Geohelminths: Use in the Treatment of Selected Human Diseases
Abstract
1. Introduction
2. Inflammatory Bowel Disease
2.1. Crohn’s Disease
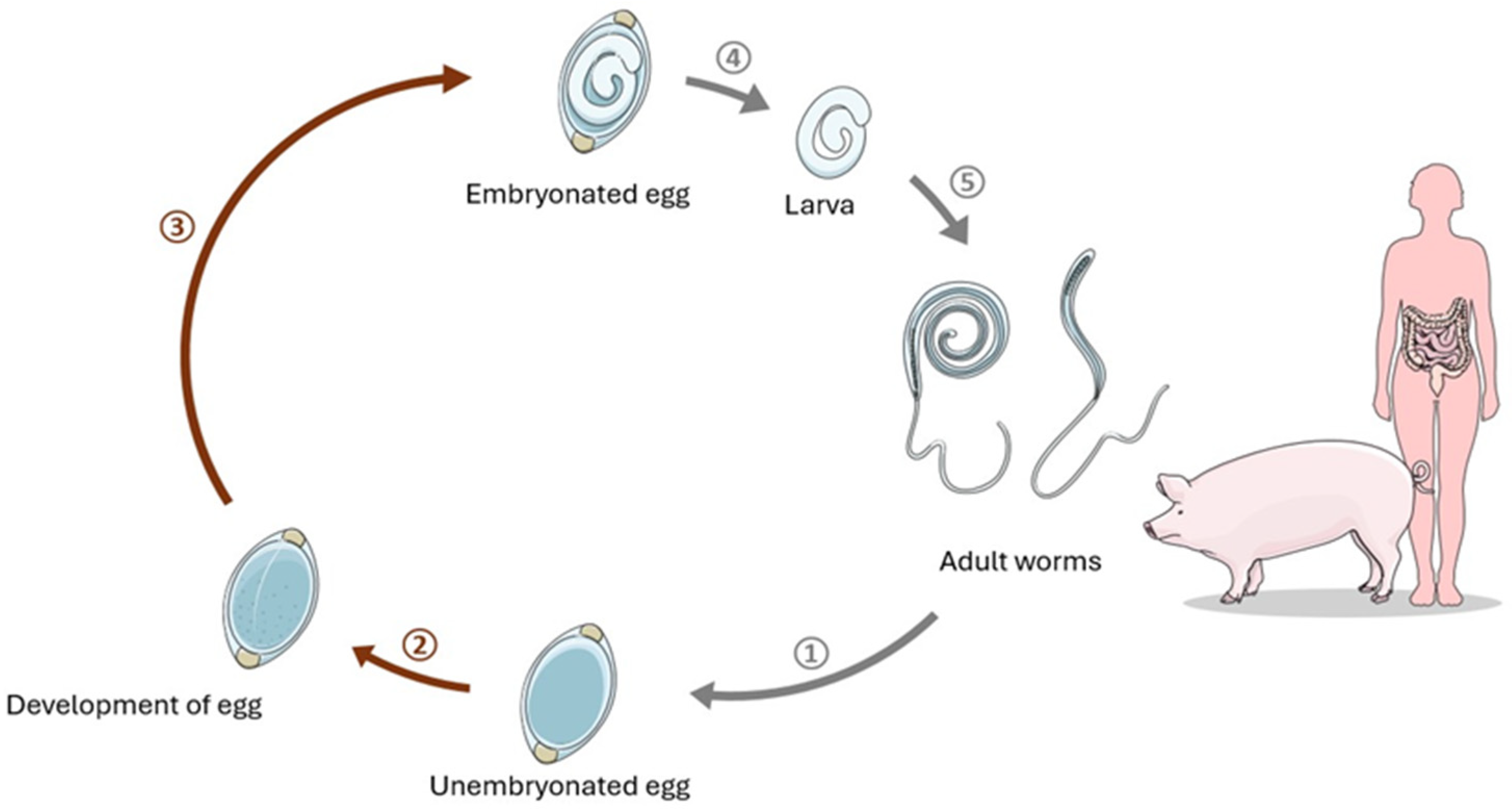
2.1.1. Trichuris suis in Crohn’s Disease Therapy
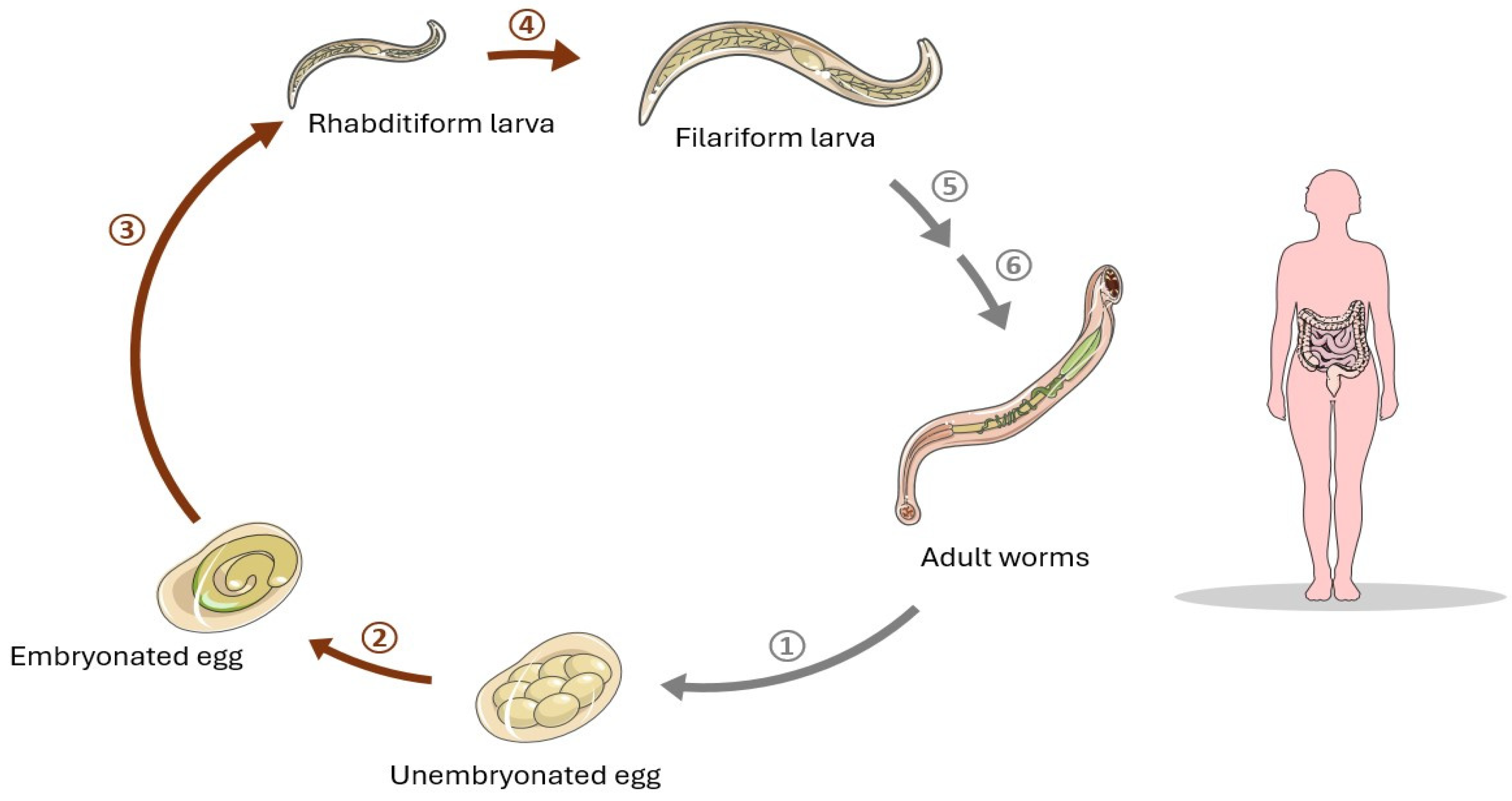
2.1.2. Necator americanus in Crohn’s Disease Therapy
2.2. Ulcerative Colitis
T. suis in Ulcerative Colitis Therapy
3. Celiac Disease
N. americanus in Celiac Disease Therapy
4. Diabetes Mellitus
5. Allergic Diseases
6. Asthma
7. Multiple Sclerosis
| Disease | Dose of Parasite | Number of Patients | Method | Result | Safety Concerns | Reference | Ref No |
|---|---|---|---|---|---|---|---|
| Crohn’s Disease | 2500 live TSO every three weeks for 24 weeks | 29 | Open-label study | 79.3% responded and 72.4% achieved remission at week 24. Mean CDAI decrease of 177.1 points. | No adverse events reported. Therapy was well-tolerated even among those receiving multiple immunosuppressants. | Summers et al., 2005 | [22] |
| Crohn’s Disease | 250, 2500, or 7500 TSO every two weeks for 12 weeks | 252 | Randomized, double-blind, placebo-controlled trial | No significant advantage over placebo in inducing remission or clinical response. | Safe but no serious adverse reactions specifically attributed to TSO. | Schölmerich et al., 2016 | [23] |
| Ulcerative Colitis | 2500 TSO every 2 weeks | 54 | Randomized controlled trial | Clinical improvement in most patients; few achieved remission. | No serious side effects reported, but full safety profile requires further research. | Summers et al., 2005 | [33] |
| Ulcerative Colitis | 7500 TSO every 2 weeks for 24 weeks | 60 | Randomized, double-blind, placebo-controlled clinical trial | No better clinical remission was achieved at week 24 compared to placebo. | 4 patients required colectomy during TSO therapy. | Prosberg et al., 2024 | [34] |
| Allergic Rhinitis | 2500 TSO every 21 days, 8 doses | 100 | Randomized, placebo-controlled, double-blind trial | No significant improvement in allergy symptoms; increased eosinophils and specific antibodies. | Increase in gastrointestinal symptoms noted; potential allergenicity not fully evaluated. | Bager et al., 2011 | [42] |
