Mosquito Gut Microbiota: A Review
Abstract
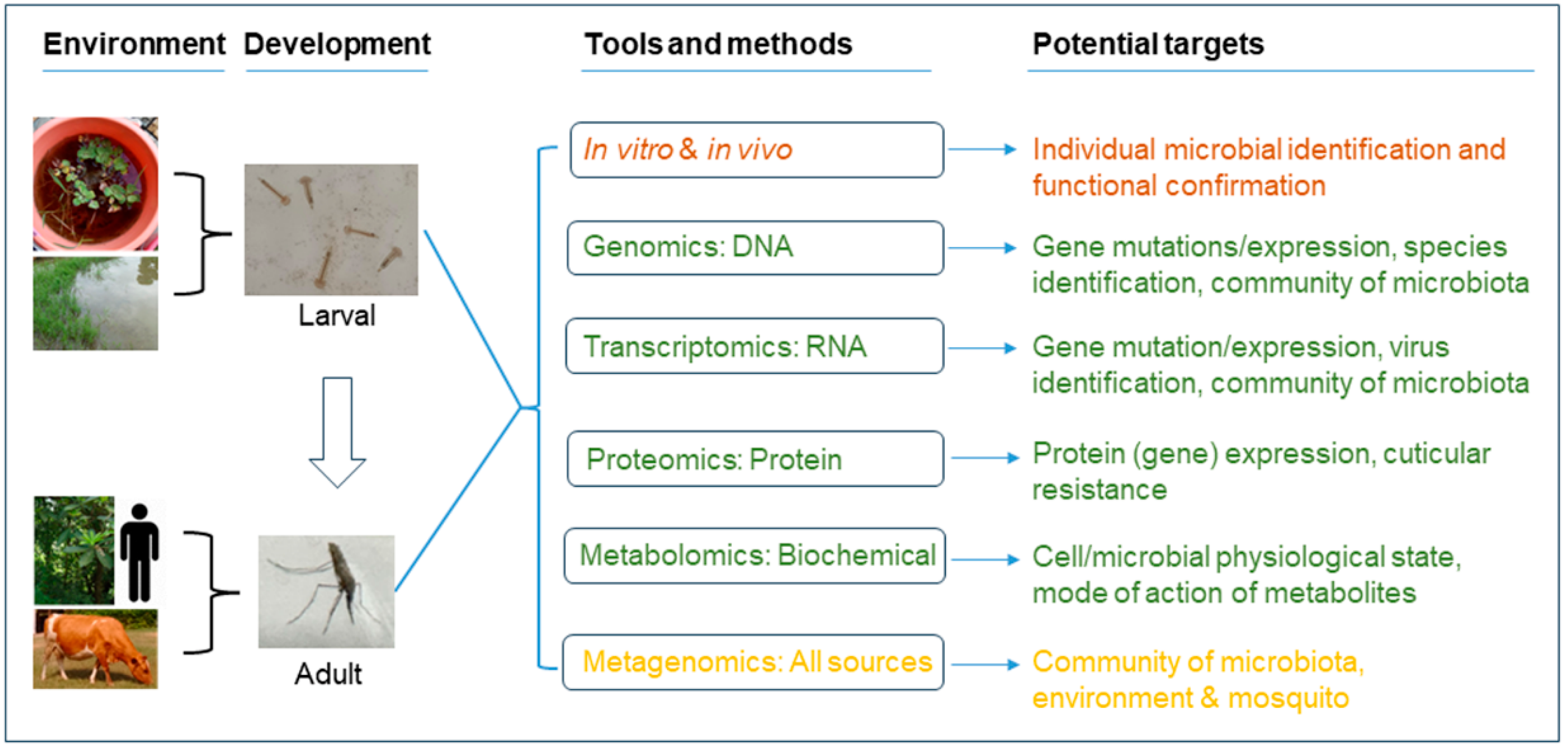
:1. Introduction
2. Methods
2.1. Protocols
2.2. Search Strategy
- (Mosquito microbiota) or (mosquito microbiome);
- (Aedes aegypti microbiota);
- (Aedes albopictus microbiota);
- (Culex microbiota);
- (Anopheles microbiota);
- (Aedes microbiota);
- (Armigeres microbiota);
- (Haemagogus microbiota);
- (Mosquito Wolbachia);
- (Mosquito Asaia);
- (Mosquito Serratia).
2.3. Study Eligibility Criteria
- Inclusion criteria:
- Exclusion criteria:
2.4. Study Selection
3. Results
3.1. Description of Search Results
3.2. Mosquito Gut Microbiota: Environment, Disease Transmission, and Mosquito Development
3.3. Key Bacteria in Mosquito Gut Microbiota: Importance for Preventing Disease Transmission
3.4. Mosquito Gut Microbiota Mediating Insecticide Resistance
3.5. Perspectives
3.6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gotschlich, E.C.; Colbert, R.A.; Gill, T. Methods in microbiome research: Past, present, and future. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101498. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017, 550, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Beaugerie, L.; Petit, J.C. Microbial-gut interactions in health and disease. Antibiotic-associated diarrhoea. Best. Pract. Res. Clin. Gastroenterol. 2004, 18, 337–352. [Google Scholar] [CrossRef]
- Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and Bacterial Resistance-A short story of an endless arms race. Int. J. Mol. Sci. 2023, 24, 5777. [Google Scholar] [CrossRef] [PubMed]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Integrative HMPRNC. The Integrative Human Microbiome Project: Dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell Host Microbe 2014, 16, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Backhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Koppel, N.; Maini Rekdal, V.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science 2017, 356, 6344. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xu, M.; Dong, W.; Deng, B.; Wang, S.; Zhang, Y.; Wang, S.; Luo, S.; Wang, W.; Qi, Y.; et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis. Int. J. Cancer 2017, 140, 2545–2556. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The impact of gut microbiota on brain and behaviour: Implications for psychiatry. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 552–558. [Google Scholar] [CrossRef]
- Spanogiannopoulos, P.; Bess, E.N.; Carmody, R.N.; Turnbaugh, P.J. The microbial pharmacists within us: A metagenomic view of xenobiotic metabolism. Nat. Rev. Microbiol. 2016, 14, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Salam, L.B.; Obayori, O.S.; Ilori, M.O.; Amund, O.O. Deciphering the cytochrome P450 genes in the microbiome of a chronically polluted soil with history of agricultural activities. Bull. Natl. Res. Cent. 2022, 46, 256. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Y.; Xu, H.; Yan, C. Genome-wide identification of P450 genes in chironomid Propsilocerus akamusi reveals candidate genes involved in gut microbiotamediated detoxification of chlorpyrifos. Insects 2022, 13, 765. [Google Scholar] [CrossRef]
- Yunta, C.; Ooi, J.M.F.; Oladepo, F.; Grafanaki, S.; Pergantis, S.A.; Tsakireli, D.; Ismail, H.M.; Paine, M.J.I. Chlorfenapyr metabolism by mosquito P450s associated with pyrethroid resistance identifies potential activation markers. Sci. Rep. 2023, 13, 14124. [Google Scholar]
- Malla, M.A.; Dubey, A.; Raj, A.; Kumar, A.; Upadhyay, N.; Yadav, S. Emerging frontiers in microbe-mediated pesticide remediation: Unveiling role of omics and In silico approaches in engineered environment. Environ. Pollut. 2022, 299, 118851. [Google Scholar] [CrossRef]
- Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 2014, 12, 168–180. [Google Scholar] [CrossRef]
- Mikaelyan, A.; Dietrich, C.; Kohler, T.; Poulsen, M.; Sillam-Dusses, D.; Brune, A. Diet is the primary determinant of bacterial community structure in the guts of higher termites. Mol. Ecol. 2015, 24, 5284–5295. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Engel, P. Mechanisms underlying gut microbiota-host interactions in insects. J. Exp. Biol. 2021, 224, jeb207696. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Roh, S.W.; Whon, T.W.; Jung, M.J.; Kim, M.S.; Park, D.S.; Yoon, C.; Nam, Y.D.; Kim, Y.J.; Choi, J.H.; et al. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Ge, J.; Tang, Z.; Tian, B.; Li, W.; Li, C.; Xu, L.; Luo, J. Dynamic gut microbiota of Apolygus lucorum across different life stages reveals potential pathogenic bacteria for facilitating the pest management. Microb. Ecol. 2023, 87, 9. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Mann, E.; Dzieciol, M.; Hess, C.; Schmitz-Esser, S.; Wagner, M.; Hess, M. Age-related differences in the luminal and mucosa-associated gut microbiome of broiler chickens and shifts associated with Campylobacter jejuni Infection. Front. Cell. Infect. Microbiol. 2016, 6, 154. [Google Scholar] [CrossRef]
- Ishigami, K.; Jang, S.; Itoh, H.; Kikuchi, Y. Obligate gut symbiotic association with caballeronia in the mulberry seed bug Paradieuches dissimilis (Lygaeoidea: Rhyparochromidae). Microb. Ecol. 2023, 86, 1307–1318. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef]
- Soltani, A.; Vatandoost, H.; Oshaghi, M.A.; Enayati, A.A.; Chavshin, A.R. The role of midgut symbiotic bacteria in resistance of Anopheles stephensi (Diptera: Culicidae) to organophosphate insecticides. Pathog. Glob. Health 2017, 111, 289–296. [Google Scholar] [CrossRef]
- Gressel, J. Microbiome facilitated pest resistance: Potential problems and uses. Pest Manag. Sci. 2018, 74, 511–515. [Google Scholar] [CrossRef]
- Chaitra, H.S.; Kalia, V.K. Gut symbionts: Hidden players of pesticide resistance in insects. Indian. J. Entomol. 2022, 84, 997–1002. [Google Scholar]
- Sato, Y.; Jang, S.; Takeshita, K.; Itoh, H.; Koike, H.; Tago, K.; Hayatsu, M.; Hori, T.; Kikuchi, Y. Insecticide resistance by a host-symbiont reciprocal detoxification. Nat. Commun. 2021, 12, 6432. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, K.; Jang, S.; Itoh, H.; Kikuchi, Y. Insecticide resistance governed by gut symbiosis in a rice pest, Cletus punctiger, under laboratory conditions. Biol. Lett. 2021, 17, 20200780. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, H.W.; Bhatt, P. Microbial adaptation and impact into the pesticide’s degradation. Arch. Microbiol. 2022, 204, 288. [Google Scholar] [CrossRef]
