Abstract
African swine fever virus (ASFV), a highly virulent double-stranded DNA virus, poses a significant threat to global pig farming, with mortality rates in domestic pigs reaching up to 100%. Originating in Kenya in 1921, ASFV has since proliferated to Western Europe, Latin America, Eastern Europe, and most recently China in 2018, resulting in substantial global agricultural losses. Antigenic epitopes, recognized by the immune system’s T cells and B cells, are pivotal in antiviral immune responses. The identification and characterization of these antigenic epitopes can offer invaluable insights into the immune response against ASFV and aid in the development of innovative immunotherapeutic strategies. Vaccine adjuvants, substances that amplify the body’s specific immune response to antigens, also play a crucial role. This review provides an overview of the progress in studying T/B-cell epitopes in ASFV proteins and ASFV vaccine adjuvants, highlighting their role in the immune response and potential use in new vaccine development.
1. Introduction
African Swine Fever (ASF) is an acute hemorrhagic viral disease, caused by African Swine Fever Virus (ASFV), that affects domestic pigs and various species of wild boars [1,2]. ASFV is a member of the Asfarviridae family and is classified as a large icosahedral double-stranded DNA virus. Its linear genome spans approximately 189 kilobases and encodes for more than 180 genes. Since the 1960s, concerted efforts have been made to develop effective ASF vaccines, including ASF gene deletion vaccines, inactivated vaccines, multiepitope vaccines, attenuated live vaccines, and subunit vaccines [3,4,5]. Despite advancements, the absence of a clear understanding of immune correlates of protection and the precise identification of protective antigens has resulted in the current absence of an effective vaccine to halt the virus’s spread in pig farming. Consequently, it is essential to conduct comprehensive research into ASFV’s protective antigens, antigenic epitopes, and innovative vaccine adjuvants.
Much like most viral infections, both humoral immunity and cellular immune responses play crucial roles in ASFV protective immunity [6]. The serum from pigs in the recovery phase of ASFV infection possessed the ability to neutralize the infection of homologous and some heterologous strains both in vivo and in vitro, potentially by inhibiting virus attachment and internalization [7,8,9]. Anti-ASFV antibodies typically became detectable around six days post-infection, and could persist for an extended period once they had survived. However, despite the presence of these antibodies, their ability to cross-neutralize in vitro did not correlate with ASFV cross-protection in pigs [10]. Thus, the specific role of antibodies in ASFV protection remains unclear. ASFV serogroup classification, based on erythrocyte adsorption inhibition tests, suggests that ASF protective immunity may be serotype-specific, as ASFV within the same serogroup can cross-protect each other, while viruses outside the serotype cannot. Furthermore, a wealth of data supports the pivotal role of T-cell immune responses in ASFV control [6,11,12,13]. Depletion of pig lymphocytes indicated that cytotoxic CD8 lymphocytes were vital for ASFV clearance and protection against the virus. Moreover, pigs vaccinated with DNA vaccines exhibited partial protection during an ASFV attack, even though no anti-ASFV antibodies were detected in the protected pigs. Additionally, the failure of adjuvant-formulated inactivated ASFV and recombinant vaccines to offer strong protection highlighted the critical importance of cytotoxic T lymphocytes (CTLs) in the protective immune response against the virus [14].
Antigenic epitopes, the unique structural features on antigen molecules, exhibit specific antigenic functions. They are differentiated into B-cell and T-cell epitopes based on their interactions with corresponding antigen receptors on immune cells [15]. The investigation of antigenic epitopes is crucial for elucidating virus-induced immune responses and forms the foundation for the development of antiviral strategies, thereby representing a dynamic field of research in virology. According to the Immune Epitope Database (IEDB), several ASFV epitopes have been identified [16]. Despite the significant progress in ASFV antigenic epitope research, which has advanced the development of antigenic epitope diagnostic methods and vaccines, the identification and application of ASFV antigenic epitopes continue to pose challenges.
Vaccine adjuvants are substances that can enhance the body’s specific immune response to antigens [17,18,19]. When formulated with vaccines, they can effectively enhance the immune response to vaccine antigens. Adjuvants were first discovered in 1920 by French scientist Gaston Ramon. They usually do not have immunogenicity, but can guide humoral and cellular immune responses to produce specific immunity against pathogens [19]. Currently, adjuvants are used to enhance the immune response of vaccines and effectively reduce the dose of vaccine antigens, which is crucial in the production process of veterinary vaccines.
In this review, we mainly focus on the recent research progress of ASFV’s T-cell epitopes, B-cell epitopes, and vaccine adjuvants, aiming to provide basic information for the formulation of ASFV vaccine development and control strategies.
2. Advancement in the Development of Antigenic Epitopes of ASFV
2.1. Antigenic Epitope-Identified Proteins in ASFV
The ASFV virion primarily consists of five components, including the viral genome, core shell, inner envelope, capsid, and outer envelope. The ASFV genome encodes approximately 150 to 200 proteins, of which about 60 are structural proteins. A large body of literature has confirmed the presence of B-cell and T-cell epitopes in ASFV. These antigenic epitope-identified proteins include p72, p37, p30, and CD2v (Figure 1).
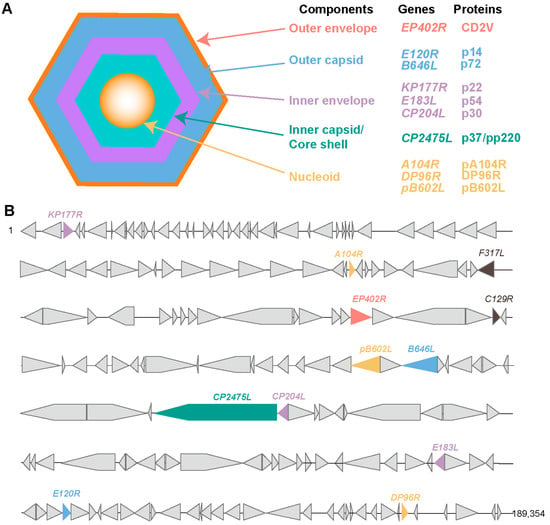
Figure 1.
Schematic representation of ASFV structure and location of antigenic epitope-identified proteins in ASFV genomes. (A), Schematic diagram of the ASFV structure. ASFV is composed of an Outer envelope, outer capsid, inner envelope, inner capsid, and nucleoid. (B), Location of antigenic epitope-identified proteins in ASFV genomes.
2.2. Current Methods for the Identification of Antigenic Epitopes of the ASFV
2.2.1. Methods for the Identification of B-Cell Epitopes
ASFV protein B-cell epitope identification is a systematic procedure involving protein expression, mouse immunization, monoclonal antibody production, and bioinformatics-guided epitope analysis. Epitope validation is further accomplished via peptide synthesis, alanine scanning in conjunction with Enzyme-Linked Immunosorbent Assay (ELISA), and dot-ELISA characterization. These methodologies have led to the discovery of numerous B-cell epitopes, including the extracellular domain of CD2v and p37 [20,21]. These findings are pivotal for elucidating immune response mechanisms against ASFV and facilitating effective vaccine development.
Given the multitude of ASFV proteins, predicting B-cell epitopes using bioinformatics tools remains an important and challenging task [22]. The complexity inherent to antigen recognition complicates epitope prediction. For linear B-cell epitopes, a prevalent approach involves the use of the ABCpred server, trained on the Bcipep database via a recurrent neural network (RNN), for analysis and selection. Epitopes with higher scores indicate a greater likelihood of B-cell epitope prediction. Subsequent secondary structure prediction is performed using DNAStar Protean software 11. The protein’s secondary structure significantly impacts the epitope. The robust chemical bonds of α-helices and β-sheets allow for the maintenance of protein structure, making such epitopes less likely to be antigenic due to their internal location and difficulty in antibody fitting. Conversely, the flexibility of β-turns and random coils in proteins allows for a looser structure, easily twisted and displayed on the protein surface. Such structures are more likely to serve as antigenic epitopes due to their prominence and ease of antibody fitting. For instance, Song et al. utilized ABCpred and DNAStar to predict the B-cell epitopes of pB602L, p30, p72, and CD2v proteins [12]. They evaluated the affinity of ASFV positive serum for synthetic peptides through peptide synthesis and dot blot analysis, successfully identifying immunodominant B-cell epitopes and incorporating them into nanoparticle vaccine antigen design [12].
