Identification of Potential Vectors and Detection of Rift Valley Fever Virus in Mosquitoes Collected Before and During the 2022 Outbreak in Rwanda
Abstract
:1. Introduction
2. Materials and Methods
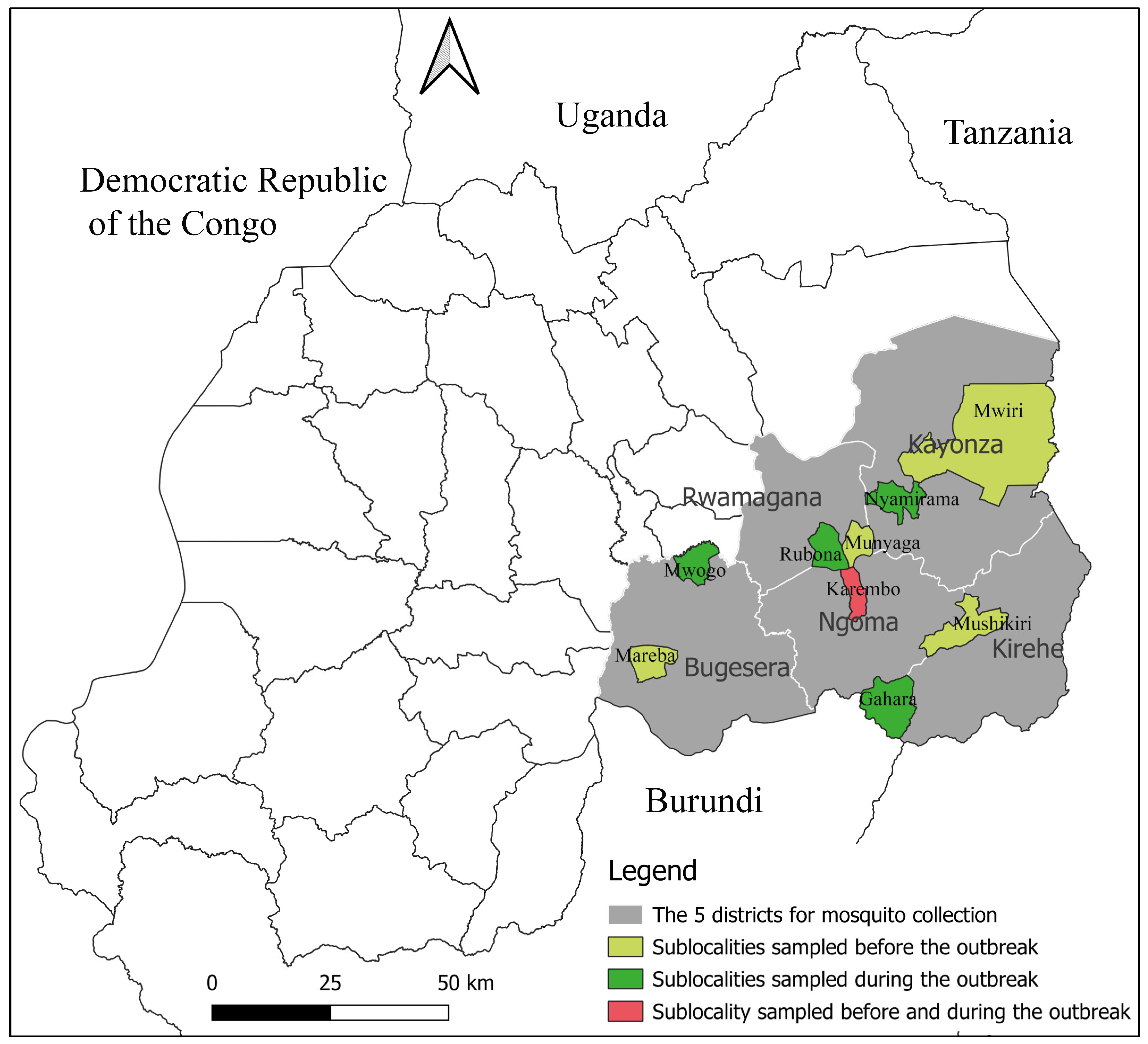
2.1. Study Area and Design
2.2. Mosquito Collection and Identification
2.3. Mosquito Homogenization
2.4. Viral RNA Extraction and RVFV Detection by RT-PCR
2.5. Virus Isolation on Vero Cells
2.6. RVFV RNA Sequencing
2.7. Data Analysis
3. Results
3.1. Mosquito Distribution
3.2. Trapped Mosquito Species and Their Abundance
3.3. RVFV Detection and Isolation
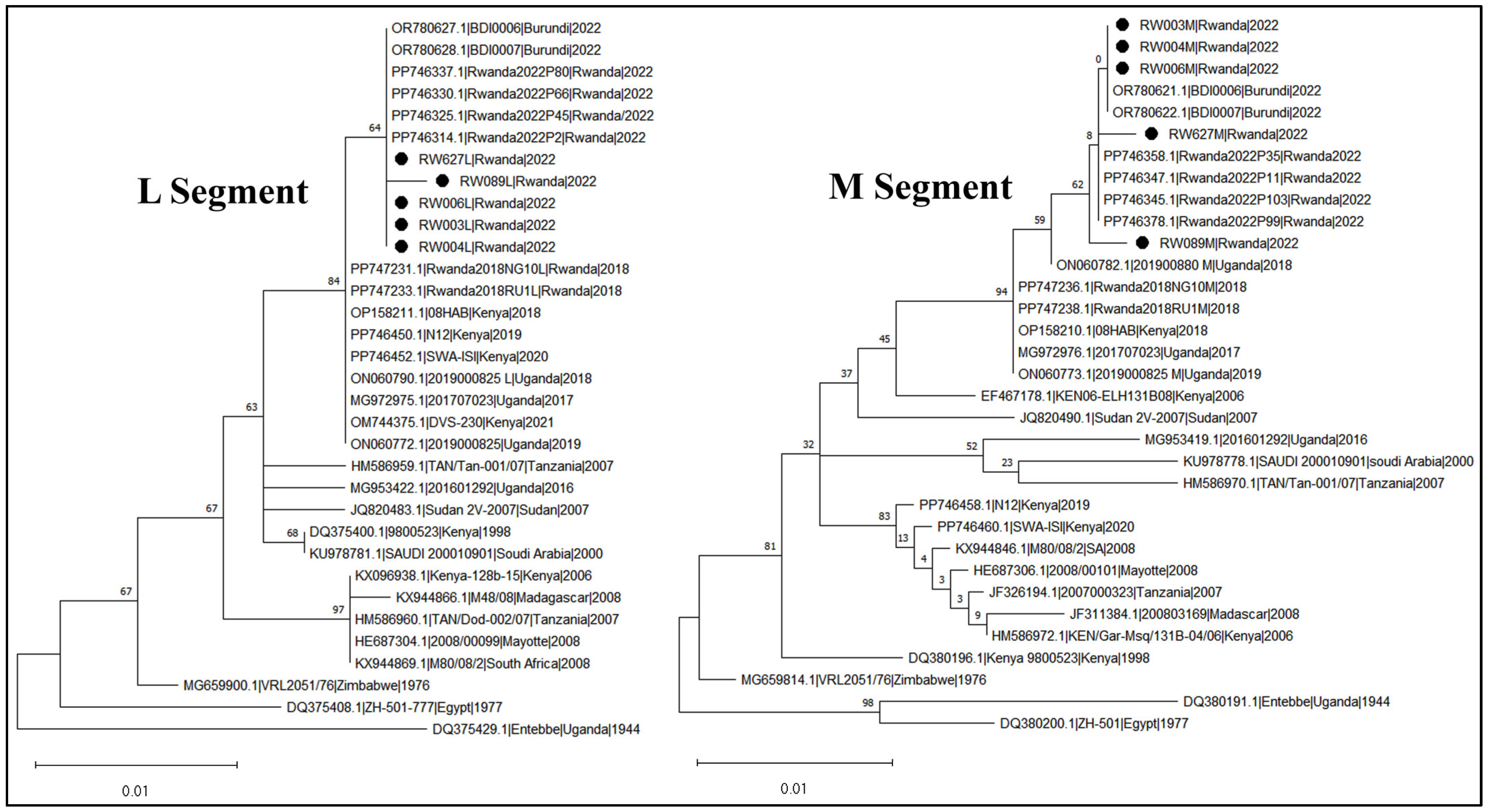
3.4. RVFV Sequencing and Phylogeny
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuhn, J.H.; Brown, K.; Adkins, S.; de la Torre, J.C.; Digiaro, M.; Ergünay, K.; Firth, A.E.; Hughes, H.R.; Junglen, S.; Lambert, A.J.; et al. Promotion of order Bunyavirales to class Bunyaviricetes to accommodate a rapidly increasing number of related polyploviricotine viruses. J. Virol. 2024, 98, e01069-24. [Google Scholar] [CrossRef]
- Daubney, R.; Hudson, J.R. Virus Disease of Sheep. J. Path 1931, XXXIV, 545. [Google Scholar] [CrossRef]
- WHO Rift Valley Fever Key Facts. 2018. Available online: https://www.who.int/health-topics/rift-valley-fever#tab=tab_1 (accessed on 1 April 2024).
- OIE Aetiology, Epidemiology, Diagnosis, Prevention and Control References. OIE Tech Dis Cards. 2019. Available online: https://www.woah.org/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/RIFT_VALLEY_FEVER.pdf (accessed on 16 June 2024).