- Itoh, H.; Hori, T.; Sato, Y.; Nagayama, A.; Tago, K.; Hayatsu, M.; Kikuchi, Y. Infection dynamics of insecticide-degrading symbionts from soil to insects in response to insecticide spraying. ISME J. 2018, 12, 909–920. [Google Scholar] [CrossRef]
- Jaffar, S.; Ahmad, S.; Lu, Y. Contribution of insect gut microbiota and their associated enzymes in insect physiology and biodegradation of pesticides. Front. Microbiol. 2022, 13, 979383. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, T.; Yuan, M.; Li, Z.; Jin, R.; Ren, Z.; Qin, Y.; Yu, C.; Cai, Y.; Shu, R.; et al. Microbiome variation correlates with the insecticide susceptibility in different geographic strains of a significant agricultural pest, Nilaparvata lugens. NPJ Biofilms Microbiomes 2023, 9, 2. [Google Scholar] [CrossRef]
- Daisley, B.A.; Trinder, M.; McDowell, T.W.; Collins, S.L.; Sumarah, M.W.; Reid, G. Microbiota-mediated modulation of organophosphate insecticide toxicity by species-dependent interactions with Lactobacilli in a Drosophila melanogaster insect model. Appl. Environ. Microbiol. 2018, 84, e02820-17. [Google Scholar] [CrossRef]
- Gao, H.; Cui, C.; Wang, L.; Jacobs-Lorena, M.; Wang, S. Mosquito microbiota and implications for disease control. Trends Parasitol. 2020, 36, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Garrigos, M.; Garrido, M.; Panisse, G.; Veiga, J.; Martinez-de la Puente, J. Interactions between West Nile Virus and the microbiota of Culex pipiens vectors: A literature review. Pathogens 2023, 12, 1287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, L.; Aksoy, S. Microbiota in disease-transmitting vectors. Nat. Rev. Microbiol. 2023, 21, 604–618. [Google Scholar] [CrossRef]
- Lee, H.; Halverson, S.; Ezinwa, N. Mosquito-borne diseases. Prim. Care 2018, 45, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Tolle, M.A. Mosquito-borne diseases. Curr. Probl. Pediatr. Adolesc. Health Care 2009, 39, 97–140. [Google Scholar] [CrossRef]
- WHO. Mosquito-Borne Diseases Geneva; WHO: Geneva, Switzerland, 2020.
- Song, X.; Zhong, Z.; Gao, L.; Weiss, B.L.; Wang, J. Metabolic interactions between disease-transmitting vectors and their microbiota. Trends Parasitol. 2022, 38, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L.; Sharma, A.; Shouche, Y.; Severson, D.W. Dynamics of midgut microflora and dengue virus impact on life history traits in Aedes aegypti. Acta Trop. 2014, 140, 151–157. [Google Scholar] [CrossRef]
- Rodpai, R.; Boonroumkaew, P.; Sadaow, L.; Sanpool, O.; Janwan, P.; Thanchomnang, T.; Intapan, P.M.; Maleewong, W. Microbiome composition and microbial community structure in mosquito vectors Aedes aegypti and Aedes albopictus in Northeastern Thailand, a dengue-endemic area. Insects 2023, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Cortes, A.; Damania, A.; Granada, Y.; Zuluaga, S.; Mejia, R.; Triana-Chavez, O. Association of midgut bacteria and their metabolic pathways with Zika infection and insecticide resistance in colombian Aedes aegypti populations. Viruses 2022, 14, 2197. [Google Scholar] [CrossRef] [PubMed]
- Djihinto, O.Y.; Medjigbodo, A.A.; Gangbadja, A.R.A.; Saizonou, H.M.; Lagnika, H.O.; Nanmede, D.; Djossou, L.; Bohounton, R.; Sovegnon, P.M.; Fanou, M.J.; et al. Malaria-transmitting vectors microbiota: Overview and interactions with Anopheles mosquito biology. Front. Microbiol. 2022, 13, 891573. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef]
- Nazni, W.A.; Hoffmann, A.A.; NoorAfizah, A.; Cheong, Y.L.; Mancini, M.V.; Golding, N.; Kamarul, G.M.R.; Arif, M.A.K.; Thohir, H.; NurSyamimi, H.; et al. Establishment of Wolbachia strain wAlbB in malaysian populations of Aedes aegypti for dengue control. Curr. Biol. 2019, 29, 4241–4248.e5. [Google Scholar] [CrossRef]
- Sanborn, M.A.; Wuertz, K.M.; Kim, H.C.; Yang, Y.; Li, T.; Pollett, S.D.; Jarman, R.G.; Berry, I.M.; Klein, T.A.; Hang, J. Metagenomic analysis reveals Culex mosquito virome diversity and Japanese encephalitis genotype V. in the Republic of Korea. Mol. Ecol. 2021, 30, 5470–5487. [Google Scholar] [CrossRef]
- Faizah, A.N.; Kobayashi, D.; Isawa, H.; Amoa-Bosompem, M.; Murota, K.; Higa, Y.; Futami, K.; Shimada, S.; Kim, K.S.; Itokawa, K.; et al. Deciphering the virome of Culex vishnui subgroup mosquitoes, the major vectors of Japanese Encephalitis, in Japan. Viruses 2020, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Demirci, M.; Yildiz Zeyrek, F. Malaria and gut microbiota: Microbial interactions in the host. Mikrobiyol. Bul. 2022, 56, 763–775. [Google Scholar] [PubMed]
- Omondi, Z.N.; Caner, A. An overview on the impact of microbiota on malaria transmission and severity: Plasmodium-vector-host axis. Acta Parasitol. 2022, 67, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Muirhead-Thomson, R.C. DDT and gammexane as residual insecticides against Anopheles gambiae in African houses. Trans. R. Soc. Trop. Med. Hyg. 1950, 43, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Cilek, J.E.; Sumners, E.; Briley, A.K.C.; Weston, J.; Richardson, A.G.; Lindroth, E.J. Potential of outdoor ultra-low-volume aerosol and thermal fog tosuppress the dengue vector, Aedes aegypti, inside dwellings. J. Am. Mosq. Control Assoc. 2020, 36, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Ruktanonchai, D.J.; Stonecipher, S.; Lindsey, N.; McAllister, J.; Pillai, S.K.; Horiuchi, K.; Delorey, M.; Biggerstaff, B.J.; Sidwa, T.; Zoretic, J.; et al. Effect of aerial insecticide spraying on West Nile virus disease—North-central Texas, 2012. Am. J. Trop. Med. Hyg. 2014, 91, 240–245. [Google Scholar] [CrossRef]
- Gleave, K.; Lissenden, N.; Richardson, M.; Ranson, H. Piperonyl butoxide (PBO) combined with pyrethroids in long-lasting insecticidal nets (LLINs) to prevent malaria in Africa. Cochrane Database Syst. Rev. 2017, 11, CD012776. [Google Scholar] [CrossRef]
- WHO. Global Report on Insecticide Resistance in Malaria Vectors: 2010–2016; WHO: Geneva, Switzerland, 2018.
