Abstract
Hemoparasitic diseases represent a significant problem with a considerable impact on tropical and subtropical areas of the world. These conditions cause economic losses associated with multi-organic failure and even the death of animals. In these areas, the hemoparasites are transmitted in an enzootic cycle when infectious cattle, such as persistently infected animals, including cows, contribute to the success of transmission. However, the factors associated with transmission have always been considered environmental issues, disregarding herd management and practices. In this sense, we conducted a cross-sectional study sampling 360 female cattle older than one year to identify infectious cattle using the PCR technique. We employed a dichotomic questionnaire for association analyses in 150 herds of the southern Andean region of Colombia. Overall prevalence with infectious cattle was 52.5% for Babesia spp., Anaplasma spp., and Trypanosoma spp., and the significant risk factors (p < 0.05) included geographic area, animal weight, purchase of cattle for fattening, disinfection of clothing after contact with neighboring animals, self-medication, separation of animals in pens, supply of mineralized salt, presence of livestock from other owners on the farm, prevention of joint trauma, documented milking routine, and sending blood samples for analysis. These practices permitted the maintenance of persistently infected animals and their movement to shed the agents to other animals in the presence of vectors. This suggests the importance of implementing comprehensive control and training measures to reduce the infectious cattle and, therefore, the profitability of dual-purpose livestock farms in the Andean region of southwestern Colombia.
1. Introduction
In tropical and subtropical areas, vector-borne pathogens cause significant economic losses due to hemoparasitism in cattle. Ixodid ticks and biting flies, which show similar distribution patterns across many regions worldwide, enhance the transmission of primary hemoparasitic agents, such as Babesia, Anaplasma, and Trypanosoma [1]. The spread of these pathogens is influenced by various epidemiological factors related to production systems and herd management practices, leading to reduced productivity, increased veterinary losses, and economic impacts [2]. Persistently infected cattle play a crucial role in sustaining the transmission cycle, with their presence associated with husbandry practices and environmental changes [3]. In South American countries, including Colombia, stable to unstable enzootic conditions with differing prevalence rates can predispose herds to ongoing transmission cycles or outbreaks when infected cattle are present [4].
Diseases of national interest, according to Resolution 003714 of the Instituto Colombiano Agropecuario ICA, include babesiosis, anaplasmosis, and trypanosomiasis due to their significant impact on livestock health and productivity [5]. These diseases primarily cause anemia, along with weight loss, increasing mortality, and making individuals more susceptible to other diseases [6]. Economic losses due to deaths from these diseases were estimated at approximately USD 108,000, broken down as follows: anaplasmosis, USD 46,057; babesiosis, USD 24,721; trypanosomosis, USD 34,881, and cases with more than one hemoparasite, USD 2370 [7].
In mountainous regions, herds are exposed to significant variations in rainfall and climate, including differences in temperature and humidity based on altitude. Extensive and semi-extensive livestock management practices, coupled with limited strategic interventions and best practices under changing climatic conditions, promote the proliferation of hematophagous arthropods and the persistence of infected cattle [8]. Herds in regions experiencing co-infestation by ectoparasites exhibit diverse epidemiological scenarios involving pathogens such as Babesia bovis, Babesia bigemina, Anaplasma marginale, Trypanosoma vivax, and Trypanosoma evansi as single or mixed infections [5,9,10]. Traditionally, these pathogens’ epidemiological dynamics were studied in limited areas, often overlooking their impact across broader geographic regions.
Therefore, the aim of this study was to determine the prevalence of these diseases in dual-purpose cattle in Colombia, providing insights into the various management practices employed across 150 herds comprising 360 dual-purpose female cattle in the southwestern Andean region, which are critical for mitigating economic losses and ad-dressing potential epidemiological challenges associated with hemoparasites in these operations. Additionally, the study aims to identify and evaluate specific risk factors contributing to the occurrence of hemotropic infections, thereby supporting the protection of regional interests within the One Health framework [11,12].
2. Materials and Methods
2.1. Study Area
The study was conducted in 24 municipalities within the Huila department, located in the southwestern Andean region of Colombia. The study was distinguished by the thermal floor diversity, encompassing climates ranging from warm to cold-humid [13]. The region exhibits a bimodal rainfall pattern, with two distinct wet seasons occurring from March to May and October to December. The annual precipitation ranges between 1500 and 2000 mm. The region experiences two distinct dry seasons, one from January to February and the other from July to August [14]. The number of days with precipitation varies between 100 and 150 in the Magdalena Valley, although less than 100 can be recorded in parts of the municipalities of Aipe and Villavieja. In the foothills, rainy days increase slightly, reaching 200 or more in isolated locations south of the department. These areas experience high humidity, with average temperatures ranging from 26 to 28 degrees Celsius [13].
