Glomerular Injury Is Associated with Severe Courses of Orthohantavirus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Statistical Analysis
3. Results
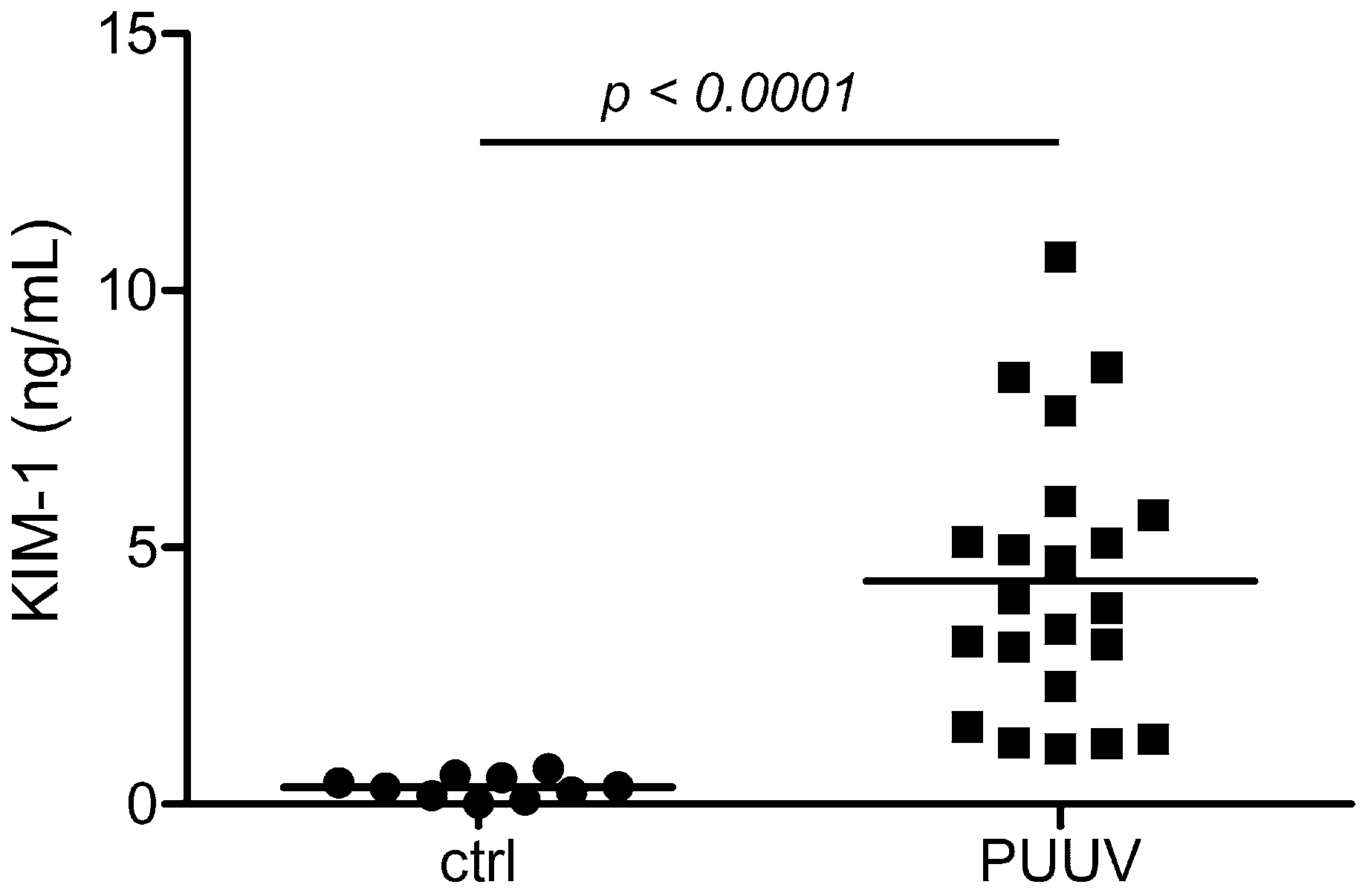
3.1. Levels of Glomerular and Tubular Injury Markers in Patients with HFRS
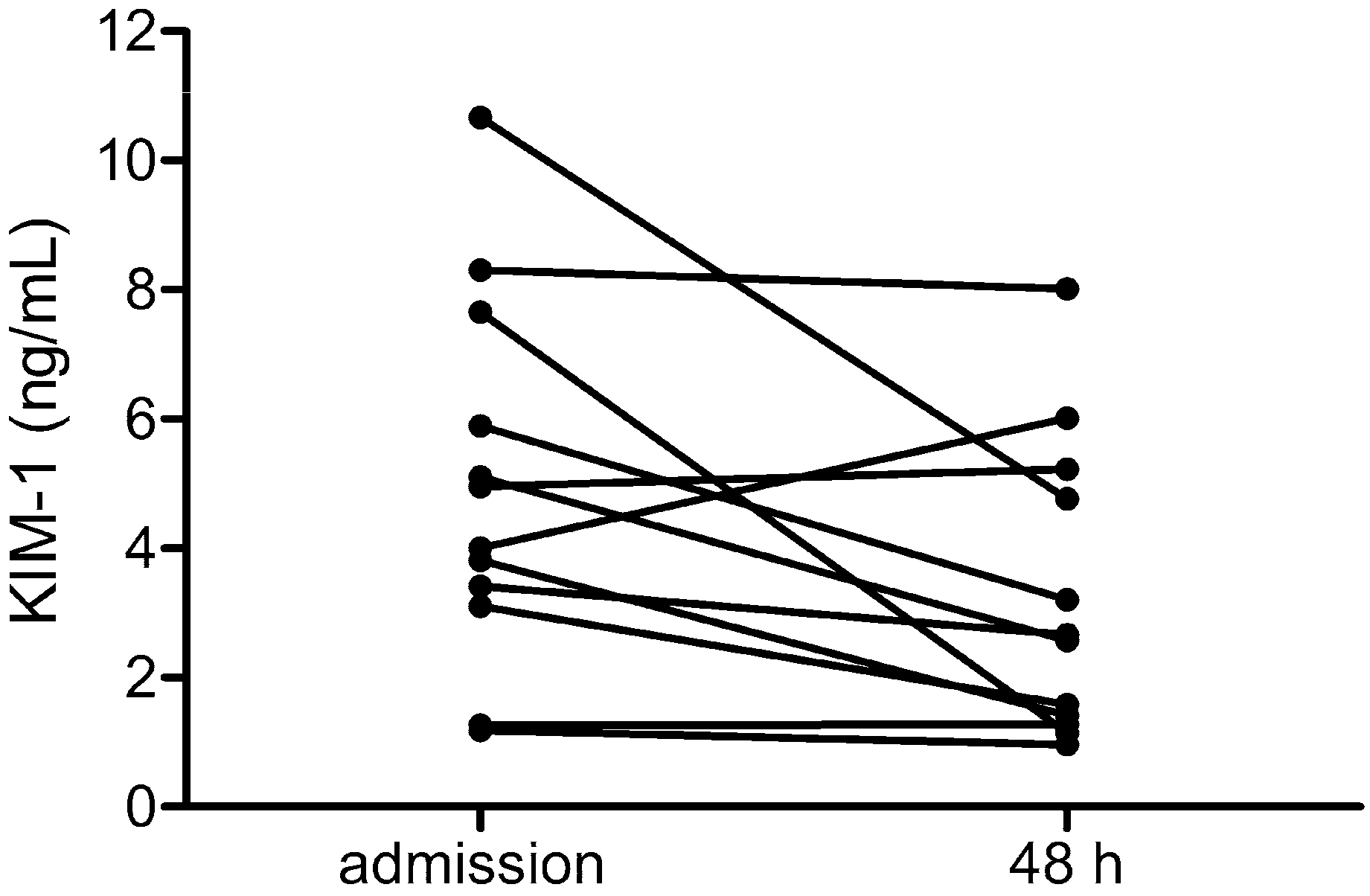
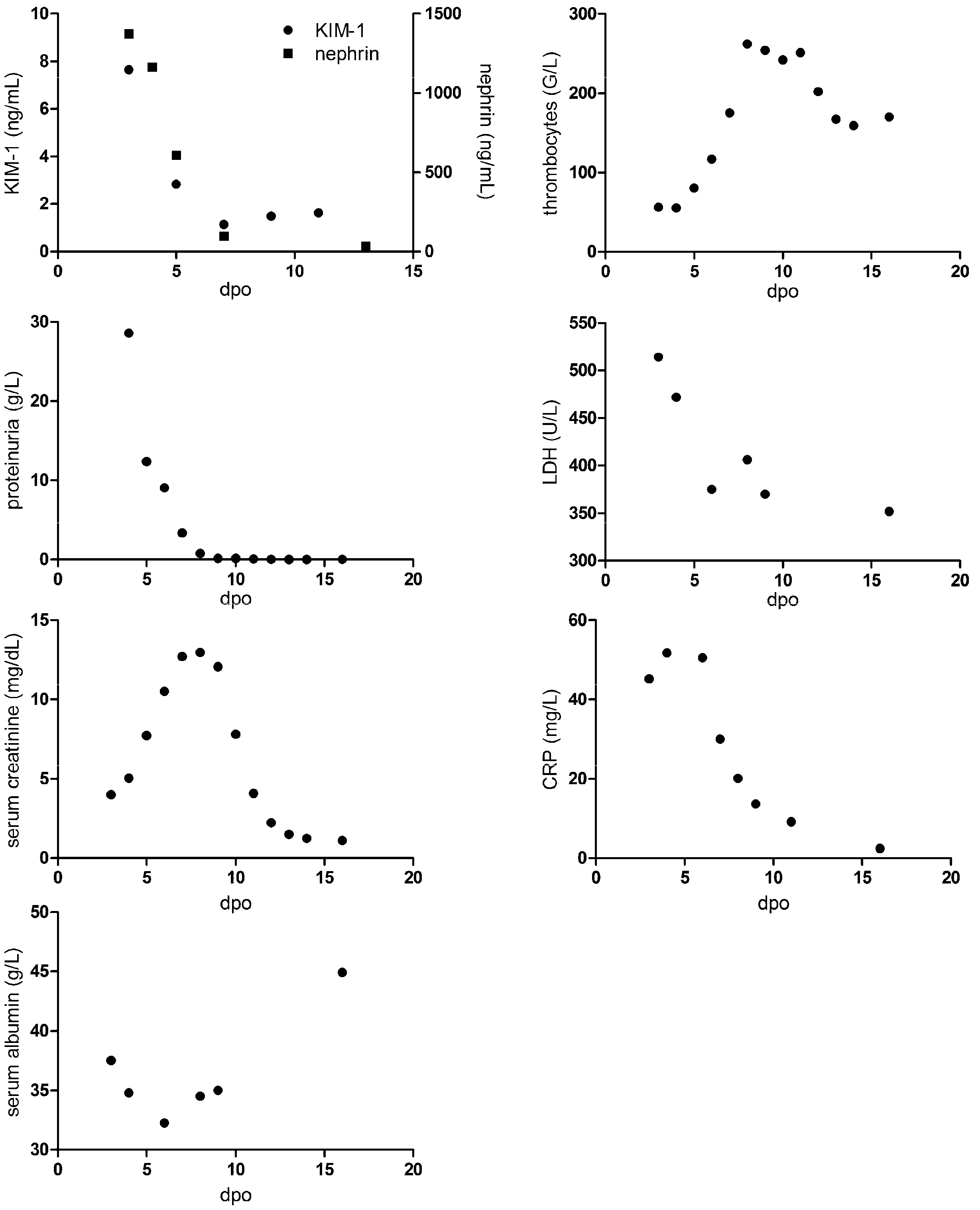
3.2. Course of Levels of KIM-1 in HFRS
3.3. Correlation of Tubular and Glomerular Injury with Disease Severity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaheri, A.; Strandin, T.; Hepojoki, J.; Sironen, T.; Henttonen, H.; Makela, S.; Mustonen, J. Uncovering the mysteries of hantavirus infections. Nat. Rev. Microbiol. 2013, 11, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Ferluga, D.; Vizjak, A. Hantavirus nephropathy. J. Am. Soc. Nephrol. 2008, 19, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Boehlke, C.; Hartleben, B.; Huber, T.B.; Hopfer, H.; Walz, G.; Neumann-Haefelin, E. Hantavirus infection with severe proteinuria and podocyte foot-process effacement. Am. J. Kidney Dis. 2014, 64, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Nusshag, C.; Stütz, A.; Hägele, S.; Speer, C.; Kälble, F.; Eckert, C.; Brenner, T.; Weigand, M.A.; Morath, C.; Reiser, J.; et al. Glomerular filtration barrier dysfunction in a self-limiting, RNA virus-induced glomerulopathy resembles findings in idiopathic nephrotic syndromes. Sci. Rep. 2020, 10, 19117. [Google Scholar] [CrossRef] [PubMed]
- Krautkrämer, E.; Grouls, S.; Stein, N.; Reiser, J.; Zeier, M. Pathogenic old world hantaviruses infect renal glomerular and tubular cells and induce disassembling of cell-to-cell contacts. J. Virol. 2011, 85, 9811–9823. [Google Scholar] [CrossRef]