- Anywaine, Z.; Lule, S.A.; Hansen, C.; Warimwe, G.; Elliott, A. Clinical manifestations of Rift Valley fever in humans: Systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2022, 16, e0010233. [Google Scholar] [CrossRef]
- Sindato, C.; Karimuribo, E.D.; Pfeiffer, D.U.; Mboera, L.E.G.; Kivaria, F.; Dautu, G.; Bernard, B.; Paweska, J.T. Spatial and temporal pattern of Rift Valley fever outbreaks in Tanzania; 1930 to 2007. PLoS ONE 2014, 9, e88897. [Google Scholar] [CrossRef]
- Murithi, R.M.; Munyua, P.; Ithondeka, P.M.; Macharia, J.M.; Hightower, A.; Luman, E.T.; Breiman, R.F.; Njenga, M.K. Rift Valley fever in Kenya: History of epizootics and identification of vulnerable districts. Epidemiol. Infect. 2011, 139, 372–380. [Google Scholar] [CrossRef]
- Carroll, S.A.; Reynes, J.; Khristova, M.L.; Andriamandimby, S.F.; Rollin, P.E.; Nichol, S.T. Genetic Evidence for Rift Valley fever Outbreaks in Madagascar Resulting from Virus Introductions from the East African Mainland rather than Enzootic Maintenance. J. Virol. 2011, 85, 6162–6167. [Google Scholar] [CrossRef]
- Kasye, M.; Teshome, D.; Eshetu, A.A. A Review on Rift Valley fever on Animal, Human Health and its Impact on Live Stock Marketing. Austin Virol. Retrovirol. 2016, 3, 1020. [Google Scholar]
- Caminade, C.; Ndione, J.A.; Diallo, M.; MacLeod, D.A.; Faye, O.; Ba, Y.; Dia, I.; Morse, A.P. Rift valley fever outbreaks in Mauritania and related environmental conditions. Int. J. Environ. Res. Public Health 2014, 11, 903–918. [Google Scholar] [CrossRef]
- Glancey, M.M.; Anyamba, A.; Linthicum, K.J. Epidemiologic and Environmental Risk Factors of Rift Valley fever in Southern Africa from 2008 to 2011. Vector-Borne Zoonotic Dis. 2015, 15, 502–511. [Google Scholar] [CrossRef]
- Sang, R.; Kioko, E.; Lutomiah, J.; Warigia, M.; Ochieng, C.; O’Guinn, M.; Lee, J.S.; Koka, H.; Godsey, M.; Hoel, D.; et al. Rift Valley fever virus epidemic in Kenya, 2006/2007, The entomologic investigations. Am. J. Trop. Med. Hyg. 2010, 83 (Suppl. 2), 28–37. [Google Scholar] [CrossRef]
- Anyamba, A.; Chretien, J.-P.; Small, J.; Tucker, C.J.; Formenty, P.B.; Richardson, J.H.; Britch, S.C.; Schnabel, D.C.; Erickson, R.L.; Linthicum, K.J. Prediction of a Rift Valley fever outbreak. Proc. Natl. Acad. Sci. USA 2009, 106, 955–959. [Google Scholar] [CrossRef]
- Linthicum, K.J.; Anyamba, A.; Tucker, C.J.; Kelley, P.W.; Myers, M.F.; Peters, C.J. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science 1999, 285, 397–400. [Google Scholar] [CrossRef]
- Mweya, C.N.; Kimera, S.I.; Stanley, G.; Misinzo, G.; Mboera, L.E.G. Climate change influences potential distribution of infected Aedes aegypti co-occurrence with dengue epidemics risk areas in Tanzania. PLoS ONE 2016, 11, e0162649. [Google Scholar] [CrossRef]
- Mweya, C.N.; Mboera, L.E.G.; Kimera, S.I. Climate influence on emerging risk areas for Rift Valley fever epidemics in Tanzania. Am. J. Trop. Med. Hyg. 2017, 97, 109–114. [Google Scholar] [CrossRef]
- Anyamba, A.; Damoah, R.; Kemp, A.; Small, J.L.; Rostal, M.K.; Bagge, W.; Cordel, C.; Brand, R.; Karesh, W.B.; Paweska, J.T. Climate Conditions During a Rift Valley Fever Post-epizootic Period in Free State, South Africa, 2014–2019. Front. Vet. Sci. 2022, 8, 730424. [Google Scholar] [CrossRef]
- Iacono, G.L.; Cunningham, A.A.; Bett, B.; Grace, D.; Redding, D.W.; Wood, J.L.N. Environmental limits of Rift Valley fever revealed using ecoepidemiological mechanistic models. Proc. Natl. Acad. Sci. USA 2018, 115, E7448–E7456. [Google Scholar] [CrossRef]
- Rissmann, M.; Stoek, F.; Pickin, M.J.; Groschup, M.H. Mechanisms of inter-epidemic maintenance of Rift Valley fever phlebovirus. Antivir. Res. 2020, 174, 104692. [Google Scholar] [CrossRef]
- Bird, B.H.; McElroy, A.K. Rift Valley fever virus: Unanswered questions. Antivir. Res. 2016, 132, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Lumley, S.; Horton, D.L.; Hernandez-Triana, L.L.M.; Johnson, N.; Fooks, A.R.; Hewson, R. Rift Valley fever virus: Strategies for maintenance, survival and vertical transmission in mosquitoes. J. Gen. Virol. 2017, 98, 875–887. [Google Scholar] [CrossRef]