| Allergic Rhinitis | 2500 TSO every 21 days over 6 months | 100 | Randomized, placebo-controlled, double-blind trial | TSO induced a Th2-polarized response and elevated IL-5, without affecting allergen-specific cytokine responses. | Elevated IL-5 and parasite-specific cytokines; no alteration in allergen-specific reactivity during peak allergy symptoms. | Bourke et al., 2012 | [43] |
| Multiple Sclerosis | 2500 TSO orally every 2 weeks for 12 weeks | 10 | Open-label, magnetic resonance imaging assessor-blinded, baseline-to-treatment study | No significant beneficial effect observed; increase in MRI lesions and relapses during treatment. | Well-tolerated but associated with gastrointestinal symptoms and eosinophilia. | Voldsgaard et al., 2015 | [49] |
| Multiple Sclerosis | 2500 TSO orally every 2 weeks for 3 months | 5 | Phase 1 study | Reduced number of new gadolinium-enhancing MRI lesions. Preliminary increase in anti-inflammatory cytokines IL-4 and IL-10. | Well-tolerated with no significant adverse effects observed during the study duration. | Jo et al., 2011 | [50] |
| Multiple Sclerosis | 2500 TSO orally every 2 weeks for 6 months | 4 | Immune monitoring study | Slight downregulation of Th1-associated cytokine IL-2, temporary increase in Th2 cytokine IL-4. Mild eosinophilia and changes in T-cell and NK cell numbers observed. | Well-tolerated with mild eosinophilia. No significant safety concerns reported. | Benzel et al., 2011 | [51] |
| Disease | Dose of Parasite | Number of Patients | Method | Result | Safety Concerns | Reference | Ref No |
|---|---|---|---|---|---|---|---|
| Asthma | 10 larvae | 32 | Randomized, placebo-controlled trial | Improvement in airway responsiveness was non-significant; well tolerated with mild symptoms. | Infection was well tolerated without significant exacerbation of asthma symptoms. | Feary et al., 2010 | [27] |
| Celiac Disease | 15 larvae (10 at week 0 and 5 at week 12) | 20 | Randomized double-blinded placebo-controlled trial | No improvement in primary outcomes; infection safe and tolerable. Experiment imposed no obvious benefit on pathology. | Infection well tolerated; some transient pain and enteritis. No serious adverse effects. | Daveson et al., 2011 | [28] |