- Temonen, M.; Vapalahti, O.; Holthofer, H.; Brummer-Korvenkontio, M.; Vaheri, A.; Lankinen, H. Susceptibility of human cells to Puumala virus infection. J. Gen. Virol. 1993, 74 Pt 3, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Bourquain, D.; Bodenstein, C.; Schurer, S.; Schaade, L. Puumala and Tula Virus Differ in Replication Kinetics and Innate Immune Stimulation in Human Endothelial Cells and Macrophages. Viruses 2019, 11, 855. [Google Scholar] [CrossRef]
- Nusshag, C.; Boegelein, L.; Schreiber, P.; Essbauer, S.; Osberghaus, A.; Zeier, M.; Krautkrämer, E. Expression Profile of Human Renal Mesangial Cells Is Altered by Infection with Pathogenic Puumala Orthohantavirus. Viruses 2022, 14, 823. [Google Scholar] [CrossRef] [PubMed]
- Hägele, S.; Müller, A.; Nusshag, C.; Reiser, J.; Zeier, M.; Krautkrämer, E. Motility of human renal cells is disturbed by infection with pathogenic hantaviruses. BMC Infect. Dis. 2018, 18, 645. [Google Scholar] [CrossRef]
- Ala-Houhala, I.; Koskinen, M.; Ahola, T.; Harmoinen, A.; Kouri, T.; Laurila, K.; Mustonen, J.; Pasternack, A. Increased glomerular permeability in patients with nephropathia epidemica caused by Puumala hantavirus. Nephrol. Dial. Transplant. 2002, 17, 246–252. [Google Scholar] [CrossRef]
- Settergren, B.; Trollfors, B.; Fasth, A.; Hultberg, B.; Norrby, S.R. Glomerular filtration rate and tubular involvement during acute disease and convalescence in patients with nephropathia epidemica. J. Infect. Dis. 1990, 161, 716–720. [Google Scholar] [CrossRef]
- Miettinen, M.H.; Makela, S.M.; Ala-Houhala, I.O.; Huhtala, H.S.; Koobi, T.; Vaheri, A.I.; Pasternack, A.I.; Porsti, I.H.; Mustonen, J.T. Tubular proteinuria and glomerular filtration 6 years after puumala hantavirus-induced acute interstitial nephritis. Nephron Clin. Pract. 2009, 112, c115–c120. [Google Scholar] [CrossRef]