- Al Nazawi, A.M.; Aqili, J.; Alzahrani, M.; McCall, P.J.; Weetman, D. Combined target site (kdr) mutations play a primary role in highly pyrethroid resistant phenotypes of Aedes aegypti from Saudi Arabia. Parasit. Vectors 2017, 10, 161. [Google Scholar] [CrossRef]
- Ochomo, E.; Bayoh, M.N.; Brogdon, W.G.; Gimnig, J.E.; Ouma, C.; Vulule, J.M.; Walker, E.D. Pyrethroid resistance in Anopheles gambiae s.s. and Anopheles arabiensis in western Kenya: Phenotypic, metabolic and target site characterizations of three populations. Med. Vet. Entomol. 2013, 27, 156–164. [Google Scholar] [CrossRef]
- Muthusamy, R.; Shivakumar, M.S. Involvement of metabolic resistance and F1534C kdr mutation in the pyrethroid resistance mechanisms of Aedes aegypti in India. Acta Trop. 2015, 148, 137–141. [Google Scholar] [CrossRef]
- Adedeji, E.O.; Ogunlana, O.O.; Fatumo, S.; Beder, T.; Ajamma, Y.; Koenig, R.; Adebiyi, E. Anopheles metabolic proteins in malaria transmission, prevention and control: A review. Parasit. Vectors 2020, 13, 465. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.U.; Souza, B.; Uddin, I.; Carrara, L.; Brito, L.P.; Costa, M.M.; Mahmood, M.A.; Khan, S.; Lima, J.B.P.; Martins, A.J. Insecticide resistance and underlying targets-site and metabolic mechanisms in Aedes aegypti and Aedes albopictus from Lahore, Pakistan. Sci. Rep. 2021, 11, 4555. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Li, Y.; Jeang, B.; Wang, X.; Cummings, R.F.; Zhong, D.; Yan, G. Emerging mosquito resistance to piperonyl butoxide-synergized pyrethroid insecticide and its mechanism. J. Med. Entomol. 2022, 59, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Dada, N.; Benedict, A.C.; López, F.; Lol, J.C.; Sheth, M.; Dzuris, N.; Padilla, N.; Lenhart, A. Comprehensive characterization of internal and cuticle surface microbiota of laboratory-reared F(1) Anopheles albimanus originating from different sites. Malar. J. 2021, 20, 414. [Google Scholar] [CrossRef] [PubMed]
- Keita, M.; Sogoba, N.; Kane, F.; Traore, B.; Zeukeng, F.; Coulibaly, B.; Sodio, A.B.; Traore, S.F.; Djouaka, R.; Doumbia, S. Multiple resistance mechanisms to pyrethroids insecticides in Anopheles gambiae sensu lato population from mali, West Africa. J. Infect. Dis. 2021, 223, S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, G.; Zhong, D.; Wang, X.; Hemming-Schroeder, E.; David, R.E.; Lee, M.C.; Zhong, S.; Yi, G.; Liu, Z.; et al. Widespread multiple insecticide resistance in the major dengue vector Aedes albopictus in Hainan Province, China. Pest Manag. Sci. 2021, 77, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Dada, N.; Sheth, M.; Liebman, K.; Pinto, J.; Lenhart, A. Whole metagenome sequencing reveals links between mosquito microbiota and insecticide resistance in malaria vectors. Sci. Rep. 2018, 8, 2084. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Sun, B.; Gurr, G.M.; Vasseur, L.; Xue, M.; You, M. Gut microbiota mediate insecticide resistance in the diamondback moth, Plutella xylostella (L.). Front. Microbiol. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Pan, Z.; Jin, C.; Ni, Y.; Fu, Z.; Jin, Y. Gut microbiota: An underestimated and unintended recipient for pesticide-induced toxicity. Chemosphere 2019, 227, 425–434. [Google Scholar] [CrossRef]
- Wang, S.; Wen, Q.; Qin, Y.; Xia, Q.; Shen, C.; Song, S. Gut microbiota and host cytochrome P450 characteristics in the pseudo germ-free model: Co-contributors to a diverse metabolic landscape. Gut Pathog. 2023, 15, 15. [Google Scholar] [CrossRef]
- Gomez-Govea, M.A.; Ramirez-Ahuja, M.L.; Contreras-Perera, Y.; Jimenez-Camacho, A.J.; Ruiz-Ayma, G.; Villanueva-Segura, O.K.; Trujillo-Rodriguez, G.J.; Delgado-Enciso, I.; Martinez-Fierro, M.L.; Manrique-Saide, P.; et al. Suppression of midgut microbiota impact pyrethroid susceptibility in Aedes aegypti. Front. Microbiol. 2022, 13, 761459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Shen, R.X.; Xing, D.; Zhao, C.P.; Gao, H.T.; Wu, J.H.; Zhang, N.; Zhang, H.D.; Chen, Y.; Zhao, T.Y.; et al. Metagenome sequencing reveals the midgut microbiota makeup of Culex pipiens quinquefasciatus and its possible relationship with insecticide resistance. Front. Microbiol. 2021, 12, 625539. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Dunlap, C.; Smartt, C.T.; Shin, D. Resistance to permethrin alters the gut microbiota of Aedes aegypti. Sci. Rep. 2021, 11, 14406. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Khan, M.M.; Bamisile, B.S.; Hafeez, M.; Qasim, M.; Rasheed, M.T.; Rasheed, M.A.; Ahmad, S.; Shahid, M.I.; Xu, Y. Role of Insect Gut Microbiota in Pesticide Degradation: A Review. Front. Microbiol. 2022, 13, 870462. [Google Scholar] [CrossRef] [PubMed]
- Giambo, F.; Teodoro, M.; Costa, C.; Fenga, C. Toxicology and microbiota: How do pesticides influence gut microbiota? A review. Int. J. Environ. Res. Public Health 2021, 18, 5510. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, T.; Wu, Y.; Zhong, D.; Zhou, G.; Su, X.; Xu, J.; Sotero, C.F.; Sadruddin, A.A.; Wu, K.; et al. Bacterial microbiota assemblage in Aedes albopictus mosquitoes and its impacts on larval development. Mol. Ecol. 2018, 27, 2972–2985. [Google Scholar] [CrossRef]
- Wang, Y.; Gilbreath, T.M.; Kukutla, P., 3rd; Yan, G.; Xu, J. Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS ONE 2011, 6, e24767. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Wang, Y.; Li, S.; Sun, X.; Lu, X.; Rajaofera, M.J.N.; Lu, Y.; Kang, L.; Zheng, A.; Zou, Z.; et al. Comparative analysis of the gut microbiota of adult mosquitoes from eight locations in Hainan, China. Front. Cell. Infect. Microbiol. 2020, 10, 596750. [Google Scholar] [CrossRef]
- Guegan, M.; Zouache, K.; Demichel, C.; Minard, G.; Tran Van, V.; Potier, P.; Mavingui, P.; Valiente Moro, C. The mosquito holobiont: Fresh insight into mosquito-microbiota interactions. Microbiome 2018, 6, 49. [Google Scholar] [CrossRef]
- Singh, A.; Allam, M.; Kwenda, S.; Khumalo, Z.T.H.; Ismail, A.; Oliver, S.V. The dynamic gut microbiota of zoophilic members of the Anopheles gambiae complex (Diptera: Culicidae). Sci. Rep. 2022, 12, 1495. [Google Scholar] [CrossRef]
- Ngo, C.T.; Aujoulat, F.; Veas, F.; Jumas-Bilak, E.; Manguin, S. Bacterial diversity associated with wild caught Anopheles mosquitoes from Dak Nong Province, Vietnam using culture and DNA fingerprint. PLoS ONE 2015, 10, e0118634. [Google Scholar] [CrossRef] [PubMed]
- Didion, E.M.; Doyle, M.; Benoit, J.B. Bacterial Communities of Lab and Field Northern House Mosquitoes (Diptera: Culicidae) Throughout Diapause. J. Med. Entomol. 2022, 59, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Akorli, J.; Gendrin, M.; Pels, N.A.; Yeboah-Manu, D.; Christophides, G.K.; Wilson, M.D. Seasonality and locality affect the diversity of Anopheles gambiae and Anopheles coluzzii midgut microbiota from Ghana. PLoS ONE 2016, 11, e0157529. [Google Scholar] [CrossRef] [PubMed]