| Wheeze in Ethiopia | Not specified | 604 (cases and controls) | Nested case-control study | Significant reduction in the risk of wheeze, suggesting a protective effect against asthma symptoms. | Well tolerated with no specific safety concerns. | Scrivener et al., 2001 | [44] |
| Asthma | Not specified | Multiple studies included | Systematic review and meta-analysis | Significant reduction in asthma risk. The highest tertile of infection intensity showed the strongest protective effect. | Not specified in meta-analysis, but general trends suggest that hookworm infections are well tolerated. | Leonardi-Bee et al., 2006 | [45] |
| Dose-ranging Study | 10, 25, 50, 100 larvae | 10 | Dose-ranging study | All administered doses achieved the target of at least 50 eggs/gram of feces. Infection elicited a modest host eosinophil response and was suitable for use in preliminary clinical therapeutic trials. | Skin itching and gastrointestinal symptoms common at higher doses, but overall well tolerated with no significant change in lung function. | Mortimer et al., 2006 | [47] |
| Celiac Disease | 10 L3s at 0 and 12 weeks | Not specified | Controlled clinical trial | Modulation of immune response to gluten, enhancing mucosal IL-1β and IL-22 while suppressing IFNγ and IL-17A levels. | Safe with no serious adverse effects noted, well tolerated with mild symptoms. Further studies recommended. | Croese et al., 2013 | [25] |
| Celiac Disease | 20 or 40 larvae | 54 | Randomized, placebo-controlled trial | No restore tolerance to sustained moderate consumption of gluten (2 g/d) but improved symptom scores after intermittent consumption of lower gluten doses. Participants reported fewer gluten-related adverse events. | Well tolerated with no serious adverse effects; mild gastrointestinal symptoms were the most commonly reported issues. | Croese et al. 2020 | [38] |
| Crohn’s Disease | Crude extracts of hookworm antigens | 153 (cases and controls) | A case-control study | Hookworm antigen decreased immune reaction in patients with Crohn’s disease by lowering shift in CD3+CD69+ cell population and interferon-g response than the controls. | Hookworm crude extracts were generally well tolerated; some transient pain and enteritis. No serious adverse effects. | Kabeerdoss et al. 2011 | [29] |