2.2.2. Methods for the Identification of T-Cell Epitopes
The most common identification process for T-cell epitopes in ASFV proteins includes ASFV protein expression, mouse immunization, IFN-γ ELISpot identification of cellular immune responses in splenic cells, bioinformatic analysis of T-cell epitopes, peptide synthesis combined with IFN-γ ELISpot, flow cytometry, and T-cell proliferation for further confirmation of T-cell epitopes. For instance, one T-cell epitope, 246 SRRSLVNPWT 255, has been identified on F317L using this method [23].
The use of bioinformatics tools for predicting ASFV T-cell epitopes is gaining traction. The most common approach for T-cell epitope prediction involves the use of software such as NetMHC 4.0 to screen potential T-cell epitopes. This software can predict peptides that bind with a multitude of SLA-I class molecules, from which high-affinity binding peptides are selected for biological synthesis and further epitope confirmation via IFN-γ ELISpot. For instance, Song et al. utilized NetMHC 4.0 to screen potential T-cell epitopes from CD2v and p72, and evaluated the cellular immune response of mice immunized with recombinant CD2v protein through peptide synthesis and IFN-γ ELISpot [12]. This validated the immunodominant T-cell epitopes, leading to the successful selection of immunodominant T-cell epitopes that were subsequently incorporated into the antigen design of nanoparticle vaccines.
2.3. Research Progress on B-Cell Epitopes in ASFV Proteins
2.3.1. p72-Derived B-Cell Epitopes
Given the complexity of the p72 trimer structure, information about specific p72 epitopes remains limited. Previous studies have identified a linear B-cell epitope located at 280FPENSHNIQTAGKQD294 [24] and 281PENSHNIQTA290 [25]. Recently, Tesfagaber et al. prepared anti-p72 mAb and identified a new linear B-cell epitope region between 283NSHNIQ288 [26]. The identification of B-cell epitopes of p72 not only plays an important role in ASF serological diagnosis but may also lay a solid foundation for further research on the antigenic function of the p72 protein.
2.3.2. CD2v-Derived B-Cell Epitopes
Multiple linear B-cell epitopes in the extracellular domain of ASFV’s CD2v have been identified through monoclonal antibodies (mAbs). For instance, a linear epitope formed by amino acids 28LDSNITNDNNINGVSWNFFNNSF51 was identified by mAbs produced by truncated CD2v protein fused with Norovirus (NoV) P particles [27]. Using mAbs produced by eukaryotic cells expressing CD2v, two epitopes, 38DINGVSWN45 and 134GTNTNIY140, were identified through peptide scanning [28]. Two linear B-cell epitopes, 128TCKKNNGTNT137 and 148VKYTNESILE157, were recognized by mAbs produced by truncated CD2v expressed in CHO-S [29]. The B-cell epitope (154SILE157) of the CD2v extracellular domain was further ensured by dot-Blot, ELISA and IFA tests [20]. Jia et al. identified three linear B-cell epitopes, 147FVKYT151, 157EYNWN161, and 195SSNY198, by screening five types of mAbs produced by truncated CD2v protein expressed in baculovirus [30]. Song et al. recently predicted and identified a major linear B-cell epitope, 160WNNSNINNFT169, which induced humoral and cellular immune responses in a mouse model, strongly suggesting that linear B-cell epitopes may promote the design and development of ASFV subunit vaccines [31]. Lu et al. identified a novel epitope, 264EPSPREP270, located in the CD2v-IR structural domain, which could be used for the design and development of subunit vaccines [32]. It is noteworthy that some of the linear B-cell epitopes mentioned above overlap; therefore, the immunogenicity of these epitopes needs to be evaluated.
2.3.3. p30-Derived B-Cell Epitopes
p30 is a protein capable of generating neutralizing antibodies [33], with its C-terminus confirmed as an immunodominant region (aa 111–130) [34]. Petrovan et al. preliminarily identified a large polypeptide fragment (120–204 aa) at the C-terminus of the p30 protein by mAb 62-35 and 142-4 [35]. Recently, a study expressed overlapping truncated fragments in E. coli, determining that the linear epitope sequence recognized by monoclonal antibody 1B4G2–4 of p30 was within the range of 157FNKVIRAHNFIQTIYGTPLK177. After conducting amino acid-to-amino acid transfer identification, the smallest linear epitope was finally identified as 164HNFIQTI170. This epitope exhibited a strong antigen index and partial alpha-helical angles and coiled regions. It also showed high conservation across different strains, making it suitable for subsequent vaccine development [36].
2.3.4. p54-Derived B-Cell Epitopes
Envelope protein p54, similar to B646L, can induce neutralizing antibodies in pigs, although these antibodies cannot provide protection against potent ASFV attacks [37]. Utilizing high-throughput analysis technology based on gene chips, Desmet et al. identified two B-cell epitopes of p54 (IVLIYLFSSRKKKAA and AA 149–161) [38]. Zheng et al. identified a novel linear B-cell epitope (110TMSAIENLR118) on the ASFV P54 protein using monoclonal antibodies, which was conserved in all reference ASFV strains from different regions in China [39]. Nanobodies were used as a new tool to identify linear B-cell epitopes on the ASFV p54 and a novel minimal linear B-cell epitope, 76QQWVEV81, was identified with core binding site as 76QQWV79 [40].
2.3.5. DP96R-Derived B-Cell Epitopes
DP96R, also known as uridine kinase (UK), encodes a protein associated with virulence, which can be utilized in the development of attenuated live vaccines [41,42,43]. Two B-cell epitopes (03THDCSLKEK11 and 55YWKGIKRGND64) were found on ASFV’s DP96R protein [44].
2.3.6. E120R-Derived B-Cell Epitopes
E120R is highly conserved among ASFV strains and serves as a target for the development of attenuated live vaccines against ASF [45]. Through incubation with a gene chip based on high-density peptide microarrays, it was discovered that the peptide sequence EEFEPIPDYDTTST of ASFV envelope protein E120R could react with ASF positive serum samples [38].
2.3.7. pA104R-Derived B-Cell Epitopes
The A104R gene is responsible for the synthesis of a protein, hypothesized to resemble a histone, which is strategically positioned at the sites of viral DNA replication and gene expression [46]. The dominant IgM epitope PEP23 (KAVKIRALK) and PEP15 (KFTVVTVKA) were identified by epitope modification [47]. Through the confirmatory analysis of the pA104R epitope using monoclonal antibodies, an immunodominant B-cell epitope (KPTITKOELYSI) was identified. This finding could potentially aid in the development of sensitive diagnostic tools and serve as a target for candidate vaccine development [48].
2.3.8. E184L-Derived B-Cell Epitopes
E184L serves as a crucial antagonist of the IFN signal and an immunogenic ASFV protein, capable of evading the host’s innate antiviral immune response [49,50]. Through meticulous localization, the antigenic epitopes for the E184L mAbs have been identified as 119 IQRQGFL 125 and 153 DPTEFF 158. These findings lay the groundwork for serological diagnosis and the development of epitope-based marker vaccines [51].
Taken together, numerous B-cell epitopes have been identified in ASFV, which guide the design of immunogenic peptides and novel vaccine molecules, and also facilitate the development of diagnostic reagents and clinical disease diagnosis (Table 1).