- Boissiere, A.; Tchioffo, M.T.; Bachar, D.; Abate, L.; Marie, A.; Nsango, S.E.; Shahbazkia, H.R.; Awono-Ambene, P.H.; Levashina, E.A.; Christen, R.; et al. Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog. 2012, 8, e1002742. [Google Scholar] [CrossRef] [PubMed]
- Seabourn, P.; Spafford, H.; Yoneishi, N.; Medeiros, M. The Aedes albopictus (Diptera: Culicidae) microbiome varies spatially and with Ascogregarine infection. PLoS Negl. Trop. Dis. 2020, 14, e0008615. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.L.; Souza-Neto, J.; Torres Cosme, R.; Rovira, J.; Ortiz, A.; Pascale, J.M.; Dimopoulos, G. Reciprocal tripartite interactions between the Aedes aegypti midgut microbiota, innate immune system and dengue virus influences vector competence. PLoS Negl. Trop. Dis. 2012, 6, e1561. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Sun, P.; Nie, K.; Zhu, Y.; Shi, M.; Xiao, C.; Liu, H.; Liu, Q.; Zhao, T.; Chen, X.; et al. A gut commensal bacterium promotes mosquito permissiveness to arboviruses. Cell Host Microbe 2019, 25, 101–112.e5. [Google Scholar] [CrossRef] [PubMed]
- Kalappa, D.M.; Subramani, P.A.; Basavanna, S.K.; Ghosh, S.K.; Sundaramurthy, V.; Uragayala, S.; Tiwari, S.; Anvikar, A.R.; Valecha, N. Influence of midgut microbiota in Anopheles stephensi on Plasmodium berghei infections. Malar. J. 2018, 17, 385. [Google Scholar] [CrossRef] [PubMed]
- Tchioffo, M.T.; Boissiere, A.; Churcher, T.S.; Abate, L.; Gimonneau, G.; Nsango, S.E.; Awono-Ambene, P.H.; Christen, R.; Berry, A.; Morlais, I. Modulation of malaria infection in Anopheles gambiae mosquitoes exposed to natural midgut bacteria. PLoS ONE 2013, 8, e81663. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, L.; Pang, X.; Zhang, R.; Zhu, Y.; Wang, P.; Gao, G.; Cheng, G. A Mesh-Duox pathway regulates homeostasis in the insect gut. Nat. Microbiol. 2017, 2, 17020. [Google Scholar] [CrossRef]
- Barnard, K.; Jeanrenaud, A.; Brooke, B.D.; Oliver, S.V. The contribution of gut bacteria to insecticide resistance and the life histories of the major malaria vector Anopheles arabiensis (Diptera: Culicidae). Sci. Rep. 2019, 9, 9117. [Google Scholar] [CrossRef] [PubMed]
- Chabanol, E.; Behrends, V.; Prevot, G.; Christophides, G.K.; Gendrin, M. Antibiotic treatment in Anopheles coluzzii affects carbon and nitrogen metabolism. Pathogens 2020, 9, 679. [Google Scholar] [CrossRef]
- Romoli, O.; Schonbeck, J.C.; Hapfelmeier, S.; Gendrin, M. Production of germ-free mosquitoes via transient colonisation allows stage-specific investigation of host-microbiota interactions. Nat. Commun. 2021, 12, 942. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Dhayal, D.; Singh, O.P.; Adak, T.; Bhatnagar, R.K. Gut microbes influence fitness and malaria transmission potential of Asian malaria vector Anopheles stephensi. Acta Trop. 2013, 128, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Fouda, M.A.; Hassan, M.I.; Al-Daly, A.G.; Hammad, K.M. Effect of midgut bacteria of Culex pipiens L. on digestion and reproduction. J. Egypt. Soc. Parasitol. 2001, 31, 767–780. [Google Scholar]
- Almeida, F.; Moura, A.S.; Cardoso, A.F.; Winter, C.E.; Bijovsky, A.T.; Suesdek, L. Effects of Wolbachia on fitness of Culex quinquefasciatus (Diptera; Culicidae). Infect. Genet. Evol. 2011, 11, 2138–2143. [Google Scholar] [CrossRef]
- Alomar, A.A.; Perez-Ramos, D.W.; Kim, D.; Kendziorski, N.L.; Eastmond, B.H.; Alto, B.W.; Caragata, E.P. Native Wolbachia infection and larval competition stress shape fitness and West Nile virus infection in Culex quinquefasciatus mosquitoes. Front. Microbiol. 2023, 14, 1138476. [Google Scholar] [CrossRef]
- Ezemuoka, L.C.; Akorli, E.A.; Aboagye-Antwi, F.; Akorli, J. Mosquito midgut Enterobacter cloacae and Serratia marcescens affect the fitness of adult female Anopheles gambiae s.l. PLoS ONE 2020, 15, e0238931. [Google Scholar] [CrossRef]
- Mitraka, E.; Stathopoulos, S.; Siden-Kiamos, I.; Christophides, G.K.; Louis, C. Asaia accelerates larval development of Anopheles gambiae. Pathog. Glob. Health 2013, 107, 305–311. [Google Scholar] [CrossRef]
- Fouda, M.A.; Hassan, M.I.; Hammad, K.M.; Hasaballah, A.I. Effects of midgut bacteria and two protease inhibitors on the reproductive potential and midgut enzymes of Culex pipiens infected with Wuchereria bancrofti. J. Egypt. Soc. Parasitol. 2013, 43, 537–546. [Google Scholar] [CrossRef]
- Gusmao, D.S.; Santos, A.V.; Marini, D.C.; Bacci, M., Jr.; Berbert-Molina, M.A.; Lemos, F.J. Culture-dependent and culture-independent characterization of microorganisms associated with Aedes aegypti (Diptera: Culicidae) (L.) and dynamics of bacterial colonization in the midgut. Acta Trop. 2010, 115, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Ricci, I.; Cappelli, A.; Damiani, C.; Ulissi, U.; Mancini, M.V.; Valzano, M.; Capone, A.; Epis, S.; Crotti, E.; et al. Mutual exclusion of Asaia and Wolbachia in the reproductive organs of mosquito vectors. Parasit. Vectors 2015, 8, 278. [Google Scholar] [CrossRef]
- Ranasinghe, K.; Gunathilaka, N.; Amarasinghe, D.; Rodrigo, W.; Udayanga, L. Diversity of midgut bacteria in larvae and females of Aedes aegypti and Aedes albopictus from Gampaha District, Sri Lanka. Parasit. Vectors 2021, 14, 433. [Google Scholar] [CrossRef] [PubMed]
- Demaio, J.; Pumpuni, C.B.; Kent, M.; Beier, J.C. The midgut bacterial flora of wild Aedes triseriatus, Culex pipiens, and Psorophora columbiae mosquitoes. Am. J. Trop. Med. Hyg. 1996, 54, 219–223. [Google Scholar] [CrossRef]
- Kim, C.H.; Lampman, R.L.; Muturi, E.J. Bacterial communities and midgut microbiota associated with mosquito populations from Waste Tires in East-Central Illinois. J. Med. Entomol. 2015, 52, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Zotzmann, S.; Steinbrink, A.; Schleich, K.; Frantzmann, F.; Xoumpholphakdy, C.; Spaeth, M.; Moro, C.V.; Mavingui, P.; Klimpel, S. Bacterial diversity of cosmopolitan Culex pipiens and invasive Aedes japonicus from Germany. Parasitol. Res. 2017, 116, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Straif, S.C.; Mbogo, C.N.; Toure, A.M.; Walker, E.D.; Kaufman, M.; Toure, Y.T.; Beier, J.C. Midgut bacteria in Anopheles gambiae and An. funestus (Diptera: Culicidae) from Kenya and Mali. J. Med. Entomol. 1998, 35, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Lindh, J.M.; Terenius, O.; Faye, I. 16S rRNA gene-based identification of midgut bacteria from field-caught Anopheles gambiae sensu lato and A. funestus mosquitoes reveals new species related to known insect symbionts. Appl. Environ. Microbiol. 2005, 71, 7217–7223. [Google Scholar] [CrossRef]