| Multiple Sclerosis | 25 larvae transcutaneously | 71 | Double-blind, randomized, placebo-controlled trial | Fewer new lesions and relapses observed; significant decrease in disease exacerbation and MRI changes. | Generally well tolerated with mild symptoms; further studies needed to fully assess the safety profile in larger and more diverse populations. | Tănăsescu et al., 2020 | [56] |
| Type 2 Diabetes (Insulin Resistance) | 20, 40 larvae | 40 | Randomized, placebo-controlled trial | Significant improvement in fasting glucose and insulin resistance at 1-year | Infection was well tolerated with some gastrointestinal symptoms | Pierce et al., 2023 | [39] |
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Strachan, D.P. Hay Fever, Hygiene, and Household Size. BMJ. Br. Med. J. 1989, 299, 1259–1260. [Google Scholar] [CrossRef]
- Bach, J. The Effect of Infections on Susceptibility to Autoimmune and Allergic Diseases. N. Engl. J. Med. 2002, 347, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Dagoye, D.; Bekele, Z.; Woldemichael, K.; Nida, H.; Yimam, M.; Hall, A.; Venn, A.; Britton, J.; Hubbard, R.; Lewis, S. Wheezing, Allergy, and Parasite Infection in Children in Urban and Rural Ethiopia. Am. J. Respir. Crit. Care Med. 2003, 167, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.; Martinelli, R.; Brunet, L.R. Innate immune responses to mycobacteria and the downregulation of atopic responses. Curr. Opin. Allergy Clin. Immunol. 2003, 3, 337–342. [Google Scholar] [CrossRef]
- Matricardi, P.M. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Controversial aspects of the ‘hygiene hypothesis’. Clin. Exp. Immunol. 2010, 160, 98–105. [Google Scholar] [CrossRef] [PubMed]
- von Hertzen, L.; Hanski, I.; Haahtela, T. Natural immunity. Biodiversity loss and inflammatory diseases are two global megatrends that might be related. EMBO Rep. 2011, 12, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.D.; Wray, G.A.; Perkins, S.E.; Parker, W. Reconstitution of the human biome as the most reasonable solution for epidemics of allergic and autoimmune diseases. Med. Hypotheses 2011, 77, 494–504. [Google Scholar] [CrossRef]
- Svoboda, E. The Worms Crawl In. Available online: https://www.nytimes.com/2008/07/01/health/research/01prof.html (accessed on 25 April 2024).