- Linthicum, K.J.; Davies, F.G.; Kairo, A.; Bailey, C.L. Rift Valley fever virus (family Bunyaviridae, genus Phlebovirus). Isolations from Diptera collected during an inter-epizootic period in Kenya. J. Hyg. 1985, 95, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Linthicum, K.J.; Britch, S.C.; Anyamba, A. Rift Valley Fever: An Emerging Mosquito-Borne Disease. Annu. Rev. Entomol. 2016, 61, 395–415. [Google Scholar] [CrossRef] [PubMed]
- Kariuki Njenga, M.; Bett, B. Rift Valley Fever Virus—How and Where Virus Is Maintained During Inter-epidemic Periods. Curr. Clin. Microbiol. Rep. 2019, 6, 18–24. [Google Scholar] [CrossRef]
- Tantely, L.M.; Boyer, S.; Fontenille, D. A Review of Mosquitoes Associated with Rift Valley fever virus in Madagascar. Am. J. Trop. Med. Hyg. 2015, 92, 722–729. [Google Scholar] [CrossRef]
- Tantely, L.M.; Andriamandimby, S.F.; Ambinintsoa, M.F.; Raharinirina, M.R.; Rafisandratantsoa, J.T.; Ravalohery, J.-P.; Harimanana, A.; Ranoelison, N.N.; Irinantenaina, J.; Ankasitrahana, M.F.; et al. An Entomological Investigation during a Recent Rift Valley fever Epizootic/Epidemic Reveals New Aspects of the Vectorial Transmission of the Virus in Madagascar. Pathogens 2024, 13, 258. [Google Scholar] [CrossRef]
- Lutomiah, J.; Omondi, D.; Masiga, D.; Mutai, C.; Mireji, P.O.; Ongus, J.; Linthicum, K.J.; Sang, R. Blood meal analysis and virus detection in blood-fed mosquitoes collected during the 2006–2007 Rift Valley fever outbreak in Kenya. Vector-Borne Zoonotic Dis. 2014, 14, 656–664. [Google Scholar] [CrossRef]
- Fontenille, D.; Diallo, M.; Thonnon, J.; Digoutte, J.P.; Zeller, H.G. New Vectors of Rift Valley Fever in West Africa. Emerg. Infect. Dis. 1998, 4, 289–293. [Google Scholar] [CrossRef]
- Diallo, M.; Nabeth, P.; Ba, K.; Sall, A.A.; Ba, Y.; Mondo, M.; Girault, L.; Abdalahi, M.O.; Mathiot, C. Mosquito vectors of the 1998–1999 outbreak of Rift Valley fever and other arboviruses (Bagaza, Sanar, Wesselsbron and West Nile) in Mauritania and Senegal. Med. Vet. Entomol. 2005, 19, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Meegan, J.M. The Rift Valley fever epizootic in Egypt 1977–1978 1. Description of the epizootic and virological studies. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 618–623. [Google Scholar] [CrossRef]
- Jupp, P.G.; Kemp, A.; Grobbelaar, A.; Leman, P.; Burt, F.J.; AlAhmed, A.M.; Al Mujalli, D.; Al Khamees, M.; Swanepoel, R. The 2000 epidemic of Rift Valley fever in Saudi Arabia: Mosquito vector studies. Med. Vet. Entomol. 2002, 16, 245–252. [Google Scholar] [CrossRef]
- Henninger, S.M. Does the global warming modify the local Rwandan climate? Nat. Sci. 2013, 5, 124–129. [Google Scholar] [CrossRef]
- Republic of Rwanda, Ministry of Agriculture and Animal Resources (MINAGRI). Annual Report 2021–2022. Available online: https://www.minagri.gov.rw/publications/reports (accessed on 25 July 2024).
- Republic of Rwanda, Ministry of Agriculture and Animal Resources (MINAGRI). Annual Report 2018–2019. Available online: https://www.minagri.gov.rw/publications/reports (accessed on 26 July 2024).
- NISR The Republic of Rwanda. Agricultural Household Survey 2020 Report; NISR The Republic of Rwanda: Kigali, Rwanda, 2021. [Google Scholar]
- Umuhoza, T.; Berkvens, D.; Gafarasi, I.; Rukelibuga, J.; Mushonga, B.; Biryomumaisho, S. Seroprevalence of Rift Valley fever in cattle along the Akagera-Nyabarongo rivers, Rwanda. J. S. Afr. Vet. Assoc. 2017, 88, 1–5. [Google Scholar] [CrossRef]
- Muturi, E.J.; Mwangangi, J.; Shililu, J.; Muriu, S.; Jacob, B.; Kabiru, E.; Gu, W.; Mbogo, C.; Githure, J.; Novak, R. Mosquito Species Succession and Physicochemical Factors Affecting their Abundance in Rice Fields in Mwea, Kenya. J. Med. Entomol. 2007, 44, 336–344. [Google Scholar] [CrossRef]
- Rwanda Agriculture and Animal Resources Development Board (RAB). Annual Report 2012–2013. 2013. Available online: https://www.rab.gov.rw/index.php?eID=dumpFile&t=f&f=67225&token=a888ab974e8fc10e70b622866a1fe15f21562166 (accessed on 19 August 2024).