Table 1.
Identification and application of B-cell epitopes of ASFV antigens.
2.4. Research Progress on ASFV-Specific T-Cell Epitopes
2.4.1. CD2v-Derived T-Cell Epitopes
The intracellular epitope in the CD2v protein of ASFV has been demonstrated to induce both humoral and cellular immune responses [32,56]. The CD2v protein’s proline-rich cytoplasmic domain demonstrated a high degree of conservation, with 79% to 100% amino acid identity, across various genotypes of ASFV [56]. The discovery of monoclonal antibody 1F3 (264EPSPREP270) has identified it as a T-cell epitope that was not only specific to ASFV but also remarkably conserved across different genotypes [32].
2.4.2. p72-Derived T-Cell Epitopes
The p72 protein is the major capsid protein and also one of the most immunogenic proteins of ASFV, making it an important target for detection and vaccine development [57]. Using the IEDB MHC-I binding prediction algorithm coupled with ELISPOT assay detection, Sun et al. have delineated the core peptides P351 (SRISNIKNNKY), P334 (SDYTL), and P366 (SSYGGAK) derived from the p72 protein as exhibiting elevated immunogenicity in pigs that have survived infection, offering a pivotal reference for the subsequent development of tetrameric constructs in immunological research [58].
2.4.3. F317L-Derived T-Cell Epitopes
The late F317L protein of ASFV induced immunosuppression by dampening the activation of the NF-ĸB pathway, making it a potential immunogenic antigen [59,60]. Through T-cell epitopes prediction and validation by the IFN-γ ELISpot assay, the peptide F25 (246SRRSLVNPWT255) was identified as a presumed T-cell epitope, capable of inducing a robust immune response [23].
2.4.4. C129R-Derived T-Cell Epitopes
The C129R protein of ASFV, known for its strong immunogenicity and ability to target the cyclic GMP-AMP pathway, was utilized in the development of recombinant adenovirus vaccines [10,61]. Recently, Zhai and colleagues successfully pinpointed T-cell epitopes within the C129R protein, specifically in peptides C11 (53LQNPYEAVI61), C14 (81GHVTWAVPY89), C16 (97AKPDAIMLT105), and C18 (116ALNQNVLTL124) [62].
Collectively, the validated ASFV-specific T-cell epitopes summarized in this review may play a crucial role in the design and development of novel ASFV vaccines (Table 2).

Table 2.
Identification and application of T-cell epitopes of ASFV antigens.
3. Advancement in the Development of Vaccine Adjuvants of ASFV
3.1. The Functions and Mechanism of Adjuvants in ASF Vaccines
In the development of ASFV vaccines, the selection and use of adjuvants is a critical component. Adjuvants can enhance the immunogenicity of vaccines, improve the durability of immunity, reduce vaccine dosage, and thus improve the cost-effectiveness of vaccines [17,18,19]. Currently, a variety of adjuvants have been used in the development of ASFV vaccines, including aluminum salts, oil emulsions, and polymer microspheres [63,64]. These adjuvants were shown to enhance the immune response through various mechanisms, such as stimulating immune cells, promoting antigen uptake by antigen-presenting cells (APCs), stimulating the secretion of various cytokines and chemokines, inducing the differentiation of CD4 + T cells into different types (Th1, Th2, Th17, etc.), prolonging antigen presentation time, and enhancing antigen presentation efficiency [63,64].
3.2. Types of Adjuvants for ASF Vaccines
3.2.1. Montanide™ ISA
Montanide™ ISA, the veterinary vaccine adjuvant, is a common choice in animal vaccines due to its ability to induce strong immune responses with non-toxicity, good tolerability, and the simplicity of mixing the antigen and adjuvant [65]. Zajac et al. used a recombinant adenovirus mixture containing ASFV antigens (Ad5-ASFV) with 42 multi-epitopes, combined with Montanide ISA-201™ adjuvant, to immunize pigs three times. This could induce a humoral immune response, but there was no protective effect after the immune challenge [64]. A recent study utilized an inactivated vaccine produced by gamma-irradiated ASFV in combination with Montanide™ ISA 201 VG adjuvant. This formulation was administered to five weaned piglets at three-week intervals for two immunizations. Despite all animals developing antibodies against ASFV p72, the response was insufficient to confer protection from ASFV strain attacks [66].
3.2.2. Polygen™
Polygen™ is a low molecular weight copolymer adjuvant that can cross-link in solution to form a high molecular weight gel. It has been demonstrated in studies to elicit significant interferon-gamma (IFN-γ) and interleukin-12 (IL-12) responses when used in bovine parasitic vaccines [67]. Researchers administered an inactivated vaccine, prepared by combining ASFV inactivated by gamma irradiation or binary ethylenimine (BEI) with the Polygen adjuvant, via intramuscular injection to weaned piglets. Despite the presence of ASFV-specific antibodies in all vaccinated subjects, no protective effect was observed, even after stringent homologous challenge [66,68].
3.2.3. Zoetis
Vaccine adjuvants produced by Zoetis have been used in vaccine immunization research for various pathogens, including paratyphoid salmonella and porcine reproductive and respiratory syndrome virus [69,70,71]. Pigs immunized with a cocktail of 12 ASFV antigens combined with the Zoetis adjuvant showed that the cocktail-ii- Zoetis vaccine recipients had a higher survival rate, but it did not prevent clinical disease [63]. Compared to the ENABL adjuvant, pigs vaccinated with Ad5-ASFV 4-way cocktail vaccine combined with Zoetis adjuvant exhibited higher humoral immune response [72].
3.2.4. BioMize®
BioMize® is an innovative, ready-to-use, and fully customizable adjuvant developed by VaxLiant company, offering great flexibility for vaccine development and commercialization [63,64,73]. Compared to the use of the Montanide ISA-201™ adjuvant, the Ad5-ASFV BioMize® immunogen elicited a relatively lower anti-pp62 specific IgG response [64]. Immunizing pigs with a 35 adenovirus-vectored ASFV cocktail, along with BioMize® adjuvant, generated robust ASFV-specific antibodies, IFN-γ cells, and CTL responses, yet failed to confer protection against the virulent Arm07 isolate in Eurasian wild boar [73].
3.2.5. MF59®
MF59® is an oil-in-water emulsion adjuvant that has been included in influenza vaccines approved in Europe since 1997 and has been administered to over 100 million people in more than 30 countries [74]. The BEI-inactivated ASFV vaccine, combined with the MF59 adjuvant and administered intradermally and intramuscularly in pigs, elicited a positive antibody response to ASFV. However, it did not provide effective protection against a lethal attack [75].
3.2.6. Adjuvants from Bacterial Component
The major outer membrane lipoprotein I (OprI) of Pseudomonas aeruginosa is a ligand for Toll-like receptor (TLR)-2 [76]. It can trigger dendritic cells (DC) to secrete pro-inflammatory cytokines in vivo, thereby indirectly regulating adaptive immune responses. OprI can serve as a natural adjuvant, and after fusion, it can induce a strong humoral and cytotoxic T-cell response against peptides/proteins. The application of OprI in fusion proteins has been extended to antigens encoded by ASFV’s B646L and G1340L, and the resulting proteins can induce ASFV-specific CTL activity [76,77]. The different immune functions of OprI, including promoting Th1/Th2 responses, are all attributed to the activation of TLR-2 signaling. The immunoregulatory activity of OprI fusion proteins has also been used in vaccine development. A recent study has shown that the mixture of OprI fusion proteins formulated with ISA206 adjuvant can induce strong ASFV-specific humoral and cellular immune responses in pigs, providing valuable information for the further development of subunit vaccines against ASF [11]. Heat-labile enterotoxin B (LTB), when used as an adjuvant carrying antigens, can enhance the immunogenicity of vaccines and play a role in T-cell activation and differentiation [78]. Following oral immunization with recombinant Lactobacillus lactis expressing ASFV protein-LTB fusion protein, the local mucosal immunity, humoral immunity, and Th2 cell immunity were enhanced compared with no LTB adjuvant group [79]. These findings provide new insights into the design and development of ASFV subunit vaccines.