- Baumgart, D.C.; Sandborn, W.J. Crohn’s Disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.; Peyrin-Biroulet, L. Crohn’s Disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Hansen, J.J. Immune Responses to Intestinal Microbes in Inflammatory Bowel Diseases. Curr. Allergy Asthma Rep. 2015, 15, 61. [Google Scholar] [CrossRef]
- Maloy, K.J.; Powrie, F. Intestinal Homeostasis and Its Breakdown in Inflammatory Bowel Disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.E.; Li, J.; Blum, A.M.; Metwali, A.; Qadir, K.; Urban, J.F.; Weinstock, J.V. Exposure to Schistosome Eggs Protects Mice from TNBS-Induced Colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G385–G391. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.E.; Urban, J.F.; Argo, C.K.; Weinstock, J.V. Does the Failure to Acquire Helminthic Parasites Predispose to Crohn’s Disease? FASEB J. 2000, 14, 1848–1855. [Google Scholar] [CrossRef]
- Beer, R.J.S. Experimental Infection of Man with Pig Whipworm. BMJ. Br. Med. J. 1971, 2, 44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beer, R.J.S. Morphological Descriptions of the Egg and Larval Stages of Trichuris suis Schrank, 1788. Parasitology 1973, 67, 263–278. [Google Scholar] [CrossRef]
- Beer, R.J.S. The Relationship between Trichuris Trichiura (Linnaeus 1758) of Man and Trichuris suis (Schrank 1788) of the Pig. Res Vet Sci. 1976, 20, 47–54. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Viable Embryonated Eggs of the Whipworm Trichuris suis as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05777. [Google Scholar] [CrossRef] [PubMed]
- Hoshina, T.; Sakurai, T.; Ichimura, H.; Ishiwata, K.; En, S.; Yamada, T.; Lee, K.; Shimizu, A.; Hase, K.; Kanuka, H. Safety and Tolerability of Medicinal Parasite Ova (Trichuris suis) in Healthy Japanese Volunteers: A Randomized, Double-Blind, Placebo-Controlled Trial. Parasitol. Int. 2021, 85, 102441. [Google Scholar] [CrossRef]
- Leroux, L.; Nasr, M.; Valanparambil, R.M.; Tam, M.; Rosa, B.A.; Siciliani, E.A.; Hill, D.E.; Zarlenga, D.S.; Jaramillo, M.; Weinstock, J.V.; et al. Analysis of the Trichuris suis Excretory/Secretory Proteins as a Function of Life Cycle Stage and Their Immunomodulatory Properties. Sci. Rep. 2018, 8, 15921. [Google Scholar] [CrossRef]
- Hiemstra, I.H.; Klaver, E.J.; Vrijland, K.; Kringel, H.; Andreasen, A.; Bouma, G.; Kraal, G.; Van Die, I.; Haan, J.M.M.D. Excreted/Secreted Trichuris suis Products Reduce Barrier Function and Suppress Inflammatory Cytokine Production of Intestinal Epithelial Cells. Mol. Immunol. 2014, 60, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.W. Trichuris suis Therapy in Crohn’s Disease. Gut 2005, 54, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Schölmerich, J.; Fellermann, K.; Seibold, F.; Rogler, G.; Langhorst, J.; Howaldt, S.; Novacek, G.; Petersen, A.M.; Bachmann, O.; Matthes, H.; et al. A Randomised, Double-Blind, Placebo-Controlled Trial of Trichuris suis ova in Active Crohn’s Disease. J. Crohn’s Colitis 2016, 11, 390–399. [Google Scholar] [CrossRef]
- CDC. DPDX—Intestinal Hookworm. Available online: https://www.cdc.gov/dpdx/hookworm/index.html (accessed on 20 May 2024).