2.2. Study Population
To identify risk or protection factors against diseases, initially, information on 230 variables about animal management and epidemiological practices was collected on each herd, addressing the following categories: good livestock practices (GLP), animal health, feeding, reproduction, facilities, veterinary medication, personnel, clinical history, sanitation, transportation, traceability, biosecurity, and socioeconomic factors. The sample size was calculated, including the official records of cow vaccination programs, to be 2618 female cattle from 224 farms with an expected prevalence of 50% for hemoparasites as identified by Ríos-Tobón et al. (2014) in similar cattle management [15]. A confidence interval of 95% and a design effect of 5% was used. The data were processed using Epi Info™ v 7.2.0.1 software (CDC, 2016), which estimated a sample size of 385 animals. However, the final sample size was 360 cows from 150 farms, sampled between May and June 2023 to account for potential field losses. During the sampling period, the animals were exposed to weather conditions typical of the final phase of the first humid period and the transition from the dry to the rainy season. These conditions were characterized by high levels of water evaporation, which created suitable conditions for ixodid tick development and less favorable conditions for biting flies that develop in the rainy season.
2.3. Processing Samples and DNA Extraction
Given the vector-borne transmission and the presence of infectious cattle, blood was collected from the tail vein in EDTA tubes and stored at 4 °C. For DNA extraction, a DNA2000 kit (Corpogen, Colombia, ref: BM-001) was used. 250 μL of blood was transferred to a 1.5 mL microcentrifuge tube, followed by 25 μL of Proteinase K. Then, 250 μL of BLU buffer was added and vortexed until the solution was homogenized. The solution was then incubated at 55 °C for 15 min. Then, 250 μL of ethanol (96–100%) was added and mixed vigorously. The minispin column was then placed in a collection tube, and the mixture was transferred by pipetting. It was centrifuged at 8000 rpm for 1 min, and the contents of the collection tube were discarded. The minispin column was then placed in a new collection tube, and 500 μL of WB1 buffer was added. The sample was centrifuged at 8000 rpm for 1 min, and the contents of the collection tube were discarded. The minispin column was then placed in a new collection tube, and 500 μL of WB2 buffer was added. The two previous procedures were then repeated, but this time, 800 μL of WB2 buffer was added, centrifuged at 8000 rpm for 1 min, and the contents of the collection tube discarded. The minispin column was then centrifuged at maximum speed for 3 min to dry. The minispin column was then placed in a new 1.5 μL labeled microcentrifuge tube, and 100–200 μL of EB buffer was added. The tube was sealed and incubated for 2 min at room temperature. Finally, it was centrifuged at maximum speed for 1 min to elute the genomic DNA, which was purified and stored at 2–8 °C for a few days.
2.4. PCR Technique
DNA samples were processed by endpoint polymerase chain reaction (PCR) using Taq DNA Polymerase Master Mix 2X (Invitrogen, ThermoFisher, Waltham, MA, USA, including the primer sequences in Table 1. The PCR reaction volume was 12.5 µL using Taq red mix bioline 6.25 µL, 0.5 µL each of forward and reverse primers, and 2 µL DNA (50 ng/µL). For the negative control, water was added instead of DNA. Positive and negative controls were included in each analysis. PCR was performed on a Veritu-96 Well Thermal Cycler (ThermoFisher, Waltham, MA, USA), following an initial denaturation at 95 °C for 1 min, then 35 cycles of denaturation (95 °C for 15 s), annealing (58 °C for 15 s), and extension (72 °C for 10 s). There was one final extension cycle at 72 °C for 1 min and cooling at 4 °C. A 143 pb, 100 pb, and 298 pb for Anaplasma spp., Babesia spp., and Trypanosoma spp. products were observed by electrophoresis on 1.5% agarose gel with a cyber green stain.

Table 1.
Primer sequence used for PCR amplification.
2.5. Data Analysis
The prevalence was calculated using a 95% confidence interval (CI) with data entry. Data from an epidemiological questionnaire with dichotomous responses were used for association analysis, using Pearson’s correlation to analyze the relationships between the different pathogens. A descriptive study of risk factors was then performed. Binary logistic regression models were then used, where the dependent variable was the result of the PCR tests (0: negative, 1: positive for some hemoparasites), and the risk factors were defined as independent variables. For each factor, odds ratios (ORs) and 95% confidence intervals were analyzed to determine the influence of the predictive variables on the positive or negative test result (p-value < 0.05). To visually identify the relationships between the factors and the animal management activities carried out in the herds, risk factors with a significant effect on the results of the presence or absence of the disease were analyzed using Euclidean distance and hierarchical clustering. Descriptive analyses, logistic regression models, and risk factor analyses were performed using the R statistical program 4.3.3 (R Core Team, 2024).