3.2.7. Nano-Adjuvants
Compared to traditional vaccines, nano-vaccines demonstrate enhanced efficacy due to their ability to accumulate, self-assemble in lymph nodes, and be readily uptaken by APC cells [80]. Recently, a self-assembling nano ASFV candidate vaccine (NanoFVax) targeting dendritic cells has been demonstrated to elicit a potent T-cell response, with high-level antibody responses against ASFV persisting for over 231 days [12].
In summary, the selection and optimization of adjuvants for ASFV vaccines remains an important area of research that requires further exploration and study (Table 3).

Table 3.
Research progress on adjuvants in ASFV vaccines.
4. Prospects of Antigenic Epitopes and Adjuvants in the Development of Novel ASFV Vaccines and Diagnostic Reagents
Currently, techniques for identifying B-cell epitopes are well established. However, obtaining high-quality monoclonal antibodies is challenging, especially those with neutralizing activity that can recognize conformational epitopes. For the recognition of conformational B-cell antigenic epitopes, monoclonal antibodies recognizing the whole virus are more effective than those recognizing recombinant proteins. Novel ASFV detection methods, such as ELISA and flow cytometry, rely on the identification of these B-cell epitopes. By recognizing and utilizing these epitopes, we can develop more sensitive and specific detection methods to facilitate early detection and diagnosis of ASFV infection, thereby effectively controlling the spread of ASF. The OIE recommended ELISA as the preferred serological method for ASF diagnosis [83]. Despite its lower sensitivity compared to highly sensitive early pathogen detection methods such as PCR, qPCR, multiplex PCR, LAMP, and NGS, the ELISA demonstrated significant advantages in large-scale sample testing. It was particularly suitable for detecting pig herds recovering from subacute and latent ASF infections [84]. Most commercialized ELISA kits were based on a single viral protein, which could lead to false-negative results. However, ASFV tandem proteins based on multiple B-cell epitopes showed significant improvements in sensitivity in the development of ELISA kits [85,86]. It enhanced the recognition of different types of antibodies in pigs, reduced the possibility of inaccurate negative or positive results, and allowed a comprehensive assessment of ASFV exposure by k3 derived from 27 multiple peptides of 11 ASFV proteins or the antigenic dominant domains from p30, p54, and p72 [85,86]. Compared with currently available commercial detection methods, innovative methods using multiple B-cell epitopes of multiple ASFV proteins can achieve higher sensitivity and specificity.
The identification of T-cell epitopes is crucial for research on cellular immune mechanisms and the development of subunit peptide vaccines. Apart from p30, p54, and p72, there are few reports on the research of T-cell antigenic epitopes of other proteins [23]. Since T-cells only recognize antigenic peptides presented by the Major Histocompatibility Complex (MHC) molecules on the surface of Antigen Presenting Cells (APCs), the recognition of T-cell epitopes is more challenging [87]. The development of ASFV vaccines that focused solely on humoral immune responses was insufficient, as inactivated vaccines had been shown to be ineffective in providing protection against ASFV challenge [68]. Attenuated or low-virulence ASFV strains induced protective immunity in pigs against virulent ASFV strains [88], but vaccinated pigs often experienced adverse side effects, such as chronic viremia [3]. Additionally, the use of live attenuated ASFV raised significant safety concerns [3]. Furthermore, the activation of CD8 T cells aided pigs in combating ASFV infection [89], underscoring the crucial role of antigen-specific T cell immune responses in ASFV vaccine development. This necessitates further investigation into ASFV’s T-cell epitopes.
Although inactivated and subunit vaccines for ASFV have a high safety profile and have been used in conjunction with some adjuvants, they have not demonstrated robust protective effects in current research [3,90,91]. This indicates that there are many unknowns that need to be addressed with protective antigens or epitopes, adjuvants, and non-structural viral protein antigens in the development of new ASFV vaccines. Although the only attenuated live vaccine approved by Vietnam demonstrated that 93.34% of the 5958 randomly selected immunized pigs met the technical requirements, as reported in the June 2023 Global Disease Monitoring Report from the Swine Health Information Center, potential biosecurity risks such as reversion to virulence necessitated caution [91]. Therefore, the development of new nucleic acid vaccines, genetically engineered vaccines, or multi-peptide vaccines in combination with new adjuvants is an important direction for current ASFV vaccine development. When choosing a vaccine adjuvant, many factors need to be considered, with safety being the first. A good adjuvant must be safe, well-tolerated, and easy to produce; it should have good pharmaceutical properties (such as pH, osmotic pressure, endotoxin levels, etc.) and long shelf life; and finally, it should be economically viable.
Reverse vaccinology is an application that aids in the development of novel epitope vaccines based on pathogen genome sequencing [92]. Compared to traditional vaccines, epitope vaccines are safer, non-toxic, stable, and can more directly elicit immune responses against pathogenic microorganisms [93]. Epitope vaccines based on multiple epitope peptides can also overcome the problem of low conservation between epitopes of different genotype strains and elicit stronger immune responses. However, for ASFV, the development of diagnostics and vaccines based on multiple epitopes is still insufficient [94,95]. As multi-epitope vaccines are based on the selection of antigenic epitopes and the immune response of computer-screened epitopes, they are suitable for the development of universal vaccines against different ASFV genotypes, accelerating the vaccine design process and reducing its cost.
5. Conclusions
African swine fever infection causes high mortality in pigs, resulting in significant economic losses to the pig industry. Research on African swine fever vaccines has been ongoing in recent decades. The antigenic epitopes recognized by T cells and B cells of the immune system play a key role in the antiviral immune response, and therefore the resolution and precise characterization of the T-cell antigenic epitopes and B-cell antigenic epitopes of ASFV can provide an important basis for vaccine development. Here, we describe the major potential antigenic proteins of ASFV and methods for pinpointing T- and B-cell epitopes of ASFV antigens, providing detailed localization data for these epitopes. These will hold promise for the development of safe and effective ASF vaccines. Vaccines, combined with accurate, efficient, and early diagnostic techniques, could provide the basis for the prevention, control, and eradication of ASF.