- Mustonen, J.; Makela, S.; Helin, H.; Helantera, A.; Miettinen, M.; Partanen, J.; Pasternack, A. Mesangiocapillary glomerulonephritis caused by Puumala hantavirus infection. Nephron 2001, 89, 402–407. [Google Scholar] [CrossRef]
- Shakirova, V.; Khaertynova, I.; Markelova, M.; Tarlinton, R.; Behnke, J.; Martynova, E.; Garanina, E.; Rizvanov, A.; Khaiboullina, S. Serum Cytokine Alterations Associated with Age of Patients with Nephropathia Epidemica. Biomed. Res. Int. 2022, 2022, 4685288. [Google Scholar] [CrossRef]
- Outinen, T.K.; Mäkelä, S. Severity Biomarkers in Puumala Hantavirus Infection. Viruses 2021, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Noack, D.; Travar, M.; Mrdjen, V.; Voermans, J.J.C.; van de Vijver, D.; Molenkamp, R.; Koopmans, M.P.G.; Goeijenbier, M.; Rockx, B. Serum Markers Associated with Disease Severity in a Bosnian Hemorrhagic Fever with Renal Syndrome Cohort. Viruses 2022, 14, 1377. [Google Scholar] [CrossRef]
- Klingstrom, J.; Lindgren, T.; Ahlm, C. Sex-dependent differences in plasma cytokine responses to hantavirus infection. Clin. Vaccine Immunol. 2008, 15, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Martynova, E.; Davidyuk, Y.; Kabwe, E.; Garanina, E.E.; Shakirova, V.; Pavelkina, V.; Uskova, Y.; Stott, R.J.; Foster, T.L.; Markelova, M.; et al. Cytokine, Chemokine, and Metalloprotease Activation in the Serum of Patients with Nephropathia Epidemica from the Republic of Tatarstan and the Republic of Mordovia, Russia. Pathogens 2021, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Martynova, E.; Stott-Marshall, R.J.; Shakirova, V.; Saubanova, A.; Bulatova, A.; Davidyuk, Y.N.; Kabwe, E.; Markelova, M.; Khaertynova, I.; Foster, T.L.; et al. Differential Cytokine Responses and the Clinical Severity of Adult and Pediatric Nephropathia Epidemica. Int. J. Mol. Sci. 2023, 24, 7016. [Google Scholar] [CrossRef]