- Dutuze, M.F.; Ingabire, A.; Gafarasi, I.; Uwituze, S. Identification of Bunyamwera and Possible Other Orthobunyavirus Infections and Disease in Cattle during a Rift Valley fever Outbreak in Rwanda in 2018. Am. J. Trop. Med. Hyg. 2020, 103, 183–189. [Google Scholar] [CrossRef]
- Nsengimana, I.; Juma, J.; Roesel, K.; Gasana, M.N.; Ndayisenga, F.; Muvunyi, C.M.; Hakizimana, E.; Hakizimana, J.N.; Eastwood, G.; Chengula, A.A.; et al. Genomic Epidemiology of Rift Valley fever virus Involved in the 2018 and 2022 Outbreaks in Livestock in Rwanda. Viruses 2024, 16, 1148. [Google Scholar] [CrossRef]
- Lord, C.C.; Bustamante, D.M. Sources of Error in the Estimation of Mosquito Infection Rates Used to Assess Risk of Arbovirus Transmission. Am. J. Trop. Med. Hyg. 2010, 82, 1172–1184. [Google Scholar]
- Steven, L.M.K. Analysis of the effect of human-wildlife conflict on the conservation of flora and fauna in Akagera National Park-rwanda. Int. J. Soc. Sci. Econ. Res. 2021, 6, 771–786. [Google Scholar] [CrossRef]
- Smallegange, R.C.; Schmied, W.H.; van Roey, K.J.; Verhulst, N.O.; Spitzen, J.; Mukabana, W.R.; Takken, W. Sugar-fermenting yeast as an organic source of carbon dioxide to attract the malaria mosquito Anopheles gambiae. Malar. J. 2010, 9, 292. [Google Scholar] [CrossRef]
- Huang, Y.-M. A pictorial key for the identification of the subfamilies of culicidae, genera of culicinae, and subgenera of Aedes mosquitoes of the afrotropical region (diptera: Culicidae). Proc. Entomol. Soc. Wash. 2001, 103, 1–53. [Google Scholar]
- Huang, Y.M.; Rueda, L.M. Pictorial keys to the sections, groups, and species of the Aedes (Finlaya) in the Afrotropical Region (Diptera: Culicidae). Zootaxa 2017, 4221, 131–141. [Google Scholar] [CrossRef]
- Edwards, F.W. Mosquitoes of the Ethiopian Region. Nature 1941, 138, 592–593. [Google Scholar]
- Ibrahim, M.S.; Turell, M.J.; Knauert, F.K.; Lofts, R.S. Detection of Rift Valley fever virus in mosquitoes by RT-PCR. Mol. Cell Probes 1997, 11, 49–53. [Google Scholar] [CrossRef]
- Grobbelaar, A.A.; Weyer, J.; Leman, P.A.; Kemp, A.; Paweska, J.T.; Swanepoel, R. Molecular epidemiology of Rift Valley fever virus. Emerg. Infect. Dis. 2011, 17, 2270–2276. [Google Scholar] [CrossRef]
- Genomics Sequencing Center | Fralin Life Sciences Institute | Virginia Tech. Available online: https://fralinlifesci.vt.edu/Core-Services/genomics-sequencing-center.html (accessed on 27 June 2024).
- Center for Diseases Control (CDC). Mosquito Surveillance Software | Mosquitoes | CDC. Available online: https://www.cdc.gov/mosquitoes/php/toolkit/mosquito-surveillance-software.html (accessed on 20 June 2024).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11, Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Remera, E.; Rwagasore, E.; Muvunyi, C.M.; Ahmed, A. Emergence of the first molecularly confirmed outbreak of Rift Valley fever among humans in Rwanda, calls for institutionalizing the One Health strategy. IJID One Health 2024, 4, 100035. [Google Scholar] [CrossRef]
- Hakizimana, E.; Karema, C.; Munyakanage, D.; Githure, J.; Mazarati, J.B.; Tongren, J.E.; Takken, W.; Binagwaho, A.; Koenraadt, C.J. Spatio-temporal distribution of mosquitoes and risk of malaria infection in Rwanda. Acta Trop. 2018, 182, 149–157. [Google Scholar] [CrossRef]
- Ministry of Health. Risk Assessment on Yellow Fever Virus Circulation in Rwanda; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Shoemaker, T.R.; Nyakarahuka, L.; Balinandi, S.; Ojwang, J.; Tumusiime, A.; Mulei, S.; Kyondo, J.; Lubwama, B.; Sekamatte, M.; Namutebi, A.; et al. First laboratory-confirmed outbreak of human and animal Rift Valley fever virus in Uganda in 48 years. Am. J. Trop. Med. Hyg. 2019, 100, 659–671. [Google Scholar] [CrossRef]