Author Contributions
Conceptualization, Q.W., K.Y., W.L. and C.L.; Writing—original draft preparation, Q.W., C.L., B.Z., J.Z. and Z.L.; Resources and investigation, R.G., T.G. and F.Y.; Writing—review and editing, Q.W., D.Z. and Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Wuhan Science and Technology Bureau (Grant No. 2023020302020573) and the Hubei Province Innovation Centre of Agricultural Sciences and Technology (No. 2024-620-000-001-013).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We express our gratitude to the authors of the cited works for their contribution of data to the final version of this manuscript.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Dixon, L.K.; Sun, H.; Roberts, H. African swine fever. Antiviral Res. 2019, 165, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Penrith, M.L. African swine fever. Onderstepoort J. Vet. Res. 2009, 76, 91–95. [Google Scholar]
- Vu, H.L.X.; McVey, D.S. Recent progress on gene-deleted live-attenuated African swine fever virus vaccines. NPJ Vaccines 2024, 9, 60. [Google Scholar] [CrossRef]
- Wu, K.; Liu, J.; Wang, L.; Fan, S.; Li, Z.; Li, Y.; Yi, L.; Ding, H.; Zhao, M.; Chen, J. Current State of Global African Swine Fever Vaccine Development under the Prevalence and Transmission of ASF in China. Vaccines 2020, 8, 531. [Google Scholar] [CrossRef]
- Sereda, A.D.; Balyshev, V.M.; Kazakova, A.S.; Imatdinov, A.R.; Kolbasov, D.V. Protective Properties of Attenuated Strains of African Swine Fever Virus Belonging to Seroimmunotypes I–VIII. Pathogens 2020, 9, 274. [Google Scholar] [CrossRef]
- Schafer, A.; Franzoni, G.; Netherton, C.L.; Hartmann, L.; Blome, S.; Blohm, U. Adaptive Cellular Immunity against African Swine Fever Virus Infections. Pathogens 2022, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Canter, J.A.; Aponte, T.; Ramirez-Medina, E.; Pruitt, S.; Gladue, D.P.; Borca, M.V.; Zhu, J.J. Serum Neutralizing and Enhancing Effects on African Swine Fever Virus Infectivity in Adherent Pig PBMC. Viruses 2022, 14, 1249. [Google Scholar] [CrossRef]
- Escribano, J.M.; Galindo, I.; Alonso, C. Antibody-mediated neutralization of African swine fever virus: Myths and facts. Virus Res. 2013, 173, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Puertas, P.; Escribano, J.M. Blocking antibodies inhibit complete African swine fever virus neutralization. Virus Res. 1997, 49, 115–122. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, Y.; Li, J.; Wang, A.; Meng, X.; Chen, L.; Wei, J.; Tong, W.; Kong, N.; Yu, L.; et al. Screening and identification of the dominant antigens of the African swine fever virus. Front. Vet. Sci. 2023, 10, 1175701. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, W.; Yang, S.; Song, S.; Ma, Y.; Zhou, G.; Liang, X.; Miao, C.; Li, J.; Liu, Y.; et al. Evaluation of humoral and cellular immune responses induced by a cocktail of recombinant African swine fever virus antigens fused with OprI in domestic pigs. Virol. J. 2023, 20, 104. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, M.; Zhou, L.; Tian, P.; Sun, Z.; Sun, J.; Wang, X.; Zhuang, G.; Jiang, D.; Wu, Y.; et al. A candidate nanoparticle vaccine comprised of multiple epitopes of the African swine fever virus elicits a robust immune response. J. Nanobiotechnol. 2023, 21, 424. [Google Scholar] [CrossRef]
- Jenson, J.S.; Childerstone, A.; Takamatsu, H.; Dixon, L.K.; Parkhouse, R.M. The cellular immune recognition of proteins expressed by an African swine fever virus random genomic library. J. Immunol. Methods 2000, 242, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Lacasta, A.; Ballester, M.; Monteagudo, P.L.; Rodriguez, J.M.; Salas, M.L.; Accensi, F.; Pina-Pedrero, S.; Bensaid, A.; Argilaguet, J.; Lopez-Soria, S.; et al. Expression library immunization can confer protection against lethal challenge with African swine fever virus. J. Virol. 2014, 88, 13322–13332. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J.; Sun, P.; Qin, J.; Yang, X.; Chen, D.; Zhang, Y.; Zhong, N.; Wang, Z. SARS-CoV-2 epitope-specific T cells: Immunity response feature, TCR repertoire characteristics and cross-reactivity. Front. Immunol. 2023, 14, 1146196. [Google Scholar] [CrossRef]
- Huang, C.; Cao, C.; Xu, Z.; Lin, Y.; Wu, J.; Weng, Q.; Liu, Z.; Jin, Y.; Chen, P.; Hua, Q. A blocking ELISA based on virus-like nanoparticles chimerized with an antigenic epitope of ASFV P54 for detecting ASFV antibodies. Sci. Rep. 2023, 13, 19928. [Google Scholar] [CrossRef]
- Xie, C.; Yao, R.; Xia, X. The advances of adjuvants in mRNA vaccines. NPJ Vaccines 2023, 8, 162. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; S Arunachalam, P.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Liu, S.; Ding, P.; Du, Y.; Ren, D.; Chen, Y.; Li, M.; Sun, X.; Wang, S.; Chang, Z.; Li, R.; et al. Development and characterization of monoclonal antibodies against the extracellular domain of African swine fever virus structural protein, CD2v. Front. Microbiol. 2022, 13, 1056117. [Google Scholar] [CrossRef]
- Wang, A.; Chen, Z.; Zhou, J.; Chen, Y.; Liu, Y.; Liu, H.; Liang, C.; Zhu, X.; Zhang, Y.; Xin, C.; et al. Development and characterization of monoclonal antibodies against p37 protein of African swine fever virus. Int. J. Biol. Macromol. 2024, 264, 130689. [Google Scholar] [CrossRef]
- Mima, K.A.; Katorkina, E.I.; Katorkin, S.A.; Tsybanov, S.Z.; Malogolovkin, A.S. In silico prediction of B- and T-cell epitopes in the CD2v protein of african swine fever virus (African swine fever virus, Asfivirus, Asfarviridae). Vopr. Virusol. 2020, 65, 103–112. [Google Scholar] [CrossRef]
- Huang, Y.; Zhai, W.; Wang, Z.; He, Y.; Tao, C.; Chu, Y.; Pang, Z.; Zhu, H.; Jia, H. Analysis of the Immunogenicity of African Swine Fever F317L Protein and Screening of T Cell Epitopes. Animals 2024, 14, 1331. [Google Scholar] [CrossRef]
- Heimerman, M.E.; Murgia, M.V.; Wu, P.; Lowe, A.D.; Jia, W.; Rowland, R.R. Linear epitopes in African swine fever virus p72 recognized by monoclonal antibodies prepared against baculovirus-expressed antigen. J. Vet. Diagn. Invest. 2018, 30, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Yang, S.; Shao, J.; Zhou, G.; Ma, Y.; Wen, S.; Hou, Z.; Peng, D.; Guo, H.; Liu, W.; et al. Identification of p72 epitopes of African swine fever virus and preliminary application. Front. Microbiol. 2023, 14, 1126794. [Google Scholar] [CrossRef]