- Buck, M.; Nilsson, L.K.; Brunius, C.; Dabire, R.K.; Hopkins, R.; Terenius, O. Bacterial associations reveal spatial population dynamics in Anopheles gambiae mosquitoes. Sci. Rep. 2016, 6, 22806. [Google Scholar] [CrossRef]
- Zoure, A.A.; Sare, A.R.; Yameogo, F.; Somda, Z.; Massart, S.; Badolo, A.; Francis, F. Bacterial communities associated with the midgut microbiota of wild Anopheles gambiae complex in Burkina Faso. Mol. Biol. Rep. 2020, 47, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Favia, G.; Ricci, I.; Damiani, C.; Raddadi, N.; Crotti, E.; Marzorati, M.; Rizzi, A.; Urso, R.; Brusetti, L.; Borin, S.; et al. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc. Natl. Acad. Sci. USA 2007, 104, 9047–9051. [Google Scholar] [CrossRef] [PubMed]
- Gunathilaka, N.; Ranasinghe, K.; Amarasinghe, D.; Rodrigo, W.; Mallawarachchi, H.; Chandrasena, N. Molecular characterization of culturable aerobic bacteria in the midgut of field-caught Culex tritaeniorhynchus, Culex gelidus, and Mansonia annulifera Mosquitoes in the Gampaha District of Sri Lanka. Biomed. Res. Int. 2020, 2020, 8732473. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Kim, C.H.; Bara, J.; Bach, E.M.; Siddappaji, M.H. Culex pipiens and Culex restuans mosquitoes harbor distinct microbiota dominated by few bacterial taxa. Parasit. Vectors 2016, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Chandel, K.; Mendki, M.J.; Parikh, R.Y.; Kulkarni, G.; Tikar, S.N.; Sukumaran, D.; Prakash, S.; Parashar, B.D.; Shouche, Y.S.; Veer, V. Midgut microbial community of Culex quinquefasciatus mosquito populations from India. PLoS ONE 2013, 8, e80453. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Bouchon, D.; Boutin, S.; Bellamy, L.; Zhou, L.; Engelstadter, J.; Hurst, G.D. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Bordenstein, S.R.; Slatko, B. Microbe Profile: Wolbachia: A sex selector, a viral protector and a target to treat filarial nematodes. Microbiology 2018, 164, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Kozek, W.J.; Rao, R.U. The Discovery of Wolbachia in Arthropods and Nematodes—A Historical Perspective; Karger Publishers: Basel, Switzerland, 2007; pp. 1–14. [Google Scholar]
- Li, Y.; Sun, Y.; Zou, J.; Zhong, D.; Liu, R.; Zhu, C.; Li, W.; Zhou, Y.; Cui, L.; Zhou, G.; et al. Characterizing the Wolbachia infection in field-collected Culicidae mosquitoes from Hainan Province, China. Parasit. Vectors 2023, 16, 128. [Google Scholar] [CrossRef]
- Wong, M.L.; Liew, J.W.K.; Wong, W.K.; Pramasivan, S.; Mohamed Hassan, N.; Wan Sulaiman, W.Y.; Jeyaprakasam, N.K.; Leong, C.S.; Low, V.L.; Vythilingam, I. Natural Wolbachia infection in field-collected Anopheles and other mosquito species from Malaysia. Parasit. Vectors 2020, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Nugapola, N.; De Silva, W.; Karunaratne, S. Distribution and phylogeny of Wolbachia strains in wild mosquito populations in Sri Lanka. Parasit. Vectors 2017, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yeo, H.; Puniamoorthy, N. Wolbachia infection in wild mosquitoes (Diptera: Culicidae): Implications for transmission modes and host-endosymbiont associations in Singapore. Parasit. Vectors 2020, 13, 612. [Google Scholar] [CrossRef]
- da Silva, H.; Oliveira, T.M.P.; Sallum, M.A.M. Bacterial community diversity and bacterial interaction network in eight mosquito species. Genes 2022, 13, 2052. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.A.M.; Lopez, R.P.; Cerrudo, C.S.; Perotti, M.A.; Consolo, V.F.; Beron, C.M. Wolbachia dominance influences the Culex quinquefasciatus microbiota. Sci. Rep. 2023, 13, 18980. [Google Scholar] [CrossRef] [PubMed]
- Chrostek, E.; Gerth, M. Is Anopheles gambiae a natural host of Wolbachia? mBio 2019, 10, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, C.L.; Lawrence, G.G.; Golovko, G.; Kristan, M.; Orsborne, J.; Spence, K.; Hurn, E.; Bandibabone, J.; Tantely, L.M.; Raharimalala, F.N.; et al. Novel Wolbachia strains in Anopheles malaria vectors from Sub-Saharan Africa. Wellcome Open Res. 2018, 3, 113. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Quek, S.; Jeffries, C.L.; Bandibabone, J.; Dhokiya, V.; Bamou, R.; Kristan, M.; Messenger, L.A.; Gidley, A.; Hornett, E.A.; et al. Stable high-density and maternally inherited Wolbachia infections in Anopheles moucheti and Anopheles demeilloni mosquitoes. Curr. Biol. 2021, 31, 2310–2320.e5. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Calvitti, M. Male mating performance and cytoplasmic incompatibility in a wPip Wolbachia trans-infected line of Aedes albopictus (Stegomyia albopicta). Med. Vet. Entomol. 2013, 27, 377–386. [Google Scholar] [CrossRef]
- Araujo, N.J.S.; Macedo, M.J.F.; de Morais, L.P.; da Cunha, F.A.B.; de Matos, Y.; de Almeida, R.S.; Braga, M.; Coutinho, H.D.M. Control of arboviruses vectors using biological control by Wolbachia pipientis: A short review. Arch. Microbiol. 2022, 204, 376. [Google Scholar] [CrossRef]
- Mohanty, I.; Rath, A.; Mahapatra, N.; Hazra, R.K. Wolbachia: A biological control strategy against arboviral diseases. J. Vector Borne Dis. 2016, 53, 199–207. [Google Scholar]
- Iturbe-Ormaetxe, I.; Walker, T.; O’Neill, S.L. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 2011, 12, 508–518. [Google Scholar] [CrossRef]
- Hurst, G.D.D.; Jiggins, F.M.; Hinrich Graf von der Schulenburg, J.; Bertrand, D.; West, S.A.; Goriacheva, I.I.; Zakharov, I.A.; Werren, J.H.; Stouthamer, R.; Majerus, M.E.N. Male–killing Wolbachia in two species of insect. Proc. R. Soc. Lond. B Biol. Sci. 1999, 266, 735–740. [Google Scholar] [CrossRef]
- Fujii, Y.; Kageyama, D.; Hoshizaki, S.; Ishikawa, H.; Sasaki, T. Transfection of Wolbachia in Lepidoptera: The feminizer of the adzuki bean borer Ostrinia scapulalis causes male killing in the Mediterranean flour moth Ephestia kuehniella. Proc. Biol. Sci. 2001, 268, 855–859. [Google Scholar] [CrossRef]
- Knight, J. Meet the Herod bug. Nature 2001, 412, 12–14. [Google Scholar] [CrossRef]
- Yen, J.H.; Barr, A.R. The etiological agent of cytoplasmic incompatibility in Culex pipiens. J. Invertebr. Pathol. 1973, 22, 242–250. [Google Scholar] [CrossRef]
- Dobson, S.L.; Fox, C.W.; Jiggins, F.M. The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc. Biol. Sci. 2002, 269, 437–445. [Google Scholar] [CrossRef]
- Laven, H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature 1967, 216, 383–384. [Google Scholar] [CrossRef]
- Yen, P.S.; Failloux, A.B. A Review: Wolbachia-Based Population Replacement for Mosquito Control Shares Common Points with Genetically Modified Control Approaches. Pathogens 2020, 9, 404. [Google Scholar] [CrossRef]
- Yen, J.H.; Barr, A.R. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature 1971, 232, 657–658. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q.; et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef]