- Balenghien, T.; Cardinale, E.; Chevalier, V.; Elissa, N.; Failloux, A.-B.; Nipomichene, T.N.J.J.; Nicolas, G.; Rakotoharinome, V.M.; Roger, M.; Zumbo, B. Towards a better understanding of Rift Valley fever epidemiology in the south-west of the Indian Ocean. Vet. Res. 2013, 44, 78. [Google Scholar] [CrossRef]
- LaBeaud, A.D.; Sutherland, L.J.; Muiruri, S.; Muchiri, E.M.; Gray, L.R.; Zimmerman, P.A.; Hise, A.G.; King, C.H. Arbovirus prevalence in mosquitoes, Kenya. Emerg. Infect. Dis. 2011, 17, 233–241. [Google Scholar] [CrossRef]
- Karungu, S.; Atoni, E.; Ogalo, J.; Mwaliko, C.; Agwanda, B.; Yuan, Z.; Hu, X. Mosquitoes of etiological concern in Kenya and possible control strategies. Insects 2019, 10, 173. [Google Scholar] [CrossRef]
- Tantely, M.L.; Le Goff, G.; Boyer, S.; Fontenille, D. An updated checklist of mosquito species (Diptera: Culicidae) from Madagascar. Parasite 2016, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Mhina, A.D.; Kasanga, C.J.; Sindato, C.; Karimuribo, E.D.; Mboera, L.E.G. Rift Valley fever potential mosquito vectors and their infection status in Ngorongoro District in northern Tanzania. Tanzan. J. Health Res. 2015, 17. [Google Scholar] [CrossRef]
- Stoek, F.; Barry, Y.; Ba, A.; Schulz, A.; Rissmann, M.; Wylezich, C.; Sadeghi, B.; Beyit, A.D.; Eisenbarth, A.; N’diaye, F.B.; et al. Mosquito survey in Mauritania: Detection of Rift Valley fever virus and dengue virus and the determination of feeding patterns. PLoS Negl. Trop. Dis. 2022, 16, e0010203. [Google Scholar] [CrossRef]
- Seufi, A.E.M.; Galal, F.H. Role of Culex and Anopheles mosquito species as potential vectors of Rift Valley fever virus in Sudan outbreak, 2007. BMC Infect. Dis. 2010, 10, 65. [Google Scholar] [CrossRef]
- Mohamed, R.A.; Konozy, E.; Rayah, E.E. Rift Valley Fever Virus (RVFv) Dissemination Inside Mosquitoes and Investigation of the Influence of Climate on Mosquitoes Abundance. 2013. Available online: https://www.semanticscholar.org/paper/Rift-Valley-Fever-Virus-(RVFv)-Dissemination-inside-Mohamed-Konozy/c4b819b8756db3f11117eb78860f86eca52da309 (accessed on 30 October 2024).
- Turell, M.J.; Linthicum, K.J.; Patrican, L.A.; Davies, F.G.; Kairo, A.; Bailey, C.L. Vector Competence of Selected African Mosquito (Diptera: Culicidae) Species for Rift Valley fever virus. J. Med. Entomol. 2008, 45, 102–108. [Google Scholar] [CrossRef]
- Turell, M.J.; Lee, J.S.; Richardson, J.H.; Sang, R.C.; Kioko, E.N.; Agawo, M.O.; Pecor, J.; O’Guinn, M.L. Vector competence of kenyan Culex zombaensis and Culex quinquefasciatus mosquitoes for Rift Valley fever virus. J. Am. Mosq. Control Assoc. 2007, 23, 378–382. [Google Scholar] [CrossRef]
- Ndiaye, E.H.; Fall, G.; Gaye, A.; Bob, N.S.; Talla, C.; Diagne, C.T.; Diallo, D.; Ba, Y.; Dia, I.; Kohl, A.; et al. Vector competence of Aedes vexans (Meigen), Culex poicilipes (Theobald) and Cx. quinquefasciatus Say from Senegal for West and East African lineages of Rift Valley fever virus. Parasit. Vectors 2016, 9, 94. [Google Scholar] [CrossRef]
- McIntosh, B.M.; Jupp, P.; Santos, D.; Barnard, H. Vector studies on Rift Valley fever virus in South Africa. S. Afr. Med. J. 1980, 58, 127–132. [Google Scholar]
- Turell, M.J.; Kay, B.H. Susceptibility of Selected Strains of Australian Mosquitoes (Diptera: Culicidae) to Rift Valley fever virus. J. Med. Entomol. 1998, 35, 132–135. [Google Scholar] [CrossRef]
- Moutailler, S.; Krida, G.; Schaffner, F.; Vazeille, M.; Failloux, A.-B. Potential Vectors of Rift Valley Fever Virus in the Mediterranean Region. 2008. Available online: https://www.liebertpub.com/doi/10.1089/vbz.2008.0009 (accessed on 12 December 2024).