- Croese, J.; Gaze, S.; Loukas, A. Changed Gluten Immunity in Celiac Disease by Necator Americanus Provides New Insights into Autoimmunity. Int. J. Parasitol. 2013, 43, 275–282. [Google Scholar] [CrossRef]
- Berman, J.J. Nematoda (Roundworms). In Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2012; pp. 147–159. [Google Scholar]
- Feary, J.; Venn, A.; Mortimer, K.; Brown, A.; Hooi, D.; Falcone, F.H.; Pritchard, D.; Britton, J. Experimental Hookworm Infection: A Randomized Placebo-controlled Trial in Asthma. Clin. Exp. Allergy 2010, 40, 299–306. [Google Scholar] [CrossRef]
- Daveson, J.; Jones, D.; Gaze, S.; McSorley, H.J.; Clouston, A.D.; Pascoe, A.; Cooke, S.E.; Speare, R.; Macdonald, G.A.; Anderson, R.P.; et al. Effect of Hookworm Infection on Wheat Challenge in Celiac Disease—A Randomised Double-Blinded Placebo Controlled Trial. PLoS ONE 2011, 6, e17366. [Google Scholar] [CrossRef]
- Kabeerdoss, J.; Pugazhendhi, S.; Subramanian, V.; Binder, H.J.; Ramakrishna, B.S. Exposure to Hookworms in Patients with Crohn’s Disease: A Case-Control Study. Aliment. Pharmacol. Ther. 2011, 34, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Ordás, Í.; Eckmann, L.; Talamini, M.A.; Baumgart, D.C.; Sandborn, W.J. Ulcerative Colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Segal, J.; LeBlanc, J.-F.; Hart, A. Ulcerative Colitis: An Update. Clin. Med. 2021, 21, 135–139. [Google Scholar] [CrossRef]
- Heller, F.; Florian, P.; Bojarski, C.; Richter, J.F.; Christ, M.; Hillenbrand, B.; Mankertz, J.; Gitter, A.H.; Bürgel, N.; Fromm, M.; et al. Interleukin-13 Is the Key Effector TH2 Cytokine in Ulcerative Colitis That Affects Epithelial Tight Junctions, Apoptosis, and Cell Restitution. Gastroenterology 2005, 129, 550–564. [Google Scholar] [CrossRef]
- Summers, R.W.; Elliott, D.E.; Urban, J.F.; Thompson, R.; Weinstock, J.V. Trichuris suis Therapy for Active Ulcerative Colitis: A Randomized Controlled Trial. Gastroenterology 2005, 128, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Prosberg, M.V.; Halkjær, S.I.; Lo, B.; Bremerskov-Köser, C.; Ilvemark, J.F.K.F.; Seidelin, J.B.; Kristiansen, M.F.; Kort, A.; Kallemose, T.; Bager, P.; et al. Probiotic Treatment of Ulcerative Colitis with Trichuris suis ova: A Randomized, Double-blinded, Placebo-controlled Clinical Trial (the PROCTO Trial). J. Crohn’s Colitis 2024, 18, jjae095. [Google Scholar] [CrossRef]
- Freeman, H.J.; Chopra, A.; Clandinin, M.T.; Thomson, A.B.R. Recent Advances in Celiac Disease. World J. Gastroenterol. 2011, 17, 2259. [Google Scholar] [CrossRef] [PubMed]
- McSorley, H.J.; Gaze, S.; Daveson, J.; Jones, D.; Anderson, R.P.; Clouston, A.; Ruyssers, N.E.; Speare, R.; McCarthy, J.S.; Engwerda, C.R.; et al. Suppression of Inflammatory Immune Responses in Celiac Disease by Experimental Hookworm Infection. PLoS ONE 2011, 6, 24092. [Google Scholar] [CrossRef] [PubMed]
- Croese, J.; Giacomin, P.; Navarro, S.; Clouston, A.; McCann, L.; Dougall, A.; Ferreira, I.; Susianto, A.; O’Rourke, P.; Howlett, M.; et al. Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J. Allergy Clin. Immunol. 2015, 135, 508–516. [Google Scholar] [CrossRef]