3. Results
3.1. Prevalence
Of the analyzed samples, 52.5% (189/360) were positive for hemoparasites. Among these, the most prevalent pathogen was Trypanosoma spp., followed by Babesia spp. and, finally, Anaplasma spp. Conversely, co-infections exhibited a prevalence that varied slightly compared to individual infections. However, combinations of co-infections tended to be slightly less prevalent than individual infections but still accounted for a significant proportion of cases (Table 2).

Table 2.
Prevalence of hemoparasites and co-infection.
The highest prevalence of hemoparasites was identified in the 46–65-month age cohort, whereas the lowest prevalence was found in the 86–104-month cohort. For Babesia spp., the 66–85-month cohort exhibited the highest prevalence, with the lowest prevalence observed in the 86–104-month cohort. In the case of Trypanosoma spp. and Anaplasma spp., the highest prevalence was detected in the 66–85-month cohort, while individuals aged 106 months or older showed the lowest prevalence (Table 3).

Table 3.
Positive cases (+) for the presence of hemoparasites, Babesia spp., Trypanosoma spp., and Anaplasma spp., related to the age of individuals.
Regarding the geographical region, the central region exhibited the highest prevalence of hemoparasites, followed by the northern, western, and southern regions. For Babesia spp., Trypanosoma spp., and Anaplasma spp., the central region also had the highest prevalence rates, whereas the lowest prevalence for each of these pathogens was found in the southern region (Table 4).

Table 4.
Distribution of positive cases for Babesia spp., Trypanosoma spp., and Anaplasma spp. hemoparasites in cattle by geographic region in the department of Huila.
Similarly, the prevalence of any hemoparasite was higher in warm climates (temperatures above 24 °C) at 36.1% (130/360). The most frequently identified parasite was Trypanosoma spp., representing 58.7% (111/130) of cases. This was followed by Babesia spp. and Anaplasma spp., identified in 54.5% (103/130) and 54.0% (102/130) of cases, respectively. In regions with a temperate climate (between 17 and 24 °C), the prevalence of any hemoparasitic disease was 15.8% (57/360). The most prevalent species were Trypanosoma spp. (86.0% of cases, 49/57), followed by Babesia spp. (80.7% of cases, 46/57) and Anaplasma spp. (71.9% of cases, 41/57). At least 54.2% (195/360) of the animals presented one tick, and of these, 43.6% (85/195) were positive for Babesia spp. and 44.1% (86/195) for Anaplasma spp. Similarly, 60.8% (219/360) of the cattle were observed to have flies, of which 47.94 (105/219) were infected with Trypanosoma spp.
3.2. Association Analysis
The Pearson correlation coefficient for the prevalence of hemoparasites was positive, indicating a direct relationship between the presence of one pathogen and the manifestation of another (Table 5). The disparity ratio analysis demonstrated that 13 of the 230 variables evaluated significantly impacted the presence or absence of diseases. Cattle from the central region showed a higher risk of infection with hemoparasites than those from the southern region, while the risk was elevated in the northern and western areas. Similarly, the presence of one or more hemoparasites was significantly related to the following risk factors when compared to the control group of each variable: the weight of the animals, the purchase of animals for fattening, the disinfection of clothing after contact with neighboring animals, self-medication, the use of clean clothing, reproductive status, and the separation of pens. The following protective factors against the prevalence of any of the hemoparasitic agents were identified: the provision of mineralized salt, the presence of cattle from other owners on the farm, evidence of trauma in their joints, documented milking routines, and the sending of blood samples (Table 6).

Table 5.
Pearson correlation for babesiosis, anaplasmosis, and trypanosomiasis.

Table 6.
Binary logistic regression analysis of factors associated with the presence of hemoparasites, Babesia spp., Trypanosoma spp., and Anaplasma spp.
The hierarchical relationships among the significant binary risk factors revealed two main groups. The first (in orange) concerns animal health and social and sanitary conditions. The second (in blue) concerns livestock activities, including commercialization, management, and biosecurity. The length of the tree branches indicates the degree of heterogeneity among the related variables (Figure 1).