- Crawford, J.E.; Clarke, D.W.; Criswell, V.; Desnoyer, M.; Cornel, D.; Deegan, B.; Gong, K.; Hopkins, K.C.; Howell, P.; Hyde, J.S.; et al. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat. Biotechnol. 2020, 38, 482–492. [Google Scholar] [CrossRef]
- Marris, E. Bacteria could be key to freeing South Pacific of mosquitoes. Nature 2017, 548, 17–18. [Google Scholar] [CrossRef]
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes aegypti vector competence studies: A review. Infect. Genet. Evol. 2019, 67, 191–209. [Google Scholar] [CrossRef]
- Li, F.; Hua, H.; Ali, A.; Hou, M. Characterization of a bacterial symbiont Asaia sp. in the white-backed planthopper, Sogatella furcifera, and its effects on host fitness. Front. Microbiol. 2019, 10, 2179. [Google Scholar] [CrossRef] [PubMed]
- Crotti, E.; Damiani, C.; Pajoro, M.; Gonella, E.; Rizzi, A.; Ricci, I.; Negri, I.; Scuppa, P.; Rossi, P.; Ballarini, P.; et al. Asaia, a versatile acetic acid bacterial symbiont, capable of cross-colonizing insects of phylogenetically distant genera and orders. Environ. Microbiol. 2009, 11, 3252–3264. [Google Scholar] [CrossRef] [PubMed]
- Mercant Osuna, A.; Gidley, A.; Mayi, M.P.A.; Bamou, R.; Dhokiya, V.; Antonio-Nkondjio, C.; Jeffries, C.L.; Walker, T. Diverse novel Wolbachia bacteria strains and genera-specific co-infections with Asaia bacteria in Culicine mosquitoes from ecologically diverse regions of Cameroon. Wellcome Open Res. 2023, 8, 267. [Google Scholar] [CrossRef]
- Chouaia, B.; Rossi, P.; Montagna, M.; Ricci, I.; Crotti, E.; Damiani, C.; Epis, S.; Faye, I.; Sagnon, N.; Alma, A.; et al. Molecular evidence for multiple infections as revealed by typing of Asaia bacterial symbionts of four mosquito species. Appl. Environ. Microbiol. 2010, 76, 7444–7450. [Google Scholar] [CrossRef] [PubMed]
- Omoke, D.; Kipsum, M.; Otieno, S.; Esalimba, E.; Sheth, M.; Lenhart, A.; Njeru, E.M.; Ochomo, E.; Dada, N. Western Kenyan Anopheles gambiae showing intense permethrin resistance harbour distinct microbiota. Malar. J. 2021, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Pelloquin, B.; Kristan, M.; Edi, C.; Meiwald, A.; Clark, E.; Jeffries, C.L.; Walker, T.; Dada, N.; Messenger, L.A. Overabundance of Asaia and Serratia bacteria is associated with deltamethrin insecticide susceptibility in Anopheles coluzzii from Agboville, Cote d’Ivoire. Microbiol. Spectr. 2021, 9, e0015721. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, C.L.; Tantely, L.M.; Raharimalala, F.N.; Hurn, E.; Boyer, S.; Walker, T. Diverse novel resident Wolbachia strains in Culicine mosquitoes from Madagascar. Sci. Rep. 2018, 8, 17456. [Google Scholar] [CrossRef] [PubMed]
- Schrieke, H.; Maignien, L.; Constancias, F.; Trigodet, F.; Chakloute, S.; Rakotoarivony, I.; Marie, A.; L’Ambert, G.; Makoundou, P.; Pages, N.; et al. The mosquito microbiome includes habitat-specific but rare symbionts. Comput. Struct. Biotechnol. J. 2022, 20, 410–420. [Google Scholar] [CrossRef]
- Hughes, G.L.; Dodson, B.L.; Johnson, R.M.; Murdock, C.C.; Tsujimoto, H.; Suzuki, Y.; Patt, A.A.; Cui, L.; Nossa, C.W.; Barry, R.M.; et al. Native microbiome impedes vertical transmission of Wolbachia in Anopheles mosquitoes. Proc. Natl. Acad. Sci. USA 2014, 111, 12498–12503. [Google Scholar] [CrossRef]
- Capone, A.; Ricci, I.; Damiani, C.; Mosca, M.; Rossi, P.; Scuppa, P.; Crotti, E.; Epis, S.; Angeletti, M.; Valzano, M.; et al. Interactions between Asaia, Plasmodium and Anopheles: New insights into mosquito symbiosis and implications in Malaria Symbiotic Control. Parasites Vectors 2013, 6, 182. [Google Scholar] [CrossRef]
- Grogan, C.; Bennett, M.; Moore, S.; Lampe, D. Novel Asaia bogorensis Signal Sequences for Plasmodium Inhibition in Anopheles stephensi. Front. Microbiol. 2021, 12, 633667. [Google Scholar] [CrossRef]
- Favia, G.; Ricci, I.; Marzorati, M.; Negri, I.; Alma, A.; Sacchi, L.; Bandi, C.; Daffonchio, D. Bacteria of the genus Asaia: A potential paratransgenic weapon against malaria. Adv. Exp. Med. Biol. 2008, 627, 49–59. [Google Scholar]
- Damiani, C.; Ricci, I.; Crotti, E.; Rossi, P.; Rizzi, A.; Scuppa, P.; Capone, A.; Ulissi, U.; Epis, S.; Genchi, M.; et al. Mosquito-bacteria symbiosis: The case of Anopheles gambiae and Asaia. Microb. Ecol. 2010, 60, 644–654. [Google Scholar] [CrossRef]
- Kozlova, E.V.; Hegde, S.; Roundy, C.M.; Golovko, G.; Saldana, M.A.; Hart, C.E.; Anderson, E.R.; Hornett, E.A.; Khanipov, K.; Popov, V.L.; et al. Microbial interactions in the mosquito gut determine Serratia colonization and blood-feeding propensity. ISME J. 2021, 15, 93–108. [Google Scholar] [CrossRef]
- Bai, L.; Wang, L.; Vega-Rodriguez, J.; Wang, G.; Wang, S. A gut symbiotic bacterium Serratia marcescens renders mosquito resistance to Plasmodium infection through activation of mosquito immune responses. Front. Microbiol. 2019, 10, 1580. [Google Scholar] [CrossRef]
- Dinparast Djadid, N.; Jazayeri, H.; Raz, A.; Favia, G.; Ricci, I.; Zakeri, S. Identification of the midgut microbiota of An. stephensi and An. maculipennis for their application as a paratransgenic tool against malaria. PLoS ONE 2011, 6, e28484. [Google Scholar] [CrossRef]
- Wang, S.; Dos-Santos, A.L.A.; Huang, W.; Liu, K.C.; Oshaghi, M.A.; Wei, G.; Agre, P.; Jacobs-Lorena, M. Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science 2017, 357, 1399–1402. [Google Scholar] [CrossRef]
- Gao, H.; Bai, L.; Jiang, Y.; Huang, W.; Wang, L.; Li, S.; Zhu, G.; Wang, D.; Huang, Z.; Li, X.; et al. A natural symbiotic bacterium drives mosquito refractoriness to Plasmodium infection via secretion of an antimalarial lipase. Nat. Microbiol. 2021, 6, 806–817. [Google Scholar] [CrossRef]
- Arevalo-Cortes, A.; Mejia-Jaramillo, A.M.; Granada, Y.; Coatsworth, H.; Lowenberger, C.; Triana-Chavez, O. The midgut microbiota of colombian Aedes aegypti populations with different levels of resistance to the insecticide lambda-cyhalothrin. Insects 2020, 11, 584. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Cheng, P.; Wang, Y.; Liu, H.; Wang, H.; Wang, H.; Gong, M. Differences in the intestinal microbiota between insecticide-resistant and -sensitive Aedes albopictus based on full-length 16S rRNA sequencing. Microbiologyopen 2021, 10, e1177. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Peng, H.; Wang, Y.; Zhang, C.; Guo, X.; Wang, H.; Liu, L.; Lv, W.; Cheng, P.; et al. A symbiotic gut bacterium enhances Aedes albopictus resistance to insecticide. PLoS Negl. Trop. Dis. 2022, 16, e0010208. [Google Scholar] [CrossRef]
- Dada, N.; Lol, J.C.; Benedict, A.C.; Lopez, F.; Sheth, M.; Dzuris, N.; Padilla, N.; Lenhart, A. Pyrethroid exposure alters internal and cuticle surface bacterial communities in Anopheles albimanus. ISME J. 2019, 13, 2447–2464. [Google Scholar] [CrossRef]