- Tesfagaber, W.; Wang, W.; Wang, L.; Zhao, R.; Zhu, Y.; Li, F.; Sun, E.; Liu, R.; Bu, Z.; Meng, G.; et al. A highly efficient blocking ELISA based on p72 monoclonal antibody for the detection of African swine fever virus antibodies and identification of its linear B cell epitope. Int. J. Biol. Macromol. 2024, 268, 131695. [Google Scholar] [CrossRef]
- Ren, D.; Ding, P.; Liu, S.; Zhang, N.; Chen, Y.; Li, Q.; Fan, L.; Chang, Z.; Zhang, G. Development and characterization of recombinant ASFV CD2v protein nanoparticle-induced monoclonal antibody. Int. J. Biol. Macromol. 2022, 209, 533–541. [Google Scholar] [CrossRef]
- Jiang, W.; Jiang, D.; Li, L.; Wang, J.; Wang, P.; Shi, X.; Zhao, Q.; Liu, B.; Ji, P.; Zhang, G. Identification of Two Novel Linear B Cell Epitopes on the CD2v Protein of African Swine Fever Virus Using Monoclonal Antibodies. Viruses 2022, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, A.; Yang, W.; Liang, C.; Zhou, J.; Chen, Y.; Liu, Y.; Zhou, Y.; Zhang, G. Expression of extracellular domain of ASFV CD2v protein in mammalian cells and identification of B cell epitopes. Virus Res. 2023, 323, 199000. [Google Scholar] [CrossRef]
- Jia, R.; Zhang, G.; Bai, Y.; Liu, H.; Chen, Y.; Ding, P.; Zhou, J.; Feng, H.; Li, M.; Tian, Y.; et al. Identification of Linear B Cell Epitopes on CD2V Protein of African Swine Fever Virus by Monoclonal Antibodies. Microbiol. Spectr. 2022, 10, e0105221. [Google Scholar] [CrossRef]
- Song, J.; Wang, M.; Du, Y.; Wan, B.; Zhang, A.; Zhang, Y.; Zhuang, G.; Ji, P.; Wu, Y.; Zhang, G. Identification of a linear B-cell epitope on the African swine fever virus CD2v protein. Int. J. Biol. Macromol. 2023, 232, 123264. [Google Scholar] [CrossRef]
- Lu, W.; Bai, Y.; Zhang, S.; Zhao, X.; Jin, J.; Zhu, X.; Wang, R.; Wu, Y.; Zhang, A.; Zhang, G.; et al. An Intracellular Epitope of ASFV CD2v Protein Elicits Humoral and Cellular Immune Responses. Animals 2023, 13, 1967. [Google Scholar] [CrossRef]
- Neilan, J.G.; Zsak, L.; Lu, Z.; Burrage, T.G.; Kutish, G.F.; Rock, D.L. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology 2004, 319, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Murgia, M.V.; Mogler, M.; Certoma, A.; Green, D.; Monaghan, P.; Williams, D.T.; Rowland, R.R.R.; Gaudreault, N.N. Evaluation of an African swine fever (ASF) vaccine strategy incorporating priming with an alphavirus-expressed antigen followed by boosting with attenuated ASF virus. Arch. Virol. 2019, 164, 359–370. [Google Scholar] [CrossRef]
- Petrovan, V.; Yuan, F.; Li, Y.; Shang, P.; Murgia, M.V.; Misra, S.; Rowland, R.R.R.; Fang, Y. Development and characterization of monoclonal antibodies against p30 protein of African swine fever virus. Virus Res. 2019, 269, 197632. [Google Scholar] [CrossRef]
- Tian, P.; Sun, Z.; Wang, M.; Song, J.; Sun, J.; Zhou, L.; Jiang, D.; Zhang, A.; Wu, Y.; Zhang, G. Identification of a novel linear B-cell epitope on the p30 protein of African swine fever virus using monoclonal antibodies. Virus Res. 2024, 341, 199328. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Alcaraz, C.; Eiras, A.; Yanez, R.J.; Rodriguez, J.M.; Alonso, C.; Rodriguez, J.F.; Escribano, J.M. Characterization and molecular basis of heterogeneity of the African swine fever virus envelope protein p54. J. Virol. 1994, 68, 7244–7252. [Google Scholar] [CrossRef]
- Desmet, C.; Coelho-Cruz, B.; Mehn, D.; Colpo, P.; Ruiz-Moreno, A. ASFV epitope mapping by high density peptides microarrays. Virus Res. 2024, 339, 199287. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Li, C.; Hou, H.; Chen, Y.; Zhang, A.; Han, S.; Wan, B.; Wu, Y.; He, H.; Wang, N.; et al. A Novel Linear B-Cell Epitope on the P54 Protein of African Swine Fever Virus Identified Using Monoclonal Antibodies. Viruses 2023, 15, 867. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, G.; Dong, H.; Wu, S.; Du, Y.; Wan, B.; Ji, P.; Wu, Y.; Jiang, D.; Zhuang, G.; et al. Identification of a Linear B Cell Epitope on p54 of African Swine Fever Virus Using Nanobodies as a Novel Tool. Microbiol. Spectr. 2023, 11, e0336222. [Google Scholar] [CrossRef]
- Dodantenna, N.; Cha, J.W.; Chathuranga, K.; Chathuranga, W.A.G.; Weerawardhana, A.; Ranathunga, L.; Kim, Y.; Jheong, W.; Lee, J.S. The African Swine Fever Virus Virulence Determinant DP96R Suppresses Type I IFN Production Targeting IRF3. Int. J. Mol. Sci. 2024, 25, 2099. [Google Scholar] [CrossRef]
- Qi, X.; Feng, T.; Ma, Z.; Zheng, L.; Liu, H.; Shi, Z.; Shen, C.; Li, P.; Wu, P.; Ru, Y.; et al. Deletion of DP148R, DP71L, and DP96R Attenuates African Swine Fever Virus, and the Mutant Strain Confers Complete Protection against Homologous Challenges in Pigs. J. Virol. 2023, 97, e0024723. [Google Scholar] [CrossRef]
- O’Donnell, V.; Risatti, G.R.; Holinka, L.G.; Krug, P.W.; Carlson, J.; Velazquez-Salinas, L.; Azzinaro, P.A.; Gladue, D.P.; Borca, M.V. Simultaneous Deletion of the 9GL and UK Genes from the African Swine Fever Virus Georgia 2007 Isolate Offers Increased Safety and Protection against Homologous Challenge. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Li, C.; Si, X.Y.; Wang, X.G.; Yan, Z.W.; Hou, H.Y.; You, L.Q.; Chen, Y.L.; Zhang, A.K.; Wang, N.; Sun, A.J.; et al. Preparation and epitope analysis of monoclonal antibodies against African swine fever virus DP96R protein. BMC Vet. Res. 2024, 20, 191. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Z.; Feng, T.; Ma, Z.; Xue, Q.; Wu, P.; Li, P.; Li, S.; Yang, F.; Cao, W.; et al. African Swine Fever Virus E120R Protein Inhibits Interferon Beta Production by Interacting with IRF3 To Block Its Activation. J. Virol. 2021, 95, e0082421. [Google Scholar] [CrossRef]
- Liu, R.; Sun, Y.; Chai, Y.; Li, S.; Li, S.; Wang, L.; Su, J.; Yu, S.; Yan, J.; Gao, F.; et al. The structural basis of African swine fever virus pA104R binding to DNA and its inhibition by stilbene derivatives. Proc. Natl. Acad. Sci. USA 2020, 117, 11000–11009. [Google Scholar] [CrossRef]
- Frouco, G.; Freitas, F.B.; Coelho, J.; Leitao, A.; Martins, C.; Ferreira, F. DNA-Binding Properties of African Swine Fever Virus pA104R, a Histone-Like Protein Involved in Viral Replication and Transcription. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, L.; Guo, S.; Li, L.; Yu, Y.; Liu, Z.; Tan, C.; Chen, H.; Wang, X. Characterization of the monoclonal antibody and the immunodominant B-cell epitope of African swine fever virus pA104R by using mouse model. Microbiol. Spectr. 2024, 12, e0140123. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, S.; Ma, C.; Yang, F.; Cao, W.; Liu, H.; Chen, X.; Feng, T.; Shi, Z.; Tian, H.; et al. African Swine Fever Virus E184L Protein Interacts with Innate Immune Adaptor STING to Block IFN Production for Viral Replication and Pathogenesis. J. Immunol. 2023, 210, 442–458. [Google Scholar] [CrossRef]