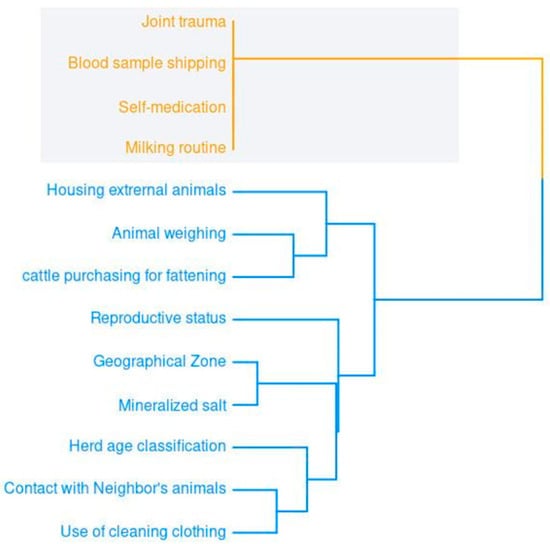
Figure 1.
Dendrogram of hierarchical relationships among the binary variables with significant representation for hemoparasite.
4. Discussion
This is the first study that reveals the prevalence of hemoparasites in infectious cattle of the Andean region of southwestern Colombia associated with risk factors. This study showed that 52.5% (189/360) of cattle were infectious, showing at least one pathogen actively involved in the maintenance of endemism because the PCR technique could identify the hemoparasites in the active phase of multiplication. Consequently, the cattle without this condition have low parasite loads, and hemoparasitism must be undiagnosed [16]. The high prevalence observed in infectious cattle is related to the infestation rates with ticks and flies. Babesia spp. and Anaplasma spp. are primarily transmitted by the tick Rhipicephalus (Boophilus) microplus, the main vector, which is widely distributed in Colombia, including the department of Huila. In the case of Anaplasma spp., transmission can also occur mechanically through hematophagous dipterans. Conversely, bovine trypanosomosis is caused by vectors from the Tabanidae family, a type of fly with high prevalence across the department of Huila. Therefore, the same animals are uninfested during periods of high arthropod activity, so enzootic transmission is unlikely into infectious cattle. In this context, the development of immune resistance following re-infection may be limited. Conversely, higher arthropod infestation rates were associated with increased exposure to hemoparasites in the cattle herd, which could be re-infected during periods of peak infestation throughout the year [17]. Therefore, it is possible that infectious animals could develop a persistent infection, maintaining high levels of parasitism and shedding them in contact with arthropod vectors [18]. This assumption is aligned with prevalence findings identified by age, which show 34.4% and 31.9% positivity in cattle between 66–85 and 46–65 months, respectively. This suggests that these animals could be persistently infected and actively participate in the transmission cycle of hemoparasites.
Regarding the transmission cycle, the environmental conditions favor the multiplication of arthropod vectors. Previous works in other areas of the country showed that transitional periods between wet and dry seasons increase the water vapor and favor the development of the non-parasite stage of ticks [19]. Similarly, a report previously showed the highest incidence of Rhipicephalus microplis in the municipalities of Huila department located in Magdalena Valley between 0 and 1000 a.s.l [20]. These findings are aligned with the highest hemoparasite prevalence identified in the north and warm climate municipalities of the Huila Department. However, the highest prevalence level of Trypanosoma spp. regarding infestation involved flies showing significant participation of these arthropods in the transmission cycle, which could involve Anaplasma spp. Similarly, the development of flies is related to rainfall levels identified in these areas that favor the biting flies [12].
About the risk factors associated with hemoparasites in cattle, this study showed that the purchase of animals from feedlots was a risk factor for the presence of at least one pathogen. In line with this, a study in England showed that the likelihood of disease spread increased when farms purchased animals from common markets. Thus, the movement of pathogen-carrying cattle between farms facilitates the spread of disease to different areas [21]. Purchasing new animals and iatrogenic transmission practices increase the likelihood of disease spreading [22]. In this study, 58.6% (211/360) of the respondents implemented quarantine measures for cattle entry. However, only 15.3% (55/360) carried out pre-diagnosis. This situation leads to the introduction of hemoparasites as quarantine does not guarantee the detection of carriers. Similarly, in a previous study in other areas of the country, the movement of persistently infected cattle for hemoparasites increased the success of transmission within farms [3].