- Hardy, H.; Hopkins, R.; Mnyone, L.; Hawkes, F.M. Manure and mosquitoes: Life history traits of two malaria vector species enhanced by larval exposure to cow dung, whilst chicken dung has a strong negative effect. Parasit. Vectors 2022, 15, 472. [Google Scholar] [CrossRef]
- Gillies, M.T.; Coetzee, M. A Supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region). The South African Institute for Medical Research: Johannesburg, South Africa, 1987. [Google Scholar]
- Nepomichene, T.N.J.J.; Raharimalala, F.N.; Andriamandimby, S.F.; Ravalohery, J.P.; Failloux, A.B.; Heraud, J.M.; Boyer, S. Vector competence of Culex antennatus and Anopheles coustani mosquitoes for Rift Valley fever virus in Madagascar. Med. Vet. Entomol. 2018, 32, 259–262. [Google Scholar] [CrossRef]
- Jupp, P.; Cornel, A. Vector competence tests with Rift Valley fever virus and five South African species of mosquito. J. Am. Mosq. Control Assoc. 1988, 4, 4–8. [Google Scholar]
- Faye, O.; Ba, H.; Ba, Y.; Freire, C.C.; Faye, O.; Ndiaye, O.; Elgady, I.O.; Zanotto, P.M.; Diallo, M.; Sall, A.A. Reemergence of Rift Valley fever, Mauritania, 2010. Emerg. Infect. Dis. 2014, 20, 300–303. [Google Scholar] [CrossRef]
- Wright, D.; Kortekaas, J.; Bowden, T.A.; Warimwe, G.M. Rift Valley fever: Biology and epidemiology. J. Gen. Virol. 2019, 100, 1187–1199. [Google Scholar] [CrossRef]
- Wichgers Schreur, P.J.; Vloet, R.P.; Kant, J.; van Keulen, L.; Gonzales, J.L.; Visser, T.M.; Koenraadt, C.J.; Vogels, C.B.; Kortekaas, J. Reproducing the Rift Valley fever virus mosquito-lamb-mosquito transmission cycle. Sci. Rep. 2021, 11, 1477. [Google Scholar] [CrossRef]
- Bron, G.M.; Wichgers Schreur, P.J.; de Jong, M.C.; van Keulen, L.; Vloet, R.P.; Koenraadt, C.J.; Kortekaas, J.; Ten Bosch, Q.A. Quantifying Rift Valley fever virus transmission efficiency in a lamb-mosquito-lamb model. Front. Cell. Infect. Microbiol. 2023, 13, 1206089. [Google Scholar] [CrossRef]
- Mweya, C.N.; Kimera, S.I.; Karimuribo, E.D.; Mboera, L.E.G. Comparison of sampling techniques for Rift Valley fever virus potential vectors, Aedes aegypti and Culex pipiens complex, in Ngorongoro district in northern Tanzania. Tanzan. J. Health Res. 2013, 15, 158–164. [Google Scholar] [CrossRef]
- Doi, T.; Behera, S.K.; Yamagata, T. On the Predictability of the Extreme Drought in East Africa During the Short Rains Season. Geophys. Res. Lett. 2022, 49, e2022GL100905. [Google Scholar] [CrossRef]
| Mosquito Species | Bugesera | Rwamagana | Ngoma | Kirehe | Kayonza | Total | % | Pools Tested | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP1 | CP2 | CP3 | CP1 | CP2 | CP3 | CP1 | CP2 | CP3 | CP1 | CP2 | CP3 | CP1 | CP2 | CP3 | ||||
| An. gambiae s.l. | 244 | 46 | 119 | 196 | 1 | 0 | 14 | 3 | 6 | 41 | 1 | 10 | 332 | 23 | 6 | 1042 | 7.0 | 47 |
| An.maculipalpis | 34 | 14 | 0 | 13 | 25 | 4 | 57 | 11 | 22 | 6 | 10 | 129 | 99 | 1 | 1 | 426 | 2.9 | 28 |
| An. squamosus | 3 | 2 | 2 | 5 | 74 | 12 | 46 | 5 | 32 | 5 | 13 | 0 | 1 | 0 | 5 | 205 | 1.4 | 17 |
| An. ziemanni | 0 | 16 | 157 | 42 | 139 | 13 | 96 | 144 | 106 | 1 | 3 | 58 | 10 | 18 | 13 | 816 | 5.5 | 47 |
| An. funestus | 0 | 0 | 0 | 0 | 0 | 1 | 44 | 34 | 0 | 1 | 0 | 0 | 0 | 3 | 4 | 87 | 0.6 | 10 |
| An. rufipes | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0.0 | 3 |
| An. coustani | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 34 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 38 | 0.3 | 2 |
| Total Anopheles | 281 | 78 | 278 | 256 | 239 | 30 | 261 | 232 | 168 | 54 | 27 | 197 | 443 | 45 | 29 | 2618 | 17.6 | 154 |