- Ramirez-Medina, E.; Vuono, E.; Rai, A.; Pruitt, S.; Espinoza, N.; Velazquez-Salinas, L.; Pina-Pedrero, S.; Zhu, J.; Rodriguez, F.; Borca, M.V.; et al. Deletion of E184L, a Putative DIVA Target from the Pandemic Strain of African Swine Fever Virus, Produces a Reduction in Virulence and Protection against Virulent Challenge. J. Virol. 2022, 96, e0141921. [Google Scholar] [CrossRef]
- Tesfagaber, W.; Lan, D.; Wang, W.; Zhao, R.; Yin, L.; Yang, M.; Zhu, Y.; Sun, E.; Liu, R.; Lin, W.; et al. Identification of two novel B cell epitopes on E184L protein of African swine fever virus using monoclonal antibodies. Virus Res. 2024, 346, 199412. [Google Scholar] [CrossRef] [PubMed]
- Urbano, A.C.; Ferreira, N.; Jordao, N.; Boinas, F.; Martins, C.; Ferreira, F. Targeted mutagenesis of the beta-strand DNA binding region of African swine fever virus histone-like protein (pA104R) impairs DNA-binding activity and antibody recognition. Antiviral Res. 2024, 221, 105784. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, M.; Zhou, L.; Tian, P.; Sun, J.; Sun, Z.; Guo, C.; Wu, Y.; Zhang, G. A novel conserved B-cell epitope in pB602L of African swine fever virus. Appl. Microbiol. Biotechnol. 2024, 108, 78. [Google Scholar] [CrossRef]
- Shi, L.F.; Ren, H.; Zhang, B.; Shi, S.Y.; Shao, H.C.; Xing, H.; Zhao, Y.Y.; Lin, Z.Z.; Zhang, Y.; Han, S.; et al. Preparation and epitope mapping of monoclonal antibodies against African swine fever virus p22 protein. Int. J. Biol. Macromol. 2024, 255, 128111. [Google Scholar] [CrossRef]
- Zhang, S.J.; Niu, B.; Liu, S.M.; Zhu, Y.M.; Zhao, D.M.; Bu, Z.G.; Hua, R.H. Identification of Two Linear Epitopes on MGF_110-13L Protein of African Swine Fever Virus with Monoclonal Antibodies. Animals 2024, 14, 1951. [Google Scholar] [CrossRef]
- Burmakina, G.; Malogolovkin, A.; Tulman, E.R.; Xu, W.; Delhon, G.; Kolbasov, D.; Rock, D.L. Identification of T-cell epitopes in African swine fever virus CD2v and C-type lectin proteins. J. Gen. Virol. 2019, 100, 259–265. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, B.; Qian, N.; Zhang, F.; Tan, X.; Lei, J.; Xiang, Y. Structure of the African swine fever virus major capsid protein p72. Cell Res. 2019, 29, 953–955. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, H.; Fan, W.; He, L.; Chen, T.; Zhou, X.; Qi, Y.; Sun, L.; Hu, R.; Luo, T.; et al. Evaluation of Cellular Immunity with ASFV Infection by Swine Leukocyte Antigen (SLA)-Peptide Tetramers. Viruses 2021, 13, 2264. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.; Feng, T.; Zhang, X.; Yang, F.; Cao, W.; Chen, H.; Liu, H.; Zhang, K.; Zhu, Z.; et al. African Swine Fever Virus F317L Protein Inhibits NF-kappaB Activation To Evade Host Immune Response and Promote Viral Replication. mSphere 2021, 6, e0065821. [Google Scholar] [CrossRef]
- Xu, L.; Hao, F.; Jeong, D.G.; Chen, R.; Gan, Y.; Zhang, L.; Yeom, M.; Lim, J.W.; Yu, Y.; Bai, Y.; et al. Mucosal and cellular immune responses elicited by nasal and intramuscular inoculation with ASFV candidate immunogens. Front. Immunol. 2023, 14, 1200297. [Google Scholar] [CrossRef]
- Dodantenna, N.; Ranathunga, L.; Chathuranga, W.A.G.; Weerawardhana, A.; Cha, J.W.; Subasinghe, A.; Gamage, N.; Haluwana, D.K.; Kim, Y.; Jheong, W.; et al. African Swine Fever Virus EP364R and C129R Target Cyclic GMP-AMP to Inhibit the cGAS-STING Signaling Pathway. J. Virol. 2022, 96, e0102222. [Google Scholar] [CrossRef]
- Zhai, W.; Huang, Y.; He, Y.; Chu, Y.; Tao, C.; Pang, Z.; Wang, Z.; Zhu, H.; Jia, H. Immunogenicity Analysis and Identification of Potential T-Cell Epitopes in C129R Protein of African Swine Fever Virus. Microorganisms 2024, 12, 1056. [Google Scholar] [CrossRef]
- Lokhandwala, S.; Petrovan, V.; Popescu, L.; Sangewar, N.; Elijah, C.; Stoian, A.; Olcha, M.; Ennen, L.; Bray, J.; Bishop, R.P.; et al. Adenovirus-vectored African Swine Fever Virus antigen cocktails are immunogenic but not protective against intranasal challenge with Georgia 2007/1 isolate. Vet. Microbiol. 2019, 235, 10–20. [Google Scholar] [CrossRef]
- Zajac, M.D.; Trujillo, J.D.; Yao, J.; Kumar, R.; Sangewar, N.; Lokhandwala, S.; Sang, H.; Mallen, K.; McCall, J.; Burton, L.; et al. Immunization of pigs with replication-incompetent adenovirus-vectored African swine fever virus multi-antigens induced humoral immune responses but no protection following contact challenge. Front. Vet. Sci. 2023, 10, 1208275. [Google Scholar] [CrossRef]
- Levast, B.; Awate, S.; Babiuk, L.; Mutwiri, G.; Gerdts, V.; van Drunen Littel-van den Hurk, S. Vaccine Potentiation by Combination Adjuvants. Vaccines 2014, 2, 297–322. [Google Scholar] [CrossRef]
- Pikalo, J.; Porfiri, L.; Akimkin, V.; Roszyk, H.; Pannhorst, K.; Kangethe, R.T.; Wijewardana, V.; Sehl-Ewert, J.; Beer, M.; Cattoli, G.; et al. Vaccination With a Gamma Irradiation-Inactivated African Swine Fever Virus Is Safe But Does Not Protect against a Challenge. Front. Immunol. 2022, 13, 832264. [Google Scholar] [CrossRef]
- Andrianarivo, A.G.; Rowe, J.D.; Barr, B.C.; Anderson, M.L.; Packham, A.E.; Sverlow, K.W.; Choromanski, L.; Loui, C.; Grace, A.; Conrad, P.A. A POLYGEN-adjuvanted killed Neospora caninum tachyzoite preparation failed to prevent foetal infection in pregnant cattle following i.v./i.m. experimental tachyzoite challenge. Int. J. Parasitol. 2000, 30, 985–990. [Google Scholar] [CrossRef]
- Blome, S.; Gabriel, C.; Beer, M. Modern adjuvants do not enhance the efficacy of an inactivated African swine fever virus vaccine preparation. Vaccine 2014, 32, 3879–3882. [Google Scholar] [CrossRef]
- Hofacre, C.L.; Rosales, A.G.; Costa, M.D.; Cookson, K.; Schaeffer, J.; Jones, M.K. Immunity and Protection Provided by Live Modified Vaccines Against Paratyphoid Salmonella in Poultry-An Applied Perspective. Avian Dis. 2021, 65, 295–302. [Google Scholar] [CrossRef]
- Mancera Gracia, J.C.; Pearce, D.S.; Masic, A.; Balasch, M. Influenza A Virus in Swine: Epidemiology, Challenges and Vaccination Strategies. Front. Vet. Sci. 2020, 7, 647. [Google Scholar] [CrossRef]
- Renukaradhya, G.J.; Meng, X.J.; Calvert, J.G.; Roof, M.; Lager, K.M. Live porcine reproductive and respiratory syndrome virus vaccines: Current status and future direction. Vaccine 2015, 33, 4069–4080. [Google Scholar] [CrossRef]
- Lokhandwala, S.; Waghela, S.D.; Bray, J.; Martin, C.L.; Sangewar, N.; Charendoff, C.; Shetti, R.; Ashley, C.; Chen, C.H.; Berghman, L.R.; et al. Induction of Robust Immune Responses in Swine by Using a Cocktail of Adenovirus-Vectored African Swine Fever Virus Antigens. Clin. Vaccine Immunol. 2016, 23, 888–900. [Google Scholar] [CrossRef]