It has been observed that farms where animals have joint trauma tend to have lower levels of hemoparasites. This is because the most used antibiotic in these herds is oxytetracycline, known for its broad spectrum and ease of use [23]. In the search for quick fixes, farmers often resort to metaphylactic measures with readily available antibiotics to treat any trauma, infection, or decline in production. As a side effect, these treatments eliminate hemoparasites. A study conducted in Colombia found that treatment with oxytetracycline for Trypanosoma spp. and Anaplasma spp. had an efficacy rate of 100% and 75.6%, respectively [11]. They also investigated the efficacy of oxytetracycline (70 mg/mL) against Babesia spp. in combination with diminazene (35 mg/mL). They concluded that the treatment reduced the presence of hemoparasites in experimental cattle and restored hematological values in animals with mild parasitemia. At the same time, they observed a similar reduction in the control group, which consisted of naturally diseased cattle with severe parasitemia [24].
Similarly, 84.2% (303/360) of cattle were self-medicated, identified as a risk factor in the analysis. In Iran, oxytetracycline resistance genes were identified: otrA in 60%, otrB in 26.7%, and 13.3% in samples infected with Anaplasma spp. [25]. In Burkina Faso, cattle tested in five out of eight zones were found to be resistant to isometamidium chloride to treat Trypanosoma spp. [26]. Similarly, it has been shown in vitro that Babesia spp. can develop resistance to the drug diminazene acetate, especially when administered at doses lower than the recommended minimum [27]. In line with this, the indiscriminate use of different types of drugs and the lack of knowledge about the correct dosage may increase or create antimicrobial resistance in some hemoparasites, especially when self-medication is a common practice among livestock farmers. In contrast, pregnant animals were found to be at higher risk of hemoparasite infection because they were immunocompromised, and it suggested the introduction of best management practices in them.
The 400–450 kg weight was a risk determinant for some hemoparasites. This is representative because it shows the average weight of female cattle in the productive stage [28]. In the case of this study, age had no significance (p > 0.05) concerning the three pathogens individually and together. However, cattle over 46 months were more susceptible to hemoparasites. In Colombia, a similar study did not obtain statistical significance for age, although the prevalence was higher in cattle between 7 and 8 years old than in those of intermediate ages [29]. Internationally, cattle over 24 months were 1.5 times more susceptible to Anaplasma spp. than younger animals due to biological or mechanical vectors [30]. Considering the above, as the animal’s weight and age increase, there is a more significant impact on its health, which results in decreased productivity.
Mineralized salt acts as a protective factor due to the importance of micro- and macroelements. It contributes to the diet, especially when it comes to low-quality food. These nutritional elements are essential for improving disease resistance and ensuring proper physiological and reproductive functioning [31]. In the same way, mineralized salt plays a fundamental role for ruminal bacteria, essential to improving the degradation and generation of nutrients by the ruminal microbiota. The increase in the production of microorganisms benefits the immune system and helps prevent various diseases in cattle. Microbiota growth indicates a better use of nutrients and increases animal immune activity. Microelements such as zinc, manganese, selenium, and copper prevent oxidative stress, and this action improves the cellular health of animals, which, in turn, contributes to the prevention of hemoparasitic diseases [32].
Finally, good livestock practices include using appropriate and exclusive clothing for production. In this study, it was observed that clothing was a risk factor. Cases have been observed where work clothing on other farms serves as a reservoir for pathogens, which, together with poor disinfection, leads to the spread of pathogens. Washing water alone is ineffective; soaps that do not contain specific components, such as methanol or polysorbate 20, are less effective at eliminating either the vector or its eggs [33]. Besides that, washing with water, with or without surfactants, at room temperature is not sufficient to eliminate the tick [34]. A recent study concluded that a temperature of 43 °C or higher for at least 30 min in immersion is sufficient to suppress the tick [35]. Therefore, establishing preventive measures and pinpointing effective management strategies are essential to enhancing herd protection and reducing the epidemiological threat posed by hemoparasitic agents [5,8].
5. Conclusions
We conclude that the cattle in the study area were found to be infectious, and the herd management practices and environment favored the agents’ transmission. These findings reinforce the importance of preventive and integrated strategies to control the vectors and borne pathogens and the importance of introducing technical support for cattle management in tropical areas. Finally, it is necessary to strengthen Huila livestock through training and knowledge transfer to producers, marketers, and professionals in the sector. Investment in education and research is the key to ensuring the region’s healthier and prosperous future.