- Krautkrämer, E.; Grouls, S.; Hettwer, D.; Rafat, N.; Tonshoff, B.; Zeier, M. Mobilization of circulating endothelial progenitor cells correlates with the clinical course of hantavirus disease. J. Virol. 2014, 88, 483–489. [Google Scholar] [CrossRef]
- Outinen, T.K.; Mäkelä, S.; Huttunen, R.; Mäenpää, N.; Libraty, D.; Vaheri, A.; Mustonen, J.; Aittoniemi, J. Urine soluble urokinase-type plasminogen activator receptor levels correlate with proteinuria in Puumala hantavirus infection. J. Intern. Med. 2014, 276, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Bunz, H.; Weyrich, P.; Peter, A.; Baumann, D.; Tschritter, O.; Guthoff, M.; Beck, R.; Jahn, G.; Artunc, F.; Häring, H.U.; et al. Urinary Neutrophil Gelatinase-Associated Lipocalin (NGAL) and proteinuria predict severity of acute kidney injury in Puumala virus infection. BMC Infect. Dis. 2015, 15, 464. [Google Scholar] [CrossRef] [PubMed]
- Libraty, D.H.; Mäkelä, S.; Vlk, J.; Hurme, M.; Vaheri, A.; Ennis, F.A.; Mustonen, J. The degree of leukocytosis and urine GATA-3 mRNA levels are risk factors for severe acute kidney injury in Puumala virus nephropathia epidemica. PLoS ONE 2012, 7, e35402. [Google Scholar] [CrossRef] [PubMed]
- Hansson, M.; Gustafsson, R.; Jacquet, C.; Chebaane, N.; Satchell, S.; Thunberg, T.; Ahlm, C.; Fors Connolly, A.M. Cystatin C and alpha1-Microglobulin Predict Severe Acute Kidney Injury in Patients with Hemorrhagic Fever with Renal Syndrome. Pathogens 2023, 9, 666. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, L.E.; Schmotz, C.; Saleem, M.A.; Lehtonen, S.; Vapalahti, O.; Vaheri, A.; Mäkelä, S.; Mustonen, J.; Strandin, T. Increased Heparanase Levels in Urine during Acute Puumala Orthohantavirus Infection Are Associated with Disease Severity. Viruses 2022, 14, 450. [Google Scholar] [CrossRef] [PubMed]