- Berticat, C.; Rousset, F.; Raymond, M.; Berthomieu, A.; Weill, M. High Wolbachia density in insecticide-resistant mosquitoes. Proc. Biol. Sci. 2002, 269, 1413–1416. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, W.; Zhang, Q.; Zhang, X.; Zhang, Z. Microbiota and transcriptome changes of Culex pipiens pallens larvae exposed to Bacillus thuringiensis israelensis. Sci. Rep. 2021, 11, 20241. [Google Scholar] [CrossRef]
- Receveur, J.P.; Pechal, J.L.; Benbow, M.E.; Donato, G.; Rainey, T.; Wallace, J.R. Changes in larval mosquito microbiota reveal non-target effects of insecticide treatments in hurricane-created habitats. Microb. Ecol. 2018, 76, 719–728. [Google Scholar] [CrossRef]
- Endersby, N.M.; Hoffmann, A.A. Effect of Wolbachia on insecticide susceptibility in lines of Aedes aegypti. Bull. Entomol. Res. 2013, 103, 269–277. [Google Scholar] [CrossRef]
- Duron, O.; Labbé, P.; Berticat, C.; Rousset, F.; Guillot, S.; Raymond, M.; Weill, M. High Wolbachia density correlates with cost ofinfection for insecticide resistant Culex Pipiens mosquitoes. Evolution 2006, 60, 303. [Google Scholar]
- Shemshadian, A.; Vatandoost, H.; Oshaghi, M.A.; Abai, M.R.; Djadid, N.D.; Karimian, F. Relationship between Wolbachia infection in Culex quinquefasciatus and its resistance to insecticide. Heliyon 2021, 7, e06749. [Google Scholar] [CrossRef]
- Ismail, H.M.; Freed, S.; Naeem, A.; Malik, S.; Ali, N. The effect of entomopathogenic fungi on enzymatic activity in chlorpyrifos-resistant mosquitoes, Culex quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 2020, 57, 204–213. [Google Scholar] [CrossRef]
- Farenhorst, M.; Mouatcho, J.C.; Kikankie, C.K.; Brooke, B.D.; Hunt, R.H.; Thomas, M.B.; Koekemoer, L.L.; Knols, B.G.; Coetzee, M. Fungal infection counters insecticide resistance in African malaria mosquitoes. Proc. Natl. Acad. Sci. USA 2009, 106, 17443–17447. [Google Scholar] [CrossRef]
- Howard, A.F.; Koenraadt, C.J.; Farenhorst, M.; Knols, B.G.; Takken, W. Pyrethroid resistance in Anopheles gambiae leads to increased susceptibility to the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Malar. J. 2010, 9, 168. [Google Scholar] [CrossRef]
- Patil, C.D.; Borase, H.P.; Salunke, B.K.; Patil, S.V. Alteration in Bacillus thuringiensis toxicity by curing gut flora: Novel approach for mosquito resistance management. Parasitol. Res. 2013, 112, 3283–3288. [Google Scholar] [CrossRef]
- Scates, S.S.; O’Neal, S.T.; Anderson, T.D. Bacteria-mediated modification of insecticide toxicity in the yellow fever mosquito, Aedes aegypti. Pestic. Biochem. Physiol. 2019, 161, 77–85. [Google Scholar] [CrossRef]
- Kikankie, C.K.; Brooke, B.D.; Knols, B.G.; Koekemoer, L.L.; Farenhorst, M.; Hunt, R.H.; Thomas, M.B.; Coetzee, M. The infectivity of the entomopathogenic fungus Beauveria bassiana to insecticide-resistant and susceptible Anopheles arabiensis mosquitoes at two different temperatures. Malar. J. 2010, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandhan, P.; Swathy, K.; Kalaimurugan, D.; Ramachandran, M.; Yuvaraj, A.; Kumar, A.N.; Manikandan, A.T.; Poovarasan, N.; Shivakumar, M.S.; Kweka, E.J. Larvicidal toxicity of Metarhizium anisopliae metabolites against three mosquito species and non-targeting organisms. PLoS ONE 2020, 15, e0232172. [Google Scholar] [CrossRef]
- Xia, Z.; Lei, L.; Zhang, H.Y.; Wei, H.L. Characterization of the ModABC Molybdate Transport System of Pseudomonas putida in Nicotine Degradation. Front. Microbiol. 2018, 9, 3030. [Google Scholar]
- Bhatt, P.; Huang, Y.; Zhan, H.; Chen, S. Insight Into Microbial Applications for the Biodegradation of Pyrethroid Insecticides. Front. Microbiol. 2019, 10, 1778. [Google Scholar] [CrossRef]
- Bhatt, P.; Rene, E.R.; Huang, Y.; Lin, Z.; Pang, S.; Zhang, W.; Chen, S. Systems biology analysis of pyrethroid biodegradation in bacteria and its effect on the cellular environment of pests and humans. J. Environ. Chem. Eng. 2021, 9, 106582. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, P.S.; Vo, D.-V.N.; Rajamohan, N.; Saravanan, R. Microbial degradation of recalcitrant pesticides: A review. Environ. Chem. Lett. 2021, 19, 3209–3228. [Google Scholar] [CrossRef]
- Singh, A.K.; Bilal, M.; Iqbal, H.M.N.; Raj, A. Trends in predictive biodegradation for sustainable mitigation of environmental pollutants: Recent progress and future outlook. Sci. Total Environ. 2021, 770, 144561. [Google Scholar] [CrossRef]
- Kumar, S.; Thomas, A.; Sahgal, A.; Verma, A.; Samuel, T.; Pillai, M.K. Effect of the synergist, piperonyl butoxide, on the development of deltamethrin resistance in yellow fever mosquito, Aedes aegypti L. (Diptera: Culicidae). Arch. Insect Biochem. Physiol. 2002, 50, 1–8. [Google Scholar] [CrossRef]
- Kumar, S.; Thomas, A.; Sahgal, A.; Verma, A.; Samuel, T.; Pillai, M.K. Variations in the insecticide-resistance spectrum of Anopheles stephensi after selection with deltamethrin or a deltamethrin-piperonyl-butoxide combination. Ann. Trop. Med. Parasitol. 2004, 98, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.L.; Cui, J.Y. Microbiome is a functional modifier of P450 drug metabolism. Curr. Pharmacol. Rep. 2019, 5, 481–490. [Google Scholar] [CrossRef]


| Mosquito Species | Insecticide | Mosquito Stage | Description of Findings | Reference |
|---|---|---|---|---|
| Ae. aegypti | Permethrin selection | Adult | Cutibacterium, Corynebacterium, Citricoccus, Leucobacter, Acinetobacter, Dietzia, and Anaerococcus spp. were more abundant in the selected strain. | [75] |
| Ae. aegypti | Lambda-cyhalothrin | Adult | Genera of Coprococcus, Ruminococcus, Bilophila, Enterobacter, Porphyromonas, Bifidobacterium, Weissella, and Delftia were enriched in the resistant group. Bacteria Bacteroides faecichinchillae decreased significantly in resistant midguts. | [47] |
| Ae. aegypti | Lambda-cyhalothrin | Adult | The presence of Pseudomonas viridiflava is associated with pyrethroid degradation. Parabacteroides, Megasphaera, Akkermansia, Lardizabala, Ruminococcus, and Coprococcus genera were enriched in susceptible mosquitoes. | [163] |
| Ae. aegypti | Permethrin, deltamethrin exposure | Adult | After exposure to permethrin, the most abundant bacterial species were Pantoea agglomerans and Pseudomonas azotoformans-fluorescens-synxantha. Elizabethkingia meningoseptica and Ps. azotoformans-fluorescens-synxantha were the most abundant after exposure to deltamethrin. | [73] |
| Ae. albopictus | Deltamethrin | Adult | Abundance of Serratia oryzae was significantly higher in the resistant strain. | [165] |
| Ae. albopictus | Deltamethrin | Adult | Acinetobacter junii and Se. oryzae significantly increased after deltamethrin treatment. | [164] |