Author Contributions
Conceptualization, S.F.-T. and W.O.B.-P.; formal analysis, C.A.M.-M., A.F.M.C., L.R.-S., and W.O.B.-P.; funding acquisition, S.F.-T. and W.O.B.-P.; investigation, S.F.-T.; methodology, C.A.M.-M. and Y.G.V.; project administration, S.F.-T. and W.O.B.-P.; validation, A.F.M.C., L.C.N.Á., and Y.G.V.; writing—original draft, C.A.M.-M., S.F.-T., A.F.M.C., L.R.-S., Y.G.V., and W.O.B.-P.; writing—review and editing, C.A.M.-M., S.F.-T., L.C.N.Á., L.R.-S., Y.G.V., and W.O.B.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Departmento del Huila—Sistema General de Regalias (SGR) project: “Análisis sanitario y genómico en ganado bovino de leche con énfasis en cría para el mejoramiento de las características productivas y competitivas en el departamento del Huila” BPIN 2021000100300.
Institutional Review Board Statement
The animals used in this study received handling and treatment under qualified veterinary supervision, following the animal experimentation rules described in the International Guiding Principles for Veterinary Research Involving Animals. The owners of animals were assigned informed consent before their inclusion, and personal or farm information was treated according to habeas data Colombian laws. This study was approved by the Ethics, Bioethics and Scientific Integrity Committee of the Corporación Colombiana de Investigación Agropecuaria AGROSAVIA under Act N.2 of 2021. The herd management data were registered after the approval of farmers, and the subsequent commitment document was signed under the requirement of Corporación Universitaria del Huila CORHUILA and the biotechnology project BPIN 2021000100300.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The authors confirm that the employed data supported the published claims, and the datasets analyzed during the study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank the Government of Huila—Department of Huila for financing and monitoring the BPIN 2021000100300 project; to the allies of the project; to the Comité de Ganaderos del Huila CGH for their participation with the livestock associations of the region; to the Corporación Colombiana de Investigación Agropecuaria AGROSAVIA for its support through the project “ID1002144: Valorac y multi animales alto valor genético Huila”; to the Corporación Universitaria del Huila CORHUILA for the execution of the project; to the National Planning Department DNP for monitoring the project; and to the Ministry of Science, Technology and Innovation for its supervision of the project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vokaty, S.; Desquesnes, M.; Applewhaite, L.; Favre, J.; Lieuw-A-Joe, R.; Parris-Aaron, M.; Bansse-Iisa, L. New Hemoparasite Information Network. Ann. N. Y. Acad. Sci. 1996, 791, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Behar, A.; Yasur-Landau, D.; Leszkowicz-Mazuz, M. Vector-Borne Diseases in Ruminants. Lester M. Shulman 2023, 441, 1–28. [Google Scholar] [CrossRef]
- Mor, N.H.; Tavera, J.V.M.; Tobón, J.C.; Guzmán Barragán, B.L.; López, G.B.; Vargas Duarte, J.J.; Corredor, D.W.S.; Tafur-Gómez, G.A. Hemoparasitism in Grazing Cattle and Risk Factors Associated with Husbandry Management in an Endemic Area of Eastern Colombia. J. Parasit. Dis. 2024, 48, 924–935. [Google Scholar] [CrossRef]
- Ferreira, G.C.M.; Canozzi, M.E.A.; Peripolli, V.; de Paula Moura, G.; Sánchez, J.; Martins, C.E.N. Prevalence of Bovine Babesia Spp., Anaplasma Marginale, and Their Co-Infections in Latin America: Systematic Review-Meta-Analysis. Ticks Tick-Borne Dis. 2022, 13, 101967. [Google Scholar] [CrossRef]
- Corrier, D.E.; Cortes, J.; Thompson, K.; Riaño, H.; Becerra, E.; Rodriguez, R. A Field Survey of Bovine Anaplasmosis, Babesiosis and Tick Vector Prevalence in the Eastern Plains of Colombia. 1978, 10, 91–92. Trop. Anim. Health Prod. 1978, 10, 91–92. [Google Scholar] [CrossRef]