- Nusshag, C.; Gruber, G.; Zeier, M.; Krautkrämer, E. Neutrophil-to-lymphocyte ratio is elevated in acute hantavirus infection and correlates with markers of disease severity. J. Med. Virol. 2024, 96, e29759. [Google Scholar] [CrossRef] [PubMed]
- Canki, E.; Kho, E.; Hoenderop, J.G.J. Urinary biomarkers in kidney disease. Clin. Chim. Acta 2024, 555, 117798. [Google Scholar] [CrossRef] [PubMed]
- Romejko, K.; Markowska, M.; Niemczyk, S. The Review of Current Knowledge on Neutrophil Gelatinase-Associated Lipocalin (NGAL). Int. J. Mol. Sci. 2023, 24, 10470. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Han, Q.; Wang, X.; Zhang, X.; Li, N.; Liu, Z. Aspartate aminotransferase to platelet ratio at admission can predict the prognosis of patients with hemorrhagic fever with renal syndrome. J. Med. Virol. 2023, 95, e29126. [Google Scholar] [CrossRef]
- Cabrera, L.E.; Tietäväinen, J.; Jokiranta, S.T.; Mäkelä, S.; Vaheri, A.; Mustonen, J.; Vapalahti, O.; Kanerva, M.; Strandin, T. Maturing neutrophils of lower density associate with thrombocytopenia in Puumala orthohantavirus-caused hemorrhagic fever with renal syndrome. Front. Immunol. 2024, 15, 1419787. [Google Scholar] [CrossRef]
- Strandin, T.; Mäkelä, S.; Mustonen, J.; Vaheri, A. Neutrophil Activation in Acute Hemorrhagic Fever with Renal Syndrome Is Mediated by Hantavirus-Infected Microvascular Endothelial Cells. Front. Immunol. 2018, 9, 2098. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, P.T.; Bourquain, D.; Bankov, K.; Auste, B.; Dabrowski, P.W.; Nitsche, A.; Krüger, D.H.; Schaade, L. Infection of human airway epithelial cells by different subtypes of Dobrava-Belgrade virus reveals gene expression patterns corresponding to their virulence potential. Virology 2016, 493, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Geimonen, E.; Neff, S.; Raymond, T.; Kocer, S.S.; Gavrilovskaya, I.N.; Mackow, E.R. Pathogenic and nonpathogenic hantaviruses differentially regulate endothelial cell responses. Proc. Natl. Acad. Sci. USA 2002, 99, 13837–13842. [Google Scholar] [CrossRef] [PubMed]
- Reynes, J.M.; Schaeffer, L.; Papadopoulos, P.; Ait-Ahmed, M.; Siby-Diakite, D.; Ripaux-Lefevre, M.; Buivan, T.P.; Lechat, S.; Vray, M.; Galempoix, J.M. Molecular Detection of Orthohantavirus puumalaense in Plasma and Urine Samples from Hospitalized Patients Presenting with a Serologically Confirmed Acute Hantavirus Infection in France. J. Clin. Microbiol. 2023, 61, e0037223. [Google Scholar] [CrossRef]
| All Patients (n = 22) Median (Range) | Moderate (n = 14) Median (Range) | Severe (n = 8) Median (Range) | p Value | |
|---|---|---|---|---|
| Gender (male/female) | 18/4 | 11/3 | 7/1 | 1.000 |
| Age (years) | 34 (22–61) | 36.5 (22–61) | 33.5 (24–44) | 0.3524 |
| LOS (days) | 5 (3–15) | 4.5 (3–5) | 8 (6–15) | 0.0001 *** |
| SCremax (mg/dL) | 5.41 (1.93–18.02) | 4.820 (1.930–9.630) | 9.935 (2.270–18.02) | 0.0027 ** |
| SAlbmin (g/L) | 35.10 (22.7–42.30) | 36.10 (33.40–42.30) | 33.3 (22.7–36.8) | 0.0098 ** |
| Plateletsmin (109/L) | 110.5 (21–291) | 135 (55–291) | 75.5 (21–154) | 0.0220 * |
| Leukocytesmax (109/L) | 10.33 (4.92–14.54) | 10.61 (5.36–14.54) | 9.28 (4.92–11.67) | 0.2295 |
| Proteinuriamax (g/L) | 3.519 (0.109–16.93) | 1.286 (0.109–9.831) | 4.217 (0.2490–16.93) | 0.0474 * |
| LDHmax (U/L) | 385 (262–514) | 372 (262–500) | 414 (269–514) | 0.7662 |