- Cadenas-Fernandez, E.; Sanchez-Vizcaino, J.M.; Kosowska, A.; Rivera, B.; Mayoral-Alegre, F.; Rodriguez-Bertos, A.; Yao, J.; Bray, J.; Lokhandwala, S.; Mwangi, W.; et al. Adenovirus-vectored African Swine Fever Virus Antigens Cocktail Is Not Protective against Virulent Arm07 Isolate in Eurasian Wild Boar. Pathogens 2020, 9, 171. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Ott, G.S.; Nest, G.V.; Rappuoli, R.; Giudice, G.D. The history of MF59((R)) adjuvant: A phoenix that arose from the ashes. Expert. Rev. Vaccines 2013, 12, 13–30. [Google Scholar] [CrossRef]
- Cadenas-Fernandez, E.; Sanchez-Vizcaino, J.M.; van den Born, E.; Kosowska, A.; van Kilsdonk, E.; Fernandez-Pacheco, P.; Gallardo, C.; Arias, M.; Barasona, J.A. High Doses of Inactivated African Swine Fever Virus Are Safe, but Do Not Confer Protection against a Virulent Challenge. Vaccines 2021, 9, 242. [Google Scholar] [CrossRef]
- Leitao, A.; Malur, A.; Cornelis, P.; Martins, C.L. Identification of a 25-aminoacid sequence from the major African swine fever virus structural protein VP72 recognised by porcine cytotoxic T lymphocytes using a lipoprotein based expression system. J. Virol. Methods 1998, 75, 113–119. [Google Scholar] [CrossRef]
- Leitao, A.; Malur, A.; Cartaxeiro, C.; Vasco, G.; Cruz, B.; Cornelis, P.; Martins, C.L. Bacterial lipoprotein based expression vectors as tools for the characterisation of African swine fever virus (ASFV) antigens. Arch. Virol. 2000, 145, 1639–1657. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, P.; Song, T.; Yang, K.; Yuan, F.; Gao, T.; Liu, Z.; Li, C.; Guo, R.; Xiao, S.; et al. A Recombinant Chimera Vaccine Composed of LTB and Mycoplasma hyopneumoniae Antigens P97R1, mhp390 and P46 Elicits Cellular Immunologic Response in Mice. Vaccines 2023, 11, 1291. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, S.; Zhang, H.; Shen, Y.; Zhang, P.; Shan, H.; Cai, X. Orally administered recombinant Lactobacillus expressing African swine fever virus antigens that induced immunity responses. Front. Microbiol. 2022, 13, 1103327. [Google Scholar] [CrossRef]
- Liao, Z.; Huang, J.; Lo, P.C.; Lovell, J.F.; Jin, H.; Yang, K. Self-adjuvanting cancer nanovaccines. J. Nanobiotechnol. 2022, 20, 345. [Google Scholar] [CrossRef]
- Lu, H.; Zhou, X.; Wu, Z.; Zhang, X.; Zhu, L.; Guo, X.; Zhang, Q.; Zhu, S.; Zhu, H.; Sun, H. Comparison of the mucosal adjuvanticities of two Toll-like receptor ligands for recombinant adenovirus-delivered African swine fever virus fusion antigens. Vet. Immunol. Immunopathol. 2021, 239, 110307. [Google Scholar] [CrossRef]
- Huang, Q.; Niu, T.; Zou, B.; Wang, J.; Xin, J.; Niu, H.; Li, N.; Jiang, Y.; Bao, J.; Zhang, D.; et al. Lactobacillus plantarum Surface-Displayed ASFV (p14.5) Can Stimulate Immune Responses in Mice. Vaccines 2022, 10, 355. [Google Scholar] [CrossRef]
- Gallardo, C.; Fernandez-Pinero, J.; Arias, M. African swine fever (ASF) diagnosis, an essential tool in the epidemiological investigation. Virus Res. 2019, 271, 197676. [Google Scholar] [CrossRef]
- Li, L.; Qiao, S.; Li, G.; Tong, W.; Dong, S.; Liu, J.; Guo, Z.; Zheng, H.; Zhao, R.; Tong, G.; et al. The Indirect ELISA and Monoclonal Antibody against African Swine Fever Virus p17 Revealed Efficient Detection and Application Prospects. Viruses 2022, 15, 50. [Google Scholar] [CrossRef]
- Afayibo, D.J.A.; Zhang, Z.; Sun, H.; Fu, J.; Zhao, Y.; Amuda, T.O.; Wu, M.; Du, J.; Guan, G.; Niu, Q.; et al. Establishment of an ELISA Based on a Recombinant Antigenic Protein Containing Multiple Prominent Epitopes for Detection of African Swine Fever Virus Antibodies. Microorganisms 2024, 12, 943. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Q.; Liu, Y.; Wang, M.; Zhang, L.; Han, L.; Chu, X.; Ding, G.; Li, Y.; Hou, Y.; et al. Indirect ELISA Using Multi-Antigenic Dominants of p30, p54 and p72 Recombinant Proteins to Detect Antibodies against African Swine Fever Virus in Pigs. Viruses 2022, 14, 2660. [Google Scholar] [CrossRef]
- Rossjohn, J.; Gras, S.; Miles, J.J.; Turner, S.J.; Godfrey, D.I.; McCluskey, J. T cell antigen receptor recognition of antigen-presenting molecules. Annu. Rev. Immunol. 2015, 33, 169–200. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Li, F.; Zhang, Z.; Chen, W.; Zhang, X.; Sun, E.; Zhu, Y.; Liu, R.; He, X.; et al. The attenuated African swine fever vaccine HLJ/18-7GD provides protection against emerging prevalent genotype II variants in China. Emerg. Microbes Infect. 2024, 13, 2300464. [Google Scholar] [CrossRef]
- Argilaguet, J.M.; Perez-Martin, E.; Nofrarias, M.; Gallardo, C.; Accensi, F.; Lacasta, A.; Mora, M.; Ballester, M.; Galindo-Cardiel, I.; Lopez-Soria, S.; et al. DNA vaccination partially protects against African swine fever virus lethal challenge in the absence of antibodies. PLoS ONE 2012, 7, e40942. [Google Scholar] [CrossRef]
- Urbano, A.C.; Ferreira, F. African swine fever control and prevention: An update on vaccine development. Emerg. Microbes Infect. 2022, 11, 2021–2033. [Google Scholar] [CrossRef]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Rai, A.; Espinoza, N.; Velazquez-Salinas, L.; Gladue, D.P. ASF Vaccine Candidate ASFV-G-∆I177L Does Not Exhibit Residual Virulence in Long-Term Clinical Studies. Pathogens 2023, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Shawan, M.; Sharma, A.R.; Halder, S.K.; Arian, T.A.; Shuvo, M.N.; Sarker, S.R.; Hasan, M.A. Advances in Computational and Bioinformatics Tools and Databases for Designing and Developing a Multi-Epitope-Based Peptide Vaccine. Int. J. Pept. Res. Ther. 2023, 29, 60. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Cai, J.H.; Diao, H.Y.; Guo, W.M.; Yang, X.; Xing, S. The progress of peptide vaccine clinical trials in gynecologic oncology. Hum. Vaccin. Immunother. 2022, 18, 2062982. [Google Scholar] [CrossRef] [PubMed]
- Simbulan, A.M.; Banico, E.C.; Sira, E.; Odchimar, N.M.O.; Orosco, F.L. Immunoinformatics-guided approach for designing a pan-proteome multi-epitope subunit vaccine against African swine fever virus. Sci. Rep. 2024, 14, 1354. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Samuel Leon Magdaleno, J.; Rajjak Shaikh, A.; Choowongkomon, K.; Li, V.; Lee, Y.; Kim, H. Designing a multi-epitope candidate vaccine by employing immunoinformatics approaches to control African swine fever spread. J. Biomol. Struct. Dyn. 2023, 41, 10214–10229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).