- Jaimes-Dueñez, J.; Tique-Oviedo, M.; Arias-Vega, L.; Castiblanco-Diaz, E.; Rivero-Rodriguez, L.; Marin-Cossio, L.; Gongora-Orjuela, A.; Jimenez-Leaño, A. Epidemiological Assessment of Anaplasma Marginale, Babesia Bigemina, and Babesia Bovis Infections in Colombian Creole Cattle Breeds: A Molecular Survey in Northeastern Colombia. Vet. Parasitol. Reg. Stud. Rep. 2024, 50, 101011. [Google Scholar] [CrossRef] [PubMed]
- Osorio Martínez, F.J.; Patiño Álvarez, A.; Linares Chaparro, C.; Romero González, L.A.; Ortiz Cardozo, J.; Reina Beltrán, J.F.; González, P.M. Colombia Sanidad Animal 2012; ICA: Bogotá, Colombia, 2013. [Google Scholar]
- Cerón, W.L.; Andreoli, R.V.; Kayano, M.T.; Canchala, T.; Ocampo-Marulanda, C.; Avila-Diaz, A.; Antunes, J. Trend Pattern of Heavy and Intense Rainfall Events in Colombia from 1981–2018: A Trend-EOF Approach. Atmosphere 2022, 13, 156. [Google Scholar] [CrossRef]
- Wells, E.; Betancourth, A.; Page, W. The Epidemiology of Bovine Trypanosomiasis in Colombia. Trop. Anim. Health Prod. 1970, 2, 111–125. [Google Scholar] [CrossRef]
- Patarroyo, J.; Villa, O.; Diazgranados, H. Epidemiology of Cattle Anaplasmosis in Colombia: I. Prevalence and Distribution of Agglutinating Antibodies. Trop. Anim. Health Prod. 1978, 10, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Betancourt Echeverri Ph, J.A.; López Valencia MSc, G.; López Sierra MSc, G.A.; Evanoff Ph, E.A.; Berrío Cataño, W.; Gómez Oquendo, J.; Velásquez Arboleda, A.F. Eficacia de La Asociación Oxitetraciclina-Isometamidium En El Control de Anaplasmosis y Tripanosomosis Bovina. CES Med. Vet. Y Zootec. 2020, 15, 49–63. [Google Scholar] [CrossRef]
- Parra Arango, J.L.; Onofre, H.G.; Cassalett Bustillo, E.R. Diagnóstico, Manejo y Control Integrado de Ectoparásitos En Bovinos Doble Propósito Del Piedemonte Llanero; Corporación Colombiana de Investigación Agropecuaria-AGROSAVIA: Bogotá, Colombia, 2018. [Google Scholar]
- Urrea, V.; Ochoa, A.; Mesa, O. Seasonality of Rainfall in Colombia. Water Resour. Res. 2019, 55, 4149–4162. [Google Scholar] [CrossRef]
- Velandia, F.; Nuñez, A.; Marquínez, G. Memoria Explicativa: Mapa Geológico Del Departamento Del Huila. Scale 2001, 1. [Google Scholar]
- Ríos-Tobón, S.; Gutiérrez-Builes, L.A.; Ríos-Osorio, L.A. Assessing Bovine Babesiosis in Rhipicephalus (Boophilus) Microplus Ticks and 3 to 9-Month-Old Cattle in the Middle Magdalena Region, Colombia. Pesqui. Veterinária Bras. 2014, 34, 313–319. [Google Scholar] [CrossRef]
- Böse, R.; Jorgensen, W.; Dalgliesh, R.; Friedhoff, K.; De Vos, A. Current State and Future Trends in the Diagnosis of Babesiosis. Vet. Parasitol. 1995, 57, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Giglioti, R.; Oliveira, H.N.; Ibelli, A.M.G.; Bilhassi, T.B.; Néo, T.A.; Santana, C.H.; Rabelo, M.D.; Machado, R.Z.; de Souza Chagas, A.C.; de Sena Oliveira, M.C. Neither Quantification by qPCR nor Quantitative Elisa Can Be Used to Discriminate Angus Cattle for Resistance/Susceptibility to Babesia Bovis. Ticks Tick-Borne Dis. 2017, 8, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.A.; Rojas, C.; Figueroa, J.V. Diagnostic Tools for the Identification of Babesia Sp. in Persistently Infected Cattle. Pathogens 2019, 8, 143. [Google Scholar] [CrossRef]
- Betancourt, J.A.; Cassalett, E. Fluctuaciones En El Número de Garrapatas Boophilus Microplus Adultas Parasitando Bovinos En El Valle Del Cauca; Corporación Colombiana de Investigación Agropecuaria: Bogotá, Colombia, 1995. [Google Scholar]
- Díaz Rivera, E.; Benavides Ortiz, E.V.; Parra Trujillo, M.H.; Riveros Escobar, E.; Arcos Dorado, J.C.; Jaramillo, F.; Londoño Arango, J.E. Frecuencia y Distribucion de Garrapatas En La Cuenca Del Alto Magdalena: Recomendaciones Para Su Control; Corporación Colombiana de Investigación Agropecuaria: Bogotá, Colombia, 1999. [Google Scholar]