- Croese, J.; Miller, G.; Marquart, L.; Llewellyn, S.; Gupta, R.; Becker, L.; Clouston, A.D.; Welch, C.; Sidorenko, J.; Wallace, L.; et al. Randomized, Placebo Controlled Trial of Experimental Hookworm Infection for Improving Gluten Tolerance in Celiac Disease. Clin. Transl. Gastroenterol. 2020, 11, e00274. [Google Scholar] [CrossRef] [PubMed]
- Pierce, D.; McDonald, M.; Merone, L.; Becker, L.; Thompson, F.; Lewis, C.; Ryan, R.Y.M.; Hii, S.F.; Zendejas-Heredia, P.A.; Traub, R.J.; et al. Effect of Experimental Hookworm Infection on Insulin Resistance in People at Risk of Type 2 Diabetes. Nat. Commun. 2023, 14, 4503. [Google Scholar] [CrossRef]
- Hoyte, F.; Nelson, H.S. Recent Advances in Allergic Rhinitis. F1000Research 2018, 7, 1333. [Google Scholar] [CrossRef]
- Dass, K.; Petrusan, A.J.; Beaumont, J.L.; Zee, P.C.; Lai, J.S.; Fishbein, A. Assessment of Sleep Disturbance in Children with Allergic Rhinitis. Ann. Allergy Asthma Immunol. 2017, 118, 505–506. [Google Scholar] [CrossRef]
- Bourke, C.D.; Mutapi, F.; Nausch, N.; Photiou, D.M.F.; Poulsen, L.K.; Kristensen, B.W.; Arnved, J.; Rønborg, S.; Roepstorff, A.; Thamsborg, S.M.; et al. Trichuris suis Ova Therapy for Allergic Rhinitis Does Not Affect Allergen-specific Cytokine Responses despite a Parasite-specific Cytokine Response. Clin. Exp. Allergy 2012, 42, 1582–1595. [Google Scholar] [CrossRef]
- Bager, P.; Kapel, C.M.O.; Roepstorff, A.; Thamsborg, S.M.; Arnved, J.; Rønborg, S.; Kristensen, B.W.; Poulsen, L.K.; Wohlfahrt, J.; Melbye, M. Symptoms after Ingestion of Pig Whipworm Trichuris suis Eggs in a Randomized Placebo-Controlled Double-Blind Clinical Trial. PLoS ONE 2011, 6, e22346. [Google Scholar] [CrossRef]
- Scrivener, S.; Yemaneberhan, H.; Zebenigus, M.; Tilahun, D.; Girma, S.; Ali, S.; McElroy, P.J.; Čustović, A.; Woodcock, A.; Pritchard, D.; et al. Independent Effects of Intestinal Parasite Infection and Domestic Allergen Exposure on Risk of Wheeze in Ethiopia: A Nested Case-Control Study. Lancet 2001, 358, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Leonardi-Bee, J.; Pritchard, D.; Britton, J. Asthma and Current Intestinal Parasite Infection. Am. J. Respir. Crit. Care Med. 2006, 174, 514–523. [Google Scholar] [CrossRef]
- Flohr, C.; Quinnell, R.J.; Britton, J. Do Helminth Parasites Protect against Atopy and Allergic Disease? Clin. Exp. Allergy 2009, 39, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, K.; Brown, A.; Feary, J.; Jagger, C.; Lewis, S.; Antoniak, M.; Pritchard, D.; Britton, J. Dose-ranging study for trials of therapeutic infection with Necator americanus in humans. Am. J. Trop. Med. Hyg. 2006, 75, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.S.; Hasseldam, H.; Bacher, I.H.; Thamsborg, S.M.; Johansen, F.F.; Kringel, H. Trichuris suis Secrete Products That Reduce Disease Severity in a Multiple Sclerosis Model. Acta Parasitol. 2017, 62, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Voldsgaard, A.I.; Bager, P.; Garde, E.; Åkeson, P.; Leffers, A.; Madsen, C.M.; Kapel, C.M.O.; Roepstorff, A.; Thamsborg, S.M.; Melbye, M.; et al. Trichuris suis Ova Therapy in Relapsing Multiple Sclerosis Is Safe but without Signals of Beneficial Effect. Mult. Scler. 2015, 21, 1723–1729. [Google Scholar] [CrossRef]