| CRPmax (mg/L) | 59.95 (17.2–150.1) | 54.65 (17.2–141.3) | 68.25 (29.8–150.1) | 0.4306 |
| Adm dpo (days) | 6 (3–9) | 6 (4–9) | 6 (3–7) | 0.3740 |
| Nephrinadm (ng/mL) | 141.6 (10.3–2571) | 95.49 (10.3–475.6) | 411.9 (172.7–2571) | 0.0020 ** |
| IgGadm (mg/L) | 455.0 (4.1–3620) | 194 (4.1–1540) | 643 (268–3620) | 0.0140 * |
| KIM-1adm (ng/mL) | 3.91 (1.084–10.66) | 3.135 (1.084–10.66) | 5.033 (1.193–8.503) | 0.1087 |
| α1-MGadm (mg/L) | 24.85 (6.2–127) | 20.3 (7.2–42) | 31.3 (6.2–127) | 0.2673 |
| Parameter | KIM-1adm (ng/mL) | Nephrinadm (ng/mL) | ||||
|---|---|---|---|---|---|---|
| R | CI | p Value | R | CI | p Value | |
| Age (years) | −0.3144 | −0.6576–0.1367 | 0.1541 | −0.0393 | −0.4845–0.4221 | 0.8695 |
| LOS (days) | 0.2014 | −0.2533–0.5831 | 0.3688 | 0.8715 | 0.6982–0.9483 | <0.0001 **** |
| SCremax (mg/dL) | 0.1846 | −0.2694–0.5716 | 0.4107 | 0.6947 | 0.3518–0.8732 | 0.0007 *** |
| Proteinuriamax (g/L) | 0.1790 | −0.2749–0.5676 | 0.4254 | 0.7616 | 0.4812–0.9006 | <0.0001 **** |
| LDHmax (U/L) | 0.0311 | −0.4070–0.4575 | 0.8909 | 0.0887 | −0.3804–0.5216 | 0.7099 |
| CRPmax (mg/L) | 0.1248 | −0.3253–0.5288 | 0.5800 | 0.4466 | −0.0091− 0.7487 | 0.0484 * |
| Plateletsmin (109/L) | 0.1243 | −0.3258–0.5285 | 0.5816 | −0.5515 | −0.8041–−0.1303 | 0.0117 * |
| Leukocytesmax (109/L) | −0.1722 | −0.5629–0.2813 | 0.4434 | −0.0165 | −0.4669–0.4406 | 0.9448 |
| SAlbmin (g/L) | 0.1767 | −0.2770–0.5660 | 0.4314 | −0.4556 | −0.7536–−0.0023 | 0.0435 * |
| Nephrinadm (ng/mL) | 0.2496 | −0.2303–0.6319 | 0.2885 | − | − | - |
| Parameter | α1-MGadm (mg/L) | IgGadm (mg/L) | ||||
|---|---|---|---|---|---|---|
| R | CI | p Value | R | CI | p Value | |
| Age (years) | −0.3752 | −0.7084–0.0947 | 0.1030 | −0.2319 | −0.6204–0.2481 | 0.3253 |