- Brennan, M.L.; Kemp, R.; Christley, R.M. Direct and Indirect Contacts between Cattle Farms in North-West England. Prev. Vet. Med. 2008, 84, 242–260. [Google Scholar] [CrossRef] [PubMed]
- Bastos, T.S.A.; Faria, A.M.; Madrid, D.M.d.C.; Bessa, L.C.d.; Linhares, G.F.C.; Fidelis, O.L.; Sampaio, P.H.; Cruz, B.C.; Cruvinel, L.B.; Nicaretta, J.E. First Outbreak and Subsequent Cases of Trypanosoma Vivax in the State of Goiás, Brazil. Rev. Bras. Parasitol. Veterinária 2017, 26, 366–371. [Google Scholar] [CrossRef]
- Lozano, M.C.; Arias, D.C. Residuos de Fármacos En Alimentos de Origen Animal: Panorama Actual En Colombia. Rev. Colomb. Cienc. Pecu. 2008, 21, 121–135. [Google Scholar] [CrossRef]
- Benavides Ortiz, E.; Vizcaíno Gerdts, O.; Polanco Palencia, N.; Mestra Pineda, A.; Betancur Hurtado, O.J. Efecto Terapéutico de Un Fármaco Frente a Los Hemoparásitos Del Bovino Babesia Bovis, Babesia Biaemina y Anaplasma Marginale. CES Med. Vet. Zootec. 2012, 7, 33–48. [Google Scholar]
- Shahbazi, P.; Nouri Gharajalar, S.; Mohebbi, K.; Taeb, J.; Hashemzadeh Farhang, H.; Nikvand, A.A.; Norouzi, R. First Survey on the Presence and Distribution of Oxytetracycline-Resistance Genes in Anaplasma Species. Acta Parasitol. 2021, 66, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Sow, A.; Sidibé, I.; Bengaly, Z.; Marcotty, T.; Séré, M.; Diallo, A.; Vitouley, H.S.; Nebié, R.; Ouédraogo, M.; Akoda, G. Field Detection of Resistance to Isometamidium Chloride and Diminazene Aceturate in Trypanosoma Vivax from the Region of the Boucle Du Mouhoun in Burkina Faso. Vet. Parasitol. 2012, 187, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Tuvshintulga, B.; Sivakumar, T.; Yokoyama, N.; Igarashi, I. Development of Unstable Resistance to Diminazene Aceturate in Babesia Bovis. Int. J. Parasitol. Drugs Drug Resist. 2019, 9, 87–92. [Google Scholar] [CrossRef]
- Zambrano, J.C.; Echeverri, J. Genetic and Environmental Variance and Covariance Parameters for Some Reproductive Traits of Holstein and Jersey Cattle in Antioquia (Colombia). Rev. Bras. Zootec. 2014, 43, 132–139. [Google Scholar] [CrossRef]
- Salamanca-Carreño, A.; Tamasaukas, R.; Cesar-Giraldo-Forero, J.; Quintero, A.D.; Hernandez-Rodríguez, M.E. Interacción Entre Factores Ambientales y Raciales Sobre La Prevalencia de Hemotrópicos En Hembras Bovinas Doble Propósito En Sabanas Inundables Araucanas, Colombia. Rev. Científica 2018, 28, 52–62. [Google Scholar]
- Okafor, C.C.; Collins, S.L.; Daniel, J.A.; Harvey, B.; Coetzee, J.F.; Whitlock, B.K. Factors Associated with Seroprevalence of Bovine Anaplasmosis in Texas. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Overton, T.; Yasui, T. Practical Applications of Trace Minerals for Dairy Cattle. J. Anim. Sci. 2014, 92, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.P. Invited Review: Mineral Absorption Mechanisms, Mineral Interactions That Affect Acid–Base and Antioxidant Status, and Diet Considerations to Improve Mineral Status. J. Dairy Sci. 2018, 101, 2763–2813. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Juliet, S.; Ajith Kumar, K.; Sunil, A.; Amithamol, K.; Nair, S.; Chandrasekhar, L.; Sujith, S.; Bandyapadhyay, A.; Rawat, A. Effects of Solvents and Surfactants against Haemaphysalis Bispinosa. Trop. Biomed. 2011, 28, 482–486. [Google Scholar] [PubMed]
- Nyahangare, E.T.; Mvumi, B.M.; McGaw, L.J.; Eloff, J.N. Addition of a Surfactant to Water Increases the Acaricidal Activity of Extracts of Some Plant Species Used to Control Ticks by Zimbabwean Smallholder Farmers. BMC Vet. Res. 2019, 15, 404. [Google Scholar] [CrossRef]
- Schimpf, D.J.; Ewert, M.M.; Lai, V.K.; Clarke, B.L. Responses of Ticks to Immersion in Hot Bathing Water: Effect of Surface Type, Water Temperature, and Soap on Tick Motor Control. PLoS ONE 2021, 16, e0261592. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).