- Jo, F.; Isaak, A.; Lee, J.; Luzzio, C.C.; Carrithers, M.D.; Cook, T.D.; Field, A.S.; Boland, J.; Fabry, Z. Probiotic Helminth Administration in Relapsing–Remitting Multiple Sclerosis: A Phase 1 Study. Mult. Scler. 2011, 17, 743–754. [Google Scholar] [CrossRef]
- Benzel, F.J.; Erdur, H.; Köhler, S.; Frentsch, M.; Thiel, A.; Harms, L.; Wandinger, K.; Rosche, B. Immune Monitoring of Trichuris suis egg Therapy in Multiple Sclerosis Patients. J. Helminthol. 2011, 86, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.; Novotná, M.; Soldán, M.M.P.; Luckey, D.; Marietta, E.; Jeraldo, P.; Chen, X.; et al. Multiple Sclerosis Patients Have a Distinct Gut Microbiota Compared to Healthy Controls. Sci. Rep. 2016, 6, 28484. [Google Scholar] [CrossRef]
- Shahi, S.K.; Freedman, S.N.; Mangalam, A.K. Gut Microbiome in Multiple Sclerosis: The Players Involved and the Roles They Play. Gut Microbes 2017, 8, 607–615. [Google Scholar] [CrossRef]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; Von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.; et al. Alterations of the Human Gut Microbiome in Multiple Sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef]
- Jenkins, T.; Pritchard, D.; Tănăsescu, R.; Telford, G.; Papaiakovou, M.; Scotti, R.; Cortés, A.; Constantinescu, C.S.; Cantacessi, C. Experimental Infection with the Hookworm, Necator Americanus, Is Associated with Stable Gut Microbial Diversity in Human Volunteers with Relapsing Multiple Sclerosis. BMC Biol. 2021, 19, 12015. [Google Scholar] [CrossRef] [PubMed]
- Tănăsescu, R.; Tench, C.R.; Constantinescu, C.S.; Telford, G.; Singh, S.; Frakich, N.; Onion, D.; Auer, D.P.; Gran, B.; Evangelou, N.; et al. Hookworm Treatment for Relapsing Multiple Sclerosis. JAMA Neurol. 2020, 77, 1089. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szuba, M.; Stachera, W.; Piwko, A.; Misiak, M.; Rutkevich, R.; Sota, M.; Atrushi, L.; Bennacer, L.; Nzekea, D.; Wu, Y.C.; et al. Geohelminths: Use in the Treatment of Selected Human Diseases. Pathogens 2024, 13, 703. https://doi.org/10.3390/pathogens13080703
Szuba M, Stachera W, Piwko A, Misiak M, Rutkevich R, Sota M, Atrushi L, Bennacer L, Nzekea D, Wu YC, et al. Geohelminths: Use in the Treatment of Selected Human Diseases. Pathogens. 2024; 13(8):703. https://doi.org/10.3390/pathogens13080703
Chicago/Turabian StyleSzuba, Magdalena, Weronika Stachera, Adrianna Piwko, Marianna Misiak, Renata Rutkevich, Marcin Sota, Lana Atrushi, Leyla Bennacer, Deborah Nzekea, Yen Ching Wu, and et al. 2024. "Geohelminths: Use in the Treatment of Selected Human Diseases" Pathogens 13, no. 8: 703. https://doi.org/10.3390/pathogens13080703
APA StyleSzuba, M., Stachera, W., Piwko, A., Misiak, M., Rutkevich, R., Sota, M., Atrushi, L., Bennacer, L., Nzekea, D., Wu, Y. C., Kim, A. T., Yu, S., Ribeiro, N., & Dybicz, M. (2024). Geohelminths: Use in the Treatment of Selected Human Diseases. Pathogens, 13(8), 703. https://doi.org/10.3390/pathogens13080703