| LOS (days) | 0.3147 | −0.1623–0.6725 | 0.1765 | 0.7572 | 0.4621–0.9013 | 0.0001 *** |
| SCremax (mg/dL) | 0.3714 | −0.0991–0.7062 | 0.1069 | 0.6311 | 0.2483–0.8434 | 0.0028 ** |
| Proteinuriamax (g/L) | 0.4226 | −0.0387–0.7354 | 0.0634 | 0.9026 | 0.7601–0.9623 | <0.0001 **** |
| LDHmax (U/L) | 0.2722 | −0.2073–0.6462 | 0.2457 | 0.2978 | −0.1668–0.6542 | 0.2022 |
| CRPmax (mg/L) | 0.5684 | 0.1544–0.8126 | 0.0089 ** | 0.1136 | −0.3588–0.5396 | 0.6335 |
| Plateletsmin (109/L) | −0.5546 | −0.8056–−0.1346 | 0.0112 * | −0.2853 | −0.6544–0.1936 | 0.2228 |
| Leukocytesmax (109/L) | 0.5865 | 0.1807–0.8216 | 0.0066 ** | −0.0888 | −0.5216–0.3804 | 0.7098 |
| SAlbmin (g/L) | −0.0211 | −0.4704–0.4370 | 0.9298 | −0.2181 | −0.6114–0.2616 | 0.3556 |
| Nephrinadm (ng/mL) | 0.5519 | 0.1308–0.8043 | 0.0116 * | 0.7672 | 0.4808–0.9057 | <0.0001 **** |
| KIM-1adm (ng/mL) | 0.3805 | −0.0887–0.7115 | 0.0980 | 0.2836 | −0.1954–0.6533 | 0.2257 |
| IgGadm (mg/L) | 0.4332 | −0.0256–0.7413 | 0.0564 | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nusshag, C.; Uhrig, J.; Gruber, G.; Schreiber, P.; Zeier, M.; Krautkrämer, E. Glomerular Injury Is Associated with Severe Courses of Orthohantavirus Infection. Pathogens 2024, 13, 693. https://doi.org/10.3390/pathogens13080693
Nusshag C, Uhrig J, Gruber G, Schreiber P, Zeier M, Krautkrämer E. Glomerular Injury Is Associated with Severe Courses of Orthohantavirus Infection. Pathogens. 2024; 13(8):693. https://doi.org/10.3390/pathogens13080693
Chicago/Turabian StyleNusshag, Christian, Josephine Uhrig, Gefion Gruber, Pamela Schreiber, Martin Zeier, and Ellen Krautkrämer. 2024. "Glomerular Injury Is Associated with Severe Courses of Orthohantavirus Infection" Pathogens 13, no. 8: 693. https://doi.org/10.3390/pathogens13080693