| Cx. quinquefasc. | 119 | 405 | 1218 | 1195 | 1144 | 120 | 830 | 461 | 451 | 33 | 20 | 474 | 3108 | 434 | 757 | 10769 | 72.7 | 487 |
| Cx. poicilipes | 4 | 0 | 58 | 1 | 3 | 0 | 2 | 1 | 1 | 2 | 6 | 0 | 0 | 4 | 17 | 99 | 0.7 | 18 |
| Cx. annulioris | 1 | 0 | 0 | 4 | 26 | 1 | 0 | 8 | 0 | 0 | 9 | 0 | 0 | 2 | 0 | 51 | 0.3 | 8 |
| Cx. spp. (NI) | 0 | 0 | 54 | 134 | 13 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 469 | 28 | 13 | 717 | 4.8 | 42 |
| Total Culex | 124 | 405 | 1330 | 1334 | 1186 | 121 | 832 | 476 | 452 | 35 | 35 | 474 | 3577 | 468 | 787 | 11636 | 78.5 | 555 |
| Ma. africana | 7 | 12 | 40 | 17 | 32 | 6 | 17 | 28 | 56 | 0 | 0 | 83 | 0 | 1 | 0 | 299 | 2.0 | 19 |
| Ma. uniformis | 0 | 0 | 43 | 17 | 36 | 6 | 2 | 24 | 30 | 2 | 0 | 1 | 3 | 9 | 0 | 173 | 1.2 | 18 |
| Total Mansonia | 7 | 12 | 83 | 34 | 68 | 12 | 19 | 52 | 86 | 2 | 0 | 84 | 3 | 10 | 0 | 472 | 3.2 | 37 |
| Coq. maculipen. | 0 | 0 | 11 | 9 | 0 | 0 | 20 | 7 | 8 | 0 | 0 | 1 | 0 | 0 | 0 | 56 | 0.4 | 6 |
| Coq. aurites | 0 | 0 | 0 | 0 | 12 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 18 | 0.1 | 5 |
| Total Coq. | 0 | 0 | 11 | 9 | 12 | 4 | 20 | 7 | 8 | 0 | 0 | 1 | 0 | 1 | 1 | 74 | 0.5 | 11 |
| Ae. aegypti | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 4 | 1 | 0 | 1 | 10 | 0.1 | 5 |
| Ae. spp. (NI) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 5 | 0.0 | 3 |
| Total Aedes | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 2 | 1 | 0 | 3 | 4 | 1 | 0 | 1 | 15 | 0.1 | 8 |
| Overall total | 412 | 495 | 1702 | 1633 | 1506 | 169 | 1132 | 769 | 715 | 91 | 65 | 760 | 4024 | 524 | 818 | 14,815 | 100 | 765 |
| Collection Period | Mosquito Species | RT-PCR * Positive Pools | RVFV Isolated | Sequence Obtained ** | Sequence ID | Site of Collection | No. Pools Tested | No. Mosq. Tested | MIR (‰) |
|---|---|---|---|---|---|---|---|---|---|
| Period 2 | Culex quinquefasciatus | 1 | 0 | 0 | _ | Rwamagana | 138 | 2462 | 0.4 |
| Period 3 (Outbreak) | Anopheles ziemanni | 2 | 0 | 2 | RW003 RW004 | Ngoma | 17 | 334 | 5.9 |
| Anopheles gambiae s.l. | 1 | 0 | 1 | RW006 | Kirehe | 10 | 141 | 7 | |
| Culex quinquefasciatus | 2 | 2 | 2 *** | RW089 RW627 | Kayonza and Rwamagana | 158 | 3020 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nsengimana, I.; Hakizimana, E.; Mupfasoni, J.; Hakizimana, J.N.; Chengula, A.A.; Kasanga, C.J.; Eastwood, G. Identification of Potential Vectors and Detection of Rift Valley Fever Virus in Mosquitoes Collected Before and During the 2022 Outbreak in Rwanda. Pathogens 2025, 14, 47. https://doi.org/10.3390/pathogens14010047
Nsengimana I, Hakizimana E, Mupfasoni J, Hakizimana JN, Chengula AA, Kasanga CJ, Eastwood G. Identification of Potential Vectors and Detection of Rift Valley Fever Virus in Mosquitoes Collected Before and During the 2022 Outbreak in Rwanda. Pathogens. 2025; 14(1):47. https://doi.org/10.3390/pathogens14010047
Chicago/Turabian StyleNsengimana, Isidore, Emmanuel Hakizimana, Jackie Mupfasoni, Jean Nepomuscene Hakizimana, Augustino A. Chengula, Christopher J. Kasanga, and Gillian Eastwood. 2025. "Identification of Potential Vectors and Detection of Rift Valley Fever Virus in Mosquitoes Collected Before and During the 2022 Outbreak in Rwanda" Pathogens 14, no. 1: 47. https://doi.org/10.3390/pathogens14010047
APA StyleNsengimana, I., Hakizimana, E., Mupfasoni, J., Hakizimana, J. N., Chengula, A. A., Kasanga, C. J., & Eastwood, G. (2025). Identification of Potential Vectors and Detection of Rift Valley Fever Virus in Mosquitoes Collected Before and During the 2022 Outbreak in Rwanda. Pathogens, 14(1), 47. https://doi.org/10.3390/pathogens14010047









