrossRef]
- Chicharro, C.; Llanes-Acevedo, I.P.; Garcia, E.; Nieto, J.; Moreno, J.; Cruz, I. Molecular typing of Leishmania infantum isolates from a leishmaniasis outbreak in Madrid, Spain, 2009 to 2012. Euro Surveill. 2013, 18, 20545. [Google Scholar] [CrossRef]

| Clinical Phase | Total No. of Tested | Total No. of Positives | Types of Assays | |||||
|---|---|---|---|---|---|---|---|---|
| IFA | qPCR | IFA and qPCR | ||||||
| No. of Tested | No. of Positives | No. of Tested | No. of Positives | No. of Tested | No. of Positives | |||
| Acute | 3565 | 216 | 3339 | 160 | 146 | 33 | 80 | 23 |
| Convalescence | 96 | 3 | 93 | 3 | 2 | 0 | 1 | 0 |
| Total | 3661 | 219 | 3432 | 163 | 148 | 33 | 81 | 23 |
| IFA | Negative | Positive | Total | |
|---|---|---|---|---|
| qPCR | ||||
| Negative | 58 | 1 | 59 | |
| Positive | 3 | 19 | 22 | |
| Total | 61 | 20 | 81 | |
| Kappa coefficient | κ = 0.87 | |||
| Sex | Age Groups | |||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Unknown | ≤22 | 23–45 | 46–64 | ≥65 | Unknown | |
| Total No. of tested | 1568 | 1706 | 387 | 296 | 604 | 626 | 827 | 1308 |
| Total No. of positives | 110 | 94 | 15 | 28 | 24 | 42 | 55 | 70 |
| Proportion of positives in total no tested (%) | 7 | 5.5 | 3.9 | 9.5 | 4 | 6.7 | 6.7 | 5.4 |
| No. of cases with available information on sex and age | 204 | 149 | ||||||
| Proportion of positives in cases with available information (%) | 53.9 | 46.1 | 18.8 | 16.1 * | 28.2 | 36.9 * | ||
| p value | p > 0.05 | * p = 0.037 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evangelidou, M.; Makka, S.; Papadogiannaki, I.; Koutantou, M.; Tegos, N.; Mpimpa, A.; Patsoula, E.; Angelakis, E. A Retrospective Study of Genetic Characterization in Suspected Visceral Leishmaniasis Cases in Greece, 2005 to 2020. Pathogens 2024, 13, 688. https://doi.org/10.3390/pathogens13080688
Evangelidou M, Makka S, Papadogiannaki I, Koutantou M, Tegos N, Mpimpa A, Patsoula E, Angelakis E. A Retrospective Study of Genetic Characterization in Suspected Visceral Leishmaniasis Cases in Greece, 2005 to 2020. Pathogens. 2024; 13(8):688. https://doi.org/10.3390/pathogens13080688
Chicago/Turabian StyleEvangelidou, Maria, Sofia Makka, Ioanna Papadogiannaki, Myrto Koutantou, Nikolaos Tegos, Anastasia Mpimpa, Eleni Patsoula, and Emmanouil Angelakis. 2024. "A Retrospective Study of Genetic Characterization in Suspected Visceral Leishmaniasis Cases in Greece, 2005 to 2020" Pathogens 13, no. 8: 688. https://doi.org/10.3390/pathogens13080688
APA StyleEvangelidou, M., Makka, S., Papadogiannaki, I., Koutantou, M., Tegos, N., Mpimpa, A., Patsoula, E., & Angelakis, E. (2024). A Retrospective Study of Genetic Characterization in Suspected Visceral Leishmaniasis Cases in Greece, 2005 to 2020. Pathogens, 13(8), 688. https://doi.org/10.3390/pathogens13080688