| Ae. stimulans | Methoprene | Larval | Increased abundances of Clostridium spp. and Lysinibacillus spp. | [169] |
| An. albimanus | Fenitrothion | Adult | Resistance selection enriches bacterial taxa, reduces diversity, and significantly increases Bacillus and Klebsiella pneumoniae. | [69] |
| An. albimanus | Alphacypermethrin or permethrin | Adult | The abundance of Pseudomonas fragi and Pa. agglomerans increased with pyrethroid exposure. | [166] |
| An. arabiensis | Deltamethrin and malathion | Adult | Susceptible mosquitoes showed greater gut bacterial diversity than resistant mosquitoes. | [93] |
| An. coluzzii | Deltamethrin | Adult | Ochrobactrum, Lysinibacillus, and Stenotrophomonas genera were significantly enriched in resistant mosquitoes; Asaia and Serratia dominated the susceptible individuals. | [150] |
| An. gambiae s.s. | Permethrin | Adult | Sphingobacterium, Lysinibacillus, Streptococcus, and Rubrobacter were associated with resistant mosquitoes; Myxococcus was associated with susceptible mosquitoes. | [149] |
| An. stephensi | Temephos selection RR > 10 | Larval | Resistant strain with 4 dominant genera, i.e., Pseudomonas, Aeromonas, Exiguobacterium, and Microbacterium | [28] |
| Cx. pipiens | Organophosphate | Adult | Resistant mosquitoes with higher loads of Wolbachia. | [167] |
| Cx. pipiens pallens | Bti exposure | Larval | The predominant bacteria changed from Actinobacteria to Firmicutes, and the abundance of Actinobacteria was gradually reduced with an increase in the concentration of Bti. At the genus level, Bacillus replaced Microbacterium as the predominant genus. | [168] |
| Cx. quinquefasciatus | Deltamethrin | Adult | At the genus level, Aeromonas, Morganella, Elizabethkingia, Enterobacter, Cedecea, and Thorsellia showed significant differences between strains. At the species level, Bacillus cereus, Enterobacter cloacae s.l., Streptomyces sp., Pseudomonas sp., and Wolbachia were more abundant in the resistant strains. | [74] |
| Mosquito Species | Insecticide | Mosquito Stage | Description of Findings | Reference |
|---|---|---|---|---|
| Ae. aegypti | Bifenthrin, Bti, temephos, methoprene | Adult, larval | Infection with Wolbachia has no effect on susceptibility to insecticides. | [170] |
| Ae. aegypti | Propoxur, naled | Larval | Broad-spectrum antibiotic treatment of larvae decreases the metabolic detoxification of propoxur and naled. Adding cultured gut bacteria isolated from mosquito larvae reduces larval mortality. | [177] |
| Ae. albopictus | Deltamethrin | Adult | Cultured bacteria Serratia oryzae and Acinetobacter junii promote resistance. | [164] |
| Ae. albopictus | Deltamethrin | Adult | The survival of Se. oryzae-enriched mosquitoes significantly increased. Three metabolic detoxification enzymes in Se. oryzae-enriched mosquitoes increased. Carboxylesterase activity was detected in Se. oryzae. Se. oryzae can degrade deltamethrin in vitro; degradation efficiency was positively correlated with time and bacterial amount. | [165] |
| An. arabiensis | Deltamethrin, malathion | Adult | Resistance mosquitoes have lower gut bacterial diversity. Supplementation of bacterial St. pyrogenes or Escherichia coli increased insecticide tolerance. Antibiotic supplementation via sugar decreased tolerance to the insecticides deltamethrin and malathion. Both R/S females had decreased α-esterase activity after gentamicin, streptomycin, vancomycin, heat-killed St. pyrogenes, or live E. coli treatment. GST, P450, and β-esterase changes are inconsistent. | [93] |
| An. gambiae s.s. | Pyrethroid | Adult | Resistance leads to increased mortality by the fungi Beauveria bassiana and Metarhizium anisopliae infection. | [175] |
| An. gambiae s.s., An. funestus, An. arabiensis | Pyrethroids, organochlorines, carbamates | Adult | Resistant mosquitoes preinfected with Be. bassiana or Me. anisopliae showed a significant increase in mortality after insecticide exposure. | [174] |
| An. stephensi | Bt | Larval | Commensal microbes in the midgut are capable of degrading insecticidal Bt proteins, decreasing larval susceptibility to Bt. Antibiotic treatment increased mortality and reached 100% mortality at a concentration of 110 μg/mL of an antibiotic mixture of penicillin, streptomycin, and erythromycin. | [176] |
| An. stephensi | Temephos selection RR > 10 | Larval | Adding symbiotic bacteria collected from the breeding place can boost the activity of α-esterase and GST enzymes. | [28] |
| Cx. pipiens | Chlorpyrifos, propoxur | Larval | Infection with Wolbachia has no effect on resistance to chlorpyrifos and propoxur. | [171] |
| Cx. quinquefasciatus | DDT | Adult | Infection with Wolbachia increases susceptibility to DDT. | [172] |
| Cx. quinquefasciatus | Chlorpyrifos selection | Larval | Activities of acetylcholinesterase (AChE), glutathione S-transferase (GST), esterase (EST), acid phosphatases (ACP), and alkaline phosphatases (ALP) increased in the chlorpyrifos-selected (Chlor-SEL) population. Activities of all enzymes were suppressed when exposed to Me. anisopliae or Be. Bassiana. | [173] |
| Mosquito Species | Insecticide | Insecticide Degradation Symbionts | Reference |
|---|---|---|---|
| Ae. aegypti | Lambda-cyhalothrin | Pseudomonas viridiflava | [163] |
| An. albimanus | Fenitrothion | † Bacillus cereus and Acinetobacter baumannii | [69] |
| An. albimanus | Permethrin, Alphacypermethrin | Pantoea agglomerans | [166] |
| An. coluzzii | Deltamethrin | Ochrobactrum, Lysinibacillus, and Stenotrophomonas genera | [150] |
| An. gambiae | Permethrin | Sphingobacterium, Lysinibacillus, and Streptococcus genera | [149] |
| An. stephensi | Temephos | Pseudomonas sp., Aeromonas sp., Exiguobacterium sp., and Microbacterium sp. | [28] |
| Multiple species | Pyrethroids | Bacteria and fungi: Bacillus spp., Raoultella ornithinolytica, Pseudomonas fluorescens, Brevibacterium sp., Acinetobacter sp., Aspergillus sp., Candida sp., Trichoderma sp., and Candia spp. | [181] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Yin, J.; Huang, X.; Zang, C.; Zhang, Y.; Cao, J.; Gong, M. Mosquito Gut Microbiota: A Review. Pathogens 2024, 13, 691. https://doi.org/10.3390/pathogens13080691
Liu H, Yin J, Huang X, Zang C, Zhang Y, Cao J, Gong M. Mosquito Gut Microbiota: A Review. Pathogens. 2024; 13(8):691. https://doi.org/10.3390/pathogens13080691
Chicago/Turabian StyleLiu, Hongmei, Jianhai Yin, Xiaodan Huang, Chuanhui Zang, Ye Zhang, Jianping Cao, and Maoqing Gong. 2024. "Mosquito Gut Microbiota: A Review" Pathogens 13, no. 8: 691. https://doi.org/10.3390/pathogens13080691
APA StyleLiu, H., Yin, J., Huang, X., Zang, C., Zhang, Y., Cao, J., & Gong, M. (2024). Mosquito Gut Microbiota: A Review. Pathogens, 13(8), 691. https://doi.org/10.3390/pathogens13080